Microstructure, Mechanical, Corrosion, and Ignition Properties of WE43 Alloy Prepared by Different Processes
Abstract
1. Introduction
2. Materials and Methods
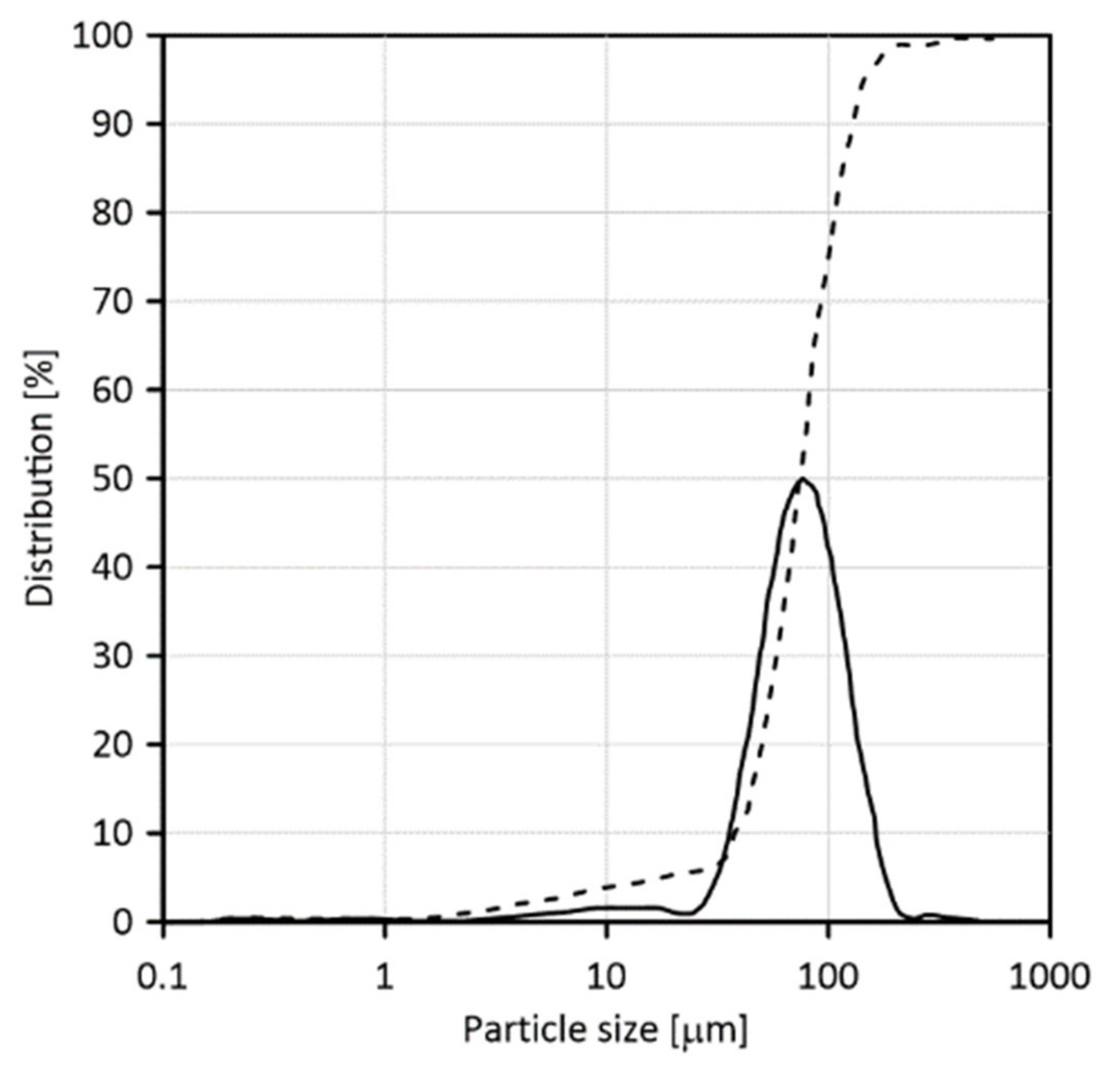
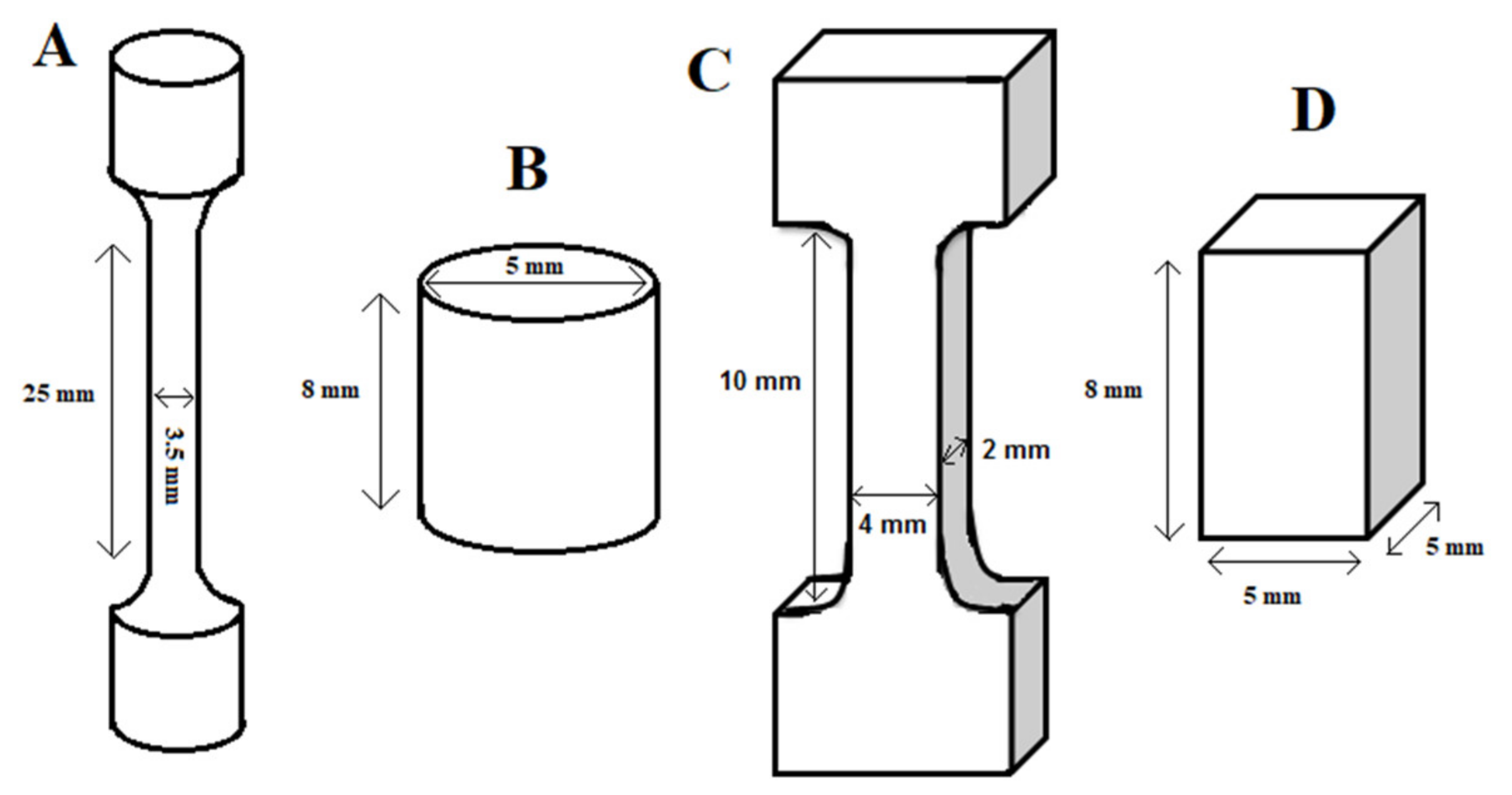
2.1. Sample Preparation
2.2. Microstructure Characterization
2.3. Mechanical Properties
2.4. Corrosion Properties
2.5. Ignition Temperature Measurement
2.6. Analysis of Oxide Mechanism
3. Results and Discussion
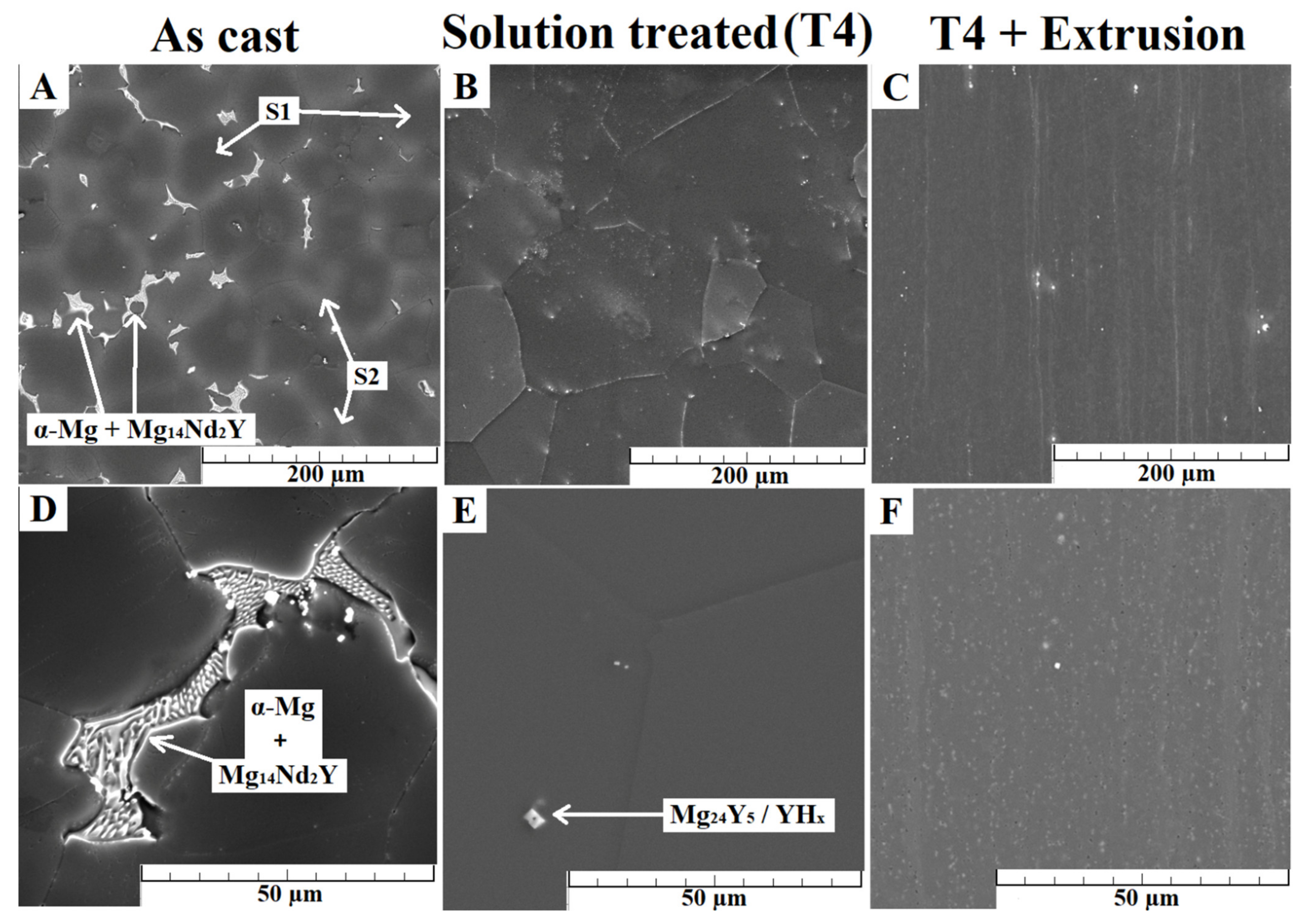
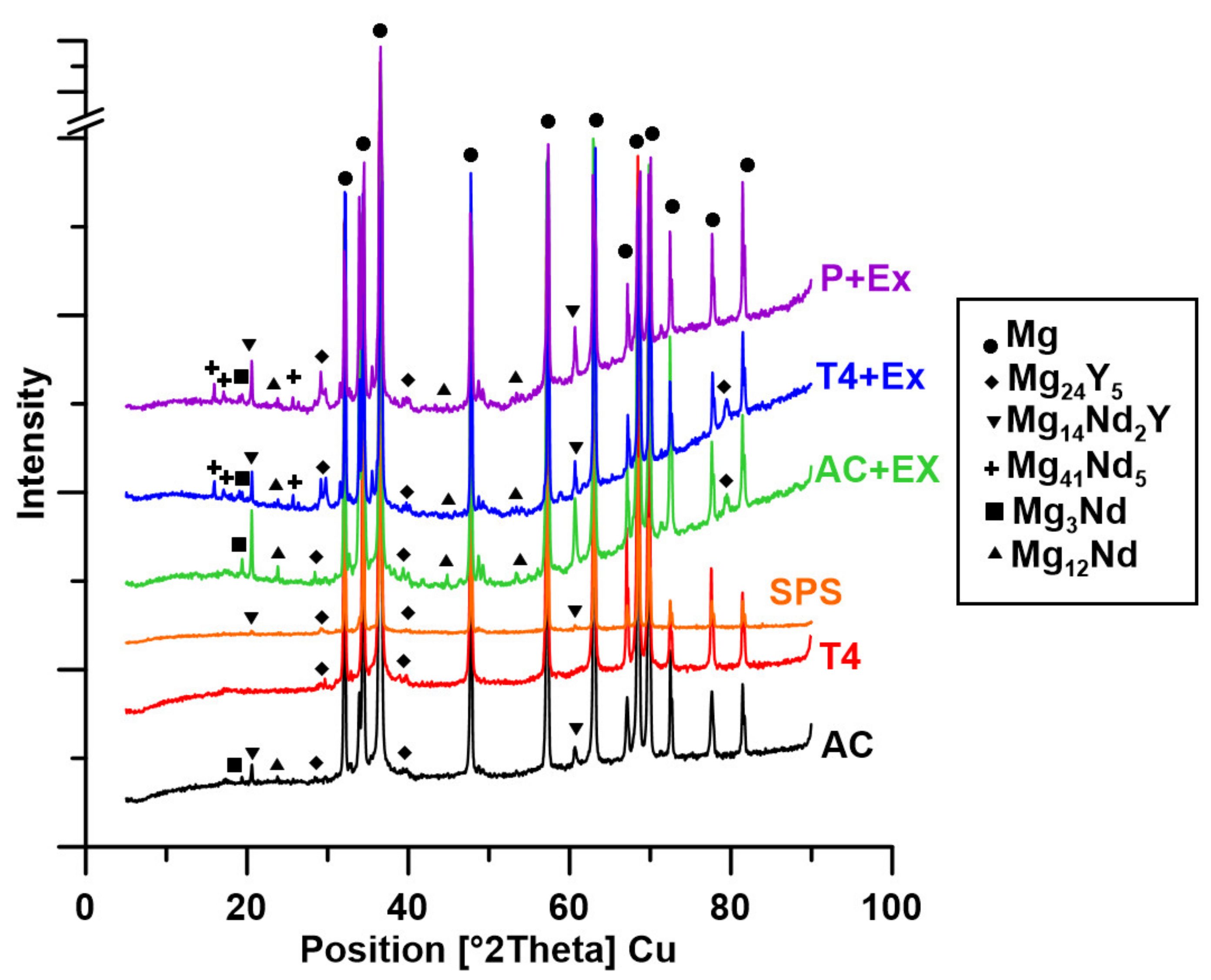
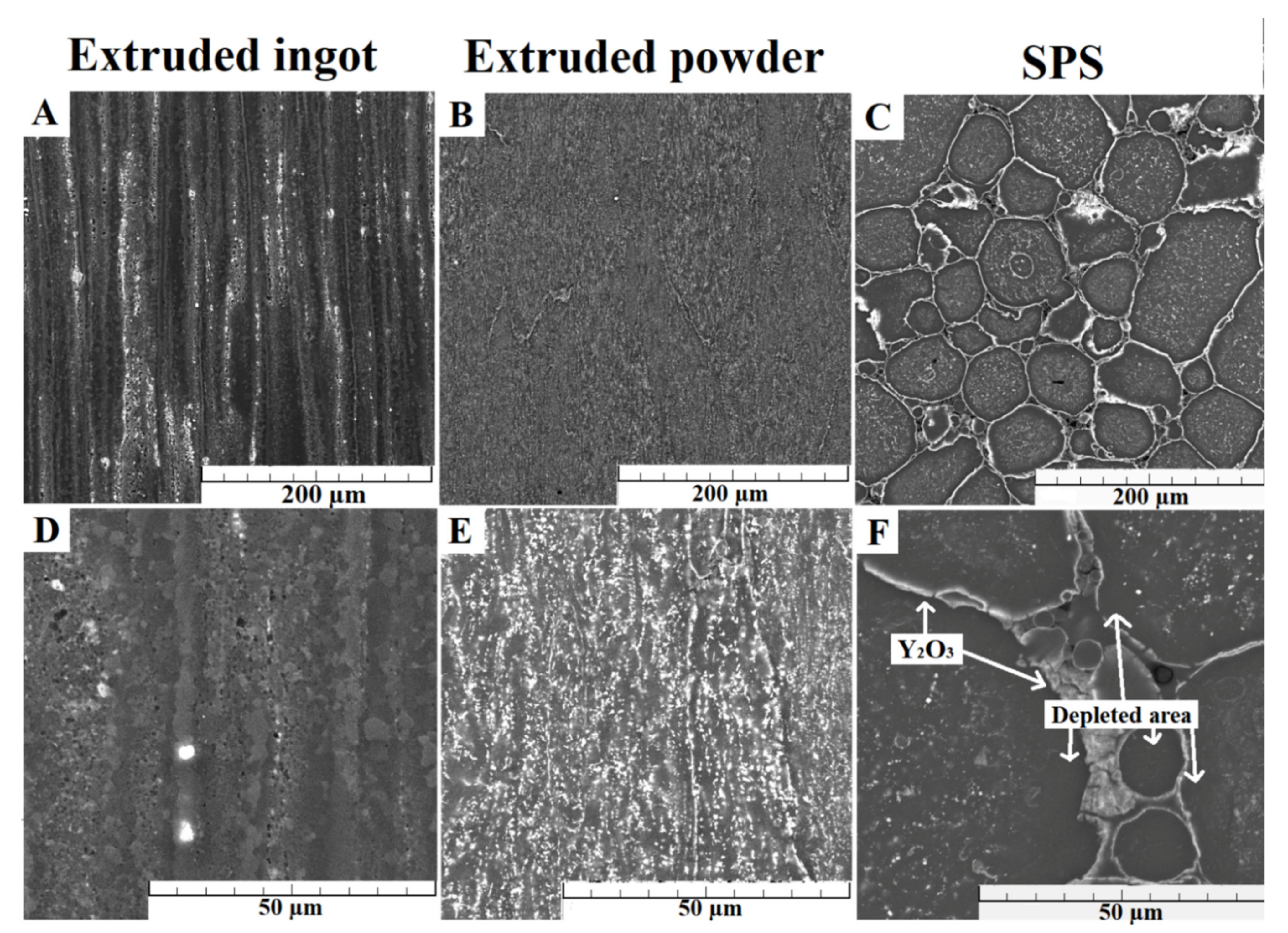
3.1. Microstructure Characterization
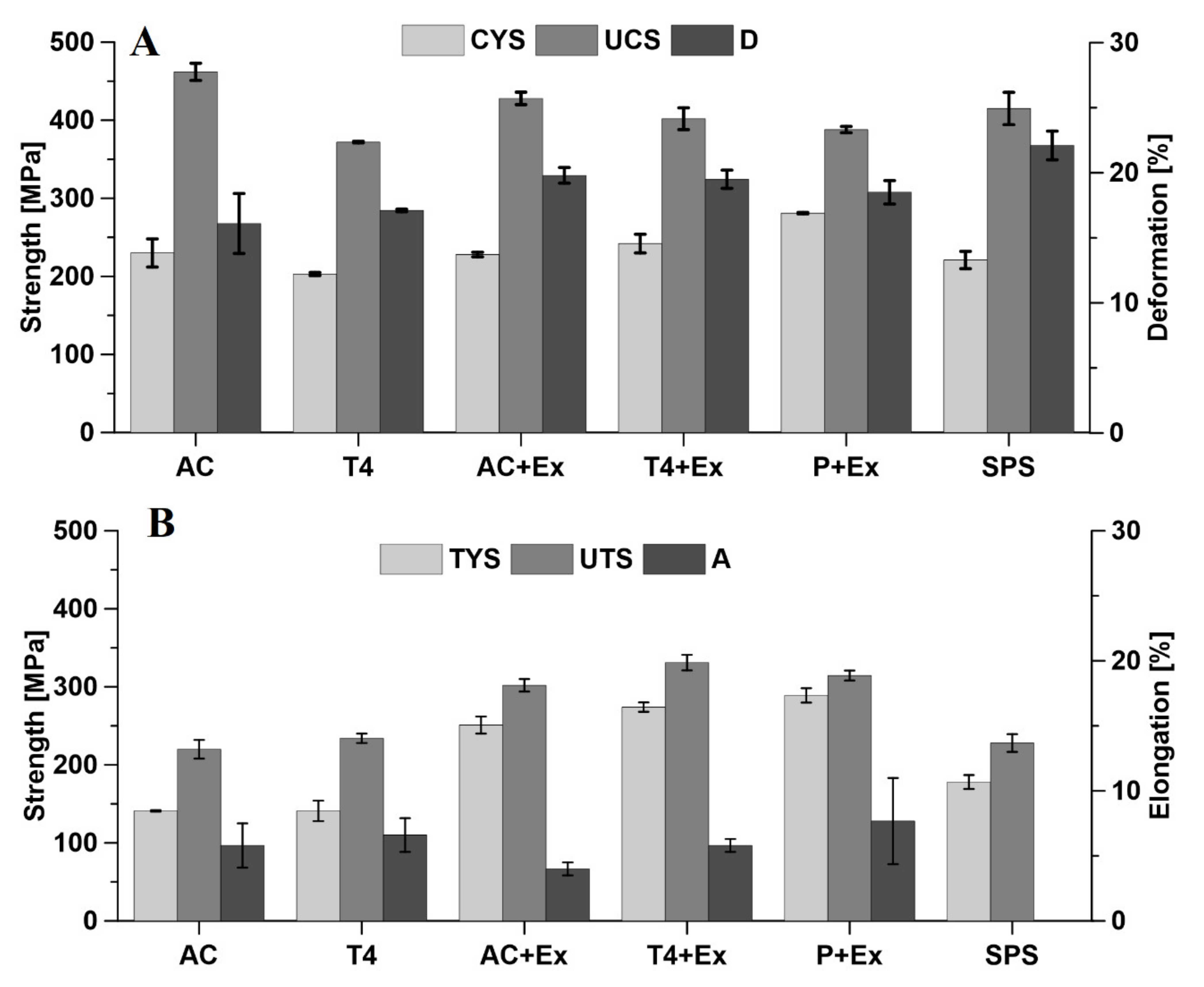
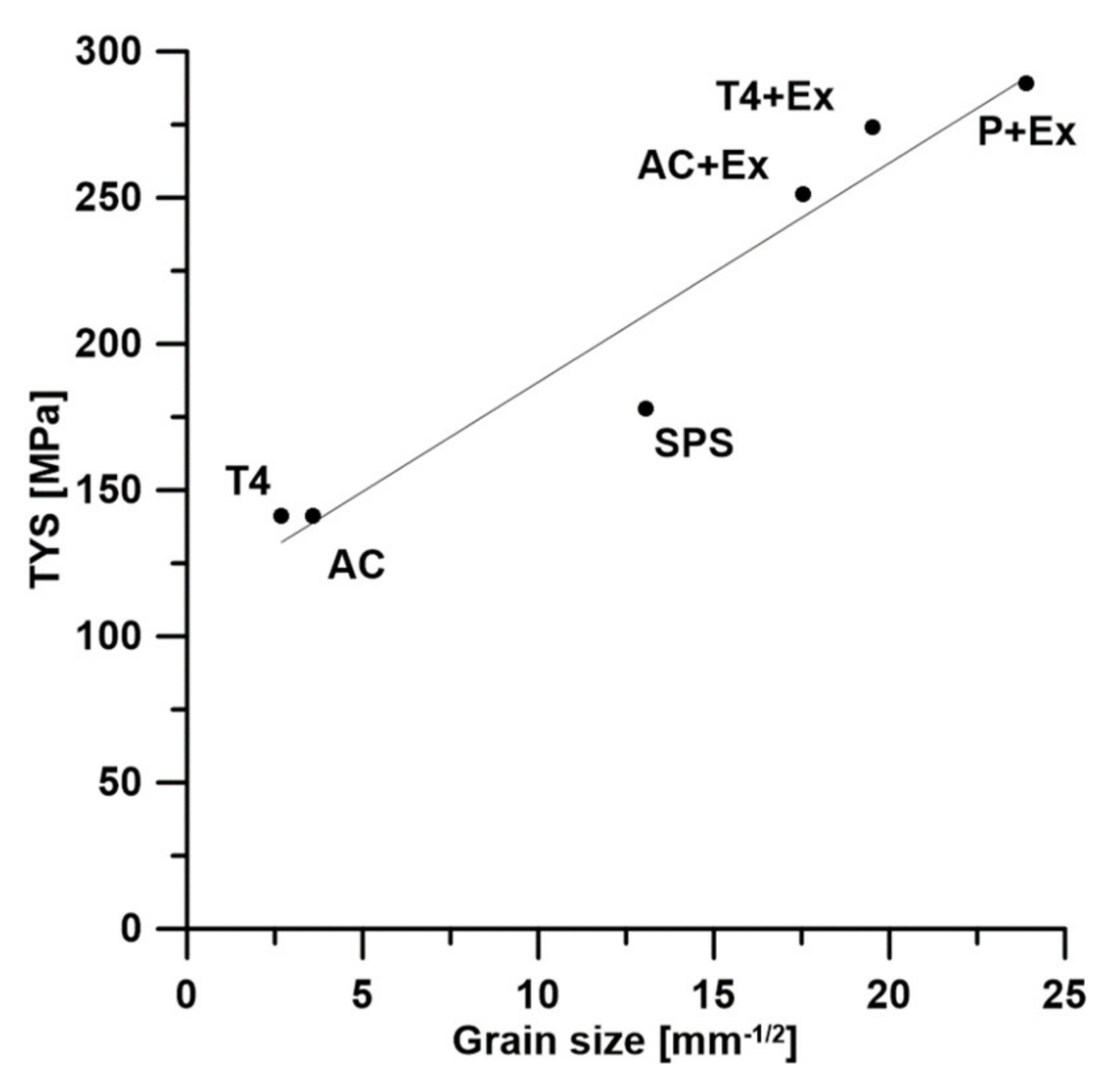
3.2. Mechanical Properties
3.3. Corrosion Properties
3.4. Ignition Temperature Characterization
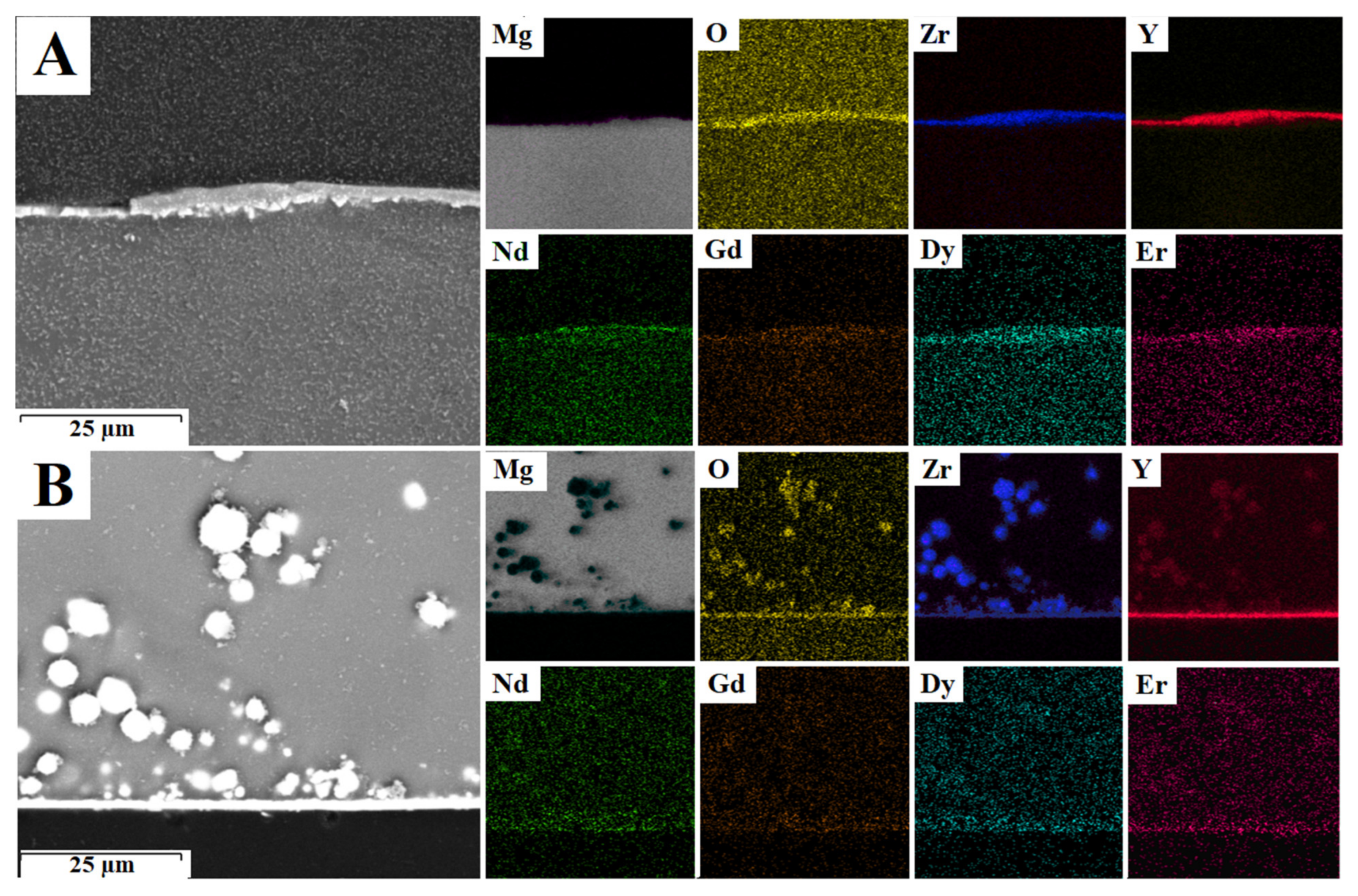
3.5. Oxide Layer Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Lu, S.; Fu, Y.; Zhang, H. Flammability and the oxidation kinetics of the magnesium alloys AZ31, WE43, and ZE10. Corros. Sci. 2015, 100, 177–185. [Google Scholar] [CrossRef]
- Mondet, M.; Barraud, E.; Lemonnier, S.; Guyon, J.; Allain, N.; Grosdidier, T. Microstructure and mechanical properties of AZ91 magnesium alloy developed by Spark Plasma Sintering. Acta Mater. 2016, 119, 55–67. [Google Scholar] [CrossRef]
- Tekumalla, S.; Si Chun, L.; Gupta, M. Preprocessing of powder to enhance mechanical and thermal response of bulk magnesium. Metal Powder Rep. 2019, 74, 137–140. [Google Scholar] [CrossRef]
- Tekumalla, S.; Gupta, M. An insight into ignition factors and mechanisms of magnesium based materials: A review. Mater. Des. 2017, 113, 84–98. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, Z.; Dong, J.; Jin, L.; Ding, W. Selective oxidation behavior of an ignition-proof Mg-Y-Ca-Ce alloy. J. Rare Earth 2013, 31, 1003–1008. [Google Scholar] [CrossRef]
- Pan, F.; Yang, M.; Chen, X. A Review on Casting Magnesium Alloys: Modification of Commercial Alloys and Development of New Alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- Aydin, D.S.; Bayindir, Z.; Hoseini, M.; Pekguleryuz, M.O. The high temperature oxidation and ignition behavior of Mg–Nd alloys part I: The oxidation of dilute alloys. J. Alloys Compd. 2013, 569, 35–44. [Google Scholar] [CrossRef]
- Yu, X.; Shen, S.; Jiang, B.; Jiang, Z.; Yang, H.; Pan, F. The effect of the existing state of Y on high temperature oxidation properties of magnesium alloys. Appl. Surf. Sci. 2016, 370, 357–363. [Google Scholar] [CrossRef]
- Ning, Z.L.; Zhao, X.Y.; Luo, A.A.; Liu, H.H.; Liang, W.Z.; Huang, Y.J.; Cao, F.Y.; Sun, J.F. The melt protection mechanism of an SO2/CO2 gas mixture for a magnesium-rare-earth based alloy. J. Alloys Compd. 2017, 722, 101–107. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Z.; Yang, W.; Fang, S.; Xu, B. Effect of yttrium, calcium and zirconium on ignition-proof principle and mechanical properties of magnesium alloys. J. Rare Earths 2012, 30, 74–78. [Google Scholar] [CrossRef]
- Go, Y.; Jo, S.M.; Park, S.H.; Kim, H.S.; You, B.S.; Kim, Y.M. Microstructure and mechanical properties of non-flammable Mg-8Al-0.3Zn-0.1Mn-0.3Ca-0.2Y alloy subjected to low-temperature, low-speed extrusion. J. Alloys Compd. 2018, 739, 69–76. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yim, C.D.; Kim, H.S.; You, B.S. Key factor influencing the ignition resistance of magnesium alloys at elevated temperatures. Scr. Mater. 2011, 65, 958–961. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Ren, X.-P.; Zhang, Y. Effect of RE on the ignition-proof, microstructure and properties of AZ91D magnesium alloy. Int. J. Miner. Metall. Mater. 2005, 12, 540–544. [Google Scholar]
- Prasad, A.; Shi, Z.; Atrens, A. Influence of Al and Y on the ignition and flammability of Mg alloys. Corros. Sci. 2012, 55, 153–163. [Google Scholar] [CrossRef]
- Han, G.; Chen, D.; Chen, G.; Huang, J. Development of non-flammable high strength extruded Mg-Al-Ca-Mn alloys with high Ca/Al ratio. J. Mater. Sci. Technol. 2018, 34, 2063–2068. [Google Scholar] [CrossRef]
- Liu, M.; Shih, D.S.; Parish, C.; Atrens, A. The ignition temperature of Mg alloys WE43, AZ31 and AZ91. Corros. Sci. 2012, 54, 139–142. [Google Scholar] [CrossRef]
- Ravi Kumar, N.V.; Blandin, J.J.; Suéry, M.; Grosjean, E. Effect of alloying elements on the ignition resistance of magnesium alloys. Scr. Mater. 2003, 49, 225–230. [Google Scholar] [CrossRef]
- Eyring, H.; Zwolinski, B. The critical temperature for combustion of metals and their alloys. In Record of Chemical Progress (Wayne State University Press); Wayne University: Detroit, MI, USA, 1947. [Google Scholar]
- Bungaro, C.; Noguera, C.; Ballone, P.; Kress, W. Early oxidation stages of Mg (0001): A density functional study. Phys. Rev. Lett. 1997, 79, 4433. [Google Scholar] [CrossRef]
- Schröder, E. Interaction effects in magnesium oxidation: A lattice-gas simulation. Comput. Mater. Sci. 2002, 24, 105–110. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Čavojský, M.; Vojtěch, D.; Beronská, N.; Fousová, M. Superior Properties of Mg–4Y–3RE–Zr Alloy Prepared by Powder Metallurgy. J. Mater. Sci. Technol. 2017, 33, 652–660. [Google Scholar] [CrossRef]
- Nie, J.F.; Muddle, B.C. Characterisation of strengthening precipitate phases in a Mg–Y–Nd alloy. Acta Mater. 2000, 48, 1691–1703. [Google Scholar] [CrossRef]
- Jiang, H.S.; Zheng, M.Y.; Qiao, X.G.; Wu, K.; Peng, Q.Y.; Yang, S.H.; Yuan, Y.H.; Luo, J.H. Microstructure and mechanical properties of WE43 magnesium alloy fabricated by direct-chill casting. Mater. Sci. Eng. A 2017, 684, 158–164. [Google Scholar] [CrossRef]
- Xiang, C.; Gupta, N.; Coelho, P.; Cho, K. Effect of microstructure on tensile and compressive behavior of WE43 alloy in as cast and heat treated conditions. Mater. Sci. Eng. A 2018, 710, 74–85. [Google Scholar] [CrossRef]
- Bütev Öcal, E.; Esen, Z.; Aydınol, K.; Dericioğlu, A.F. Comparison of the short and long-term degradation behaviors of as-cast pure Mg, AZ91 and WE43 alloys. Mater. Chem. Phys. 2020, 241, 122350. [Google Scholar] [CrossRef]
- Yang, C.; Gupta, N.; Ding, H.; Xiang, C. Effect of Microstructure on Corrosion Behavior of WE43 Magnesium Alloy in As Cast and Heat-Treated Conditions. Metals 2020, 10, 1552. [Google Scholar] [CrossRef]
- Dvorsky, D.; Kubasek, J.; Jablonska, E.; Lipov, J.; Vojtech, D. High strength AM50 magnesium alloy as a material for possible stent application in medicine. Mater. Technol. 2019, 34, 838–842. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Jakupi, P.; Danaie, M.; Botton, G.A.; Shoesmith, D.W. Tracking the corrosion of magnesium sand cast AM50 alloy in chloride environments. Corros. Sci. 2013, 75, 114–122. [Google Scholar] [CrossRef]
- Gao, G.-J.; Zeng, M.-Q.; Zhang, E.-L.; Zeng, R.-C.; Cui, L.-Y.; Xu, D.-k.; Wang, F.-Q.; Kannan, M.B. Dealloying corrosion of anodic and nanometric Mg41Nd5 in solid solution-treated Mg-3Nd-1Li-0.2Zn alloy. J. Mater. Sci. Technol. 2021, 83, 161–178. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, B.; Yang, Q.; Huang, D.; Tang, A.; Pan, F.; Han, Q. Role of second phases on the corrosion resistance of Mg-Nd-Zr alloys. J. Alloys Compd. 2020, 849, 156619. [Google Scholar] [CrossRef]
- Dvorsky, D.; Kubasek, J.; Jablonska, E.; Kaufmanova, J.; Vojtech, D. Mechanical, corrosion and biological properties of advanced biodegradable Mg-MgF2 and WE43-MgF2 composite materials prepared by spark plasma sintering. J. Alloys Compd. 2020, 825, 154016. [Google Scholar] [CrossRef]
- Minárik, P.; Zemková, M.; Lukáč, F.; Bohlen, J.; Knapek, M.; Král, R. Microstructure of the novel biomedical Mg–4Y–3Nd alloy prepared by spark plasma sintering. J. Alloys Compd. 2020, 819, 153008. [Google Scholar] [CrossRef]
- Kang, Y.; Huang, Z.; Zhao, H.; Gan, C.; Zhou, N.; Zheng, K.; Zhang, J.; Pan, F.; Huang, J.C.; Wang, S. Comparative Study of Hot Deformation Behavior and Microstructure Evolution of As-Cast and Extruded WE43 Magnesium Alloy. Metals 2020, 10, 429. [Google Scholar] [CrossRef]
- Kiełbus, A.; Rzychoń, T.; Lityńska-Dobrzyńska, L.; Dercz, G. Characterization of β and Mg41Nd5 Equilibrium Phases in Elektron 21 Magnesium Alloy after Long-Term Annealing. Solid State Phenom. 2010, 163, 106–109. [Google Scholar] [CrossRef]
- Dvorský, D.; Kubásek, J.; Průša, F.; Kristianová, E.; Vojtěch, D. Specific interface prepared by the SPS of chemically treated Mg-based powder. Mater. Chem. Phys. 2021, 261. [Google Scholar] [CrossRef]
- Dvorský, D.; Kubásek, J.; Roudnická, M.; Průša, F.; Nečas, D.; Minárik, P.; Stráská, J.; Vojtěch, D. The effect of powder size on the mechanical and corrosion properties and the ignition temperature of WE43 alloy prepared by spark plasma sintering. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]
- Zemkova, M.; Minarik, P.; Knapek, M.; Sasek, S.; Dittrich, J.; Kral, R. Microstructure and Mechanical Strength of Attritor-Milled and Spark Plasma Sintered Mg-4Y-3Nd Alloy. Crystals 2020, 10, 574. [Google Scholar] [CrossRef]
- Minarik, P.; Zemkova, M.; Knapek, M.; Sasek, S.; Dittrich, J.; Lukac, F.; Kozlik, J.; Kral, R. Effect of Short Attritor-Milling of Magnesium Alloy Powder Prior to Spark Plasma Sintering. Materials 2020, 13, 3973. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Čavojský, M.; Roudnická, M.; Vojtěch, D. WE43 magnesium alloy—Material for challenging applications. Kov. Mater. 2019, 57, 159–165. [Google Scholar] [CrossRef]
- Barnett, M.R.; Sullivan, A.; Stanford, N.; Ross, N.; Beer, A. Texture selection mechanisms in uniaxially extruded magnesium alloys. Scr. Mater. 2010, 63, 721–724. [Google Scholar] [CrossRef]
- Kleiner, S.; Uggowitzer, P.J. Mechanical anisotropy of extruded Mg–6% Al–1% Zn alloy. Mater. Sci. Eng. A 2004, 379, 258–263. [Google Scholar] [CrossRef]
- Stanford, N.; Barnett, M.R. The origin of “rare earth” texture development in extruded Mg-based alloys and its effect on tensile ductility. Mater. Sci. Eng. A 2008, 496, 399–408. [Google Scholar] [CrossRef]
- Dvorský, D.; Kubásek, J.; Voňavková, I.; Vojtěch, D. Structure, mechanical and corrosion properties of extruded Mg-Nd-Zn, Mg-Y-Zn and Mg-Y-Nd alloys. Mater. Sci. Technol. 2019, 35, 520–529. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Veselý, J.; Minárik, P.; Zemková, M.; Vojtěch, D. Characterization of the High-Strength Mg–3Nd–0.5Zn Alloy Prepared by Thermomechanical Processing. Acta Metall. Sin. 2019, 32, 321–331. [Google Scholar] [CrossRef]
- Yu, H.; Xin, Y.; Wang, M.; Liu, Q. Hall-Petch relationship in Mg alloys: A review. J. Mater. Sci. Technol. 2018, 34, 248–256. [Google Scholar] [CrossRef]
- Kang, Y.H.; Wu, D.; Chen, R.S.; Han, E.H. Microstructures and mechanical properties of the age hardened Mg–4.2Y–2.5Nd–1Gd–0.6Zr (WE43) microalloyed with Zn. J. Magnes. Alloys 2014, 2, 109–115. [Google Scholar] [CrossRef]
- Kniffka, W.; Eichmann, M.; Witt, G.; Gieseke, M.; Tandon, R.; Kiesow, T.; Wessarges, Y.; Nölke, C.; Kaierle, S. Selektives Laserstrahlschmelzen von Elektron® MAP 43 Magnesiumpulver/Selective Laser Melting of Elektron® MAP 43 Magnesium Powder. In Rapid.Tech—International Trade Show & Conference for Additive Manufacturing; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2016; pp. 244–252. [Google Scholar]
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Ding, W. Biocorrosion properties of as-extruded Mg–Nd–Zn–Zr alloy compared with commercial AZ31 and WE43 alloys. Mater. Lett. 2012, 66, 209–211. [Google Scholar] [CrossRef]
- Asqardoust, S.; Zarei Hanzaki, A.; Abedi, H.R.; Krajnak, T.; Minárik, P. Enhancing the strength and ductility in accumulative back extruded WE43 magnesium alloy through achieving bimodal grain size distribution and texture weakening. Mater. Sci. Eng. A 2017, 698, 218–229. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Du, W.; Geng, Y.; Pan, F. Effect of Nd on microstructure and mechanical properties of as-extruded Mg-Y-Zr-Nd alloy. J. Mater. Sci. Technol. 2017, 33, 926–934. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Uggowitzer, P.J.; Song, G.; Atrens, A. The influence of yttrium (Y) on the corrosion of Mg–Y binary alloys. Corros. Sci. 2010, 52, 3687–3701. [Google Scholar] [CrossRef]
- Birbilis, N.; Zhang, M.X.; Estrin, Y. Surface Grain Size Effects on the Corrosion of Magnesium. Key Eng. Mater. 2008, 384, 229–240. [Google Scholar] [CrossRef]
- Li, N.; Guo, C.; Wu, Y.H.; Zheng, Y.F.; Ruan, L.Q. Comparative study on corrossion behaviour of pure Mg and WE43 alloy in static, stirring and flowing Hank´s solution. Corros. Eng. Sci. Technol. 2012, 47, 346–351. [Google Scholar] [CrossRef]
- Argade, G.R.; Panigrahi, S.K.; Mishra, R.S. Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium. Corros. Sci. 2012, 58, 145–151. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; Huang, J.; Lian, Y.; He, G. A review on ignition mechanisms and characteristics of magnesium alloys. J. Magnes. Alloys 2020, 8, 329–344. [Google Scholar] [CrossRef]
- Dvorsky, D.; Kubasek, J.; Vojtech, D.; Minarik, P. Novel aircraft Mg-Y-Gd-Ca alloys with high ignition temperature and suppressed flammability. Mater. Lett. 2020, 264, 127313. [Google Scholar] [CrossRef]
- Dvorsky, D.; Dalibor Vojtech, J.K.; Vojtech, D.; Minárik, P.; Straska, J. The effect of Y, Gd and Ca on the ignition temperature of extruded magnesium alloys. Mater.Technol. 2020, 54, 669–675. [Google Scholar] [CrossRef]
- Fan, J.; Yang, C.; Xu, B. Effect of Ca and Y additions on oxidation behavior of magnesium alloys at high temperatures. J. Rare Earths 2012, 30, 497–502. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Granado, E.; Carvalho, W., Jr.; Kycia, S.W.; Bruno, D.; Droppa, R., Jr. X-ray powder diffraction beamline at D10B of LNLS: Application to the Ba2FeReO6 double perovskite. J. Synchrotron Radiat. 2006, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Artini, C.; Pani, M.; Plaisier, J.R.; Costa, G.A. Structural study of Nd oxidation by means of in-situ synchrotron X-ray diffraction (400 ≤ T ≤ 700 °C). Solid State Ion. 2014, 257, 38–41. [Google Scholar] [CrossRef]
- Pires, A.M.; Davolos, M.R.; Paiva-Santos, C.O.; Stucchi, E.B.; Flor, J. New X-ray powder diffraction data and Rietveld refinement for Gd2O3 monodispersed fine spherical particles. J. Solid State Chem. 2003, 171, 420–423. [Google Scholar] [CrossRef]
- Chandrasekhar, M.; Nagabhushana, H.; Sudheerkumar, K.H.; Dhananjaya, N.; Sharma, S.C.; Kavyashree, D.; Shivakumara, C.; Nagabhushana, B.M. Comparison of structural and luminescence properties of Dy2O3 nanopowders synthesized by co-precipitation and green combustion routes. Mater. Res. Bull. 2014, 55, 237–245. [Google Scholar] [CrossRef]
- Moon, R.M.; Koehler, W.C.; Child, H.R.; Raubenheimer, L.J. Magnetic structures of Er2O3 and Yb2O3. Phys. Rev. 1968, 176, 722–731. [Google Scholar] [CrossRef]
- Baldinozzi, G.; Bérar, J.F.; Calvarin-Amiri, G. Rietveld Refinement of Two-Phase Zr-Doped Y2O3. Mater. Sci. Forum 1998, 278–281, 680–685. [Google Scholar] [CrossRef]
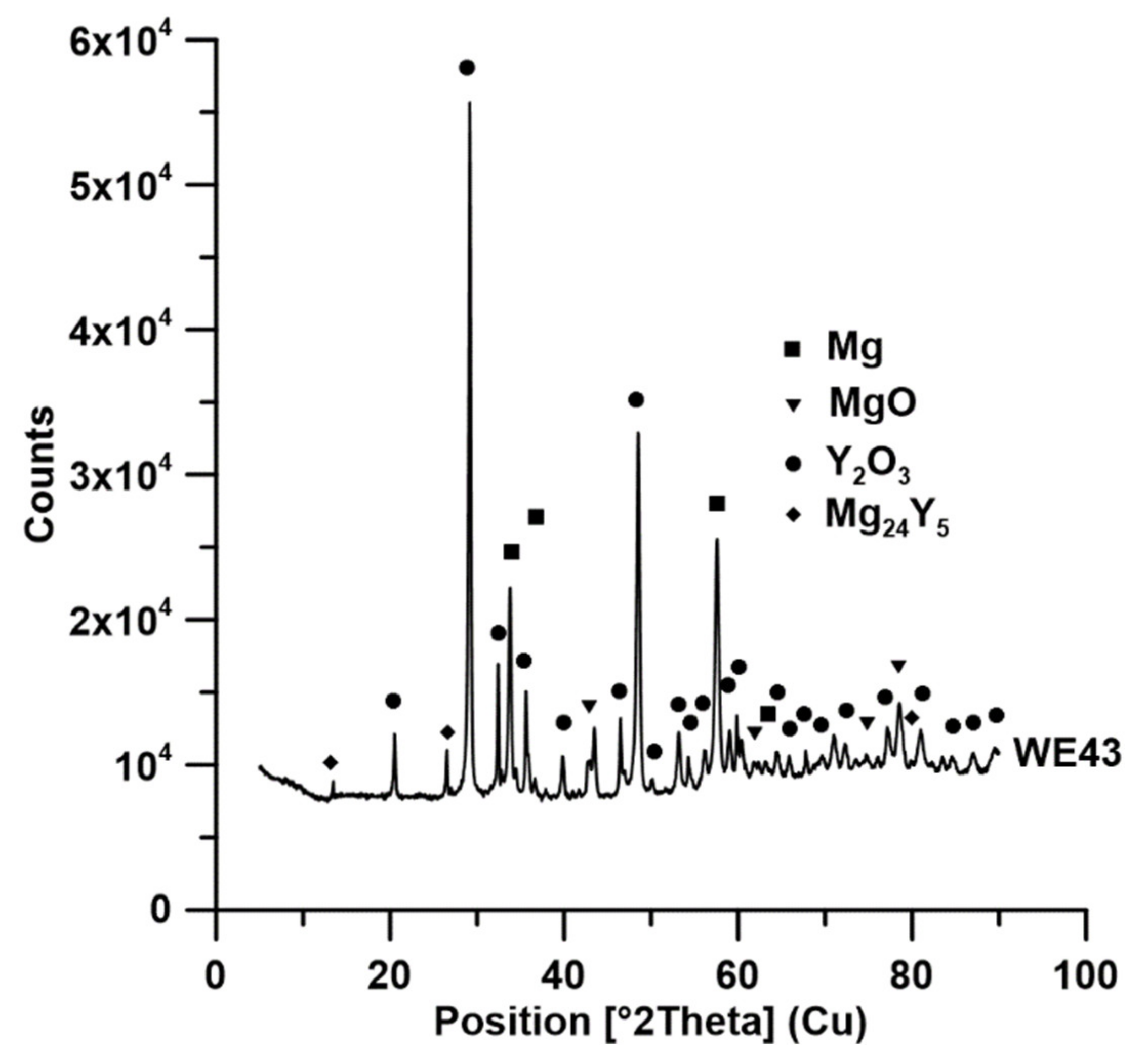
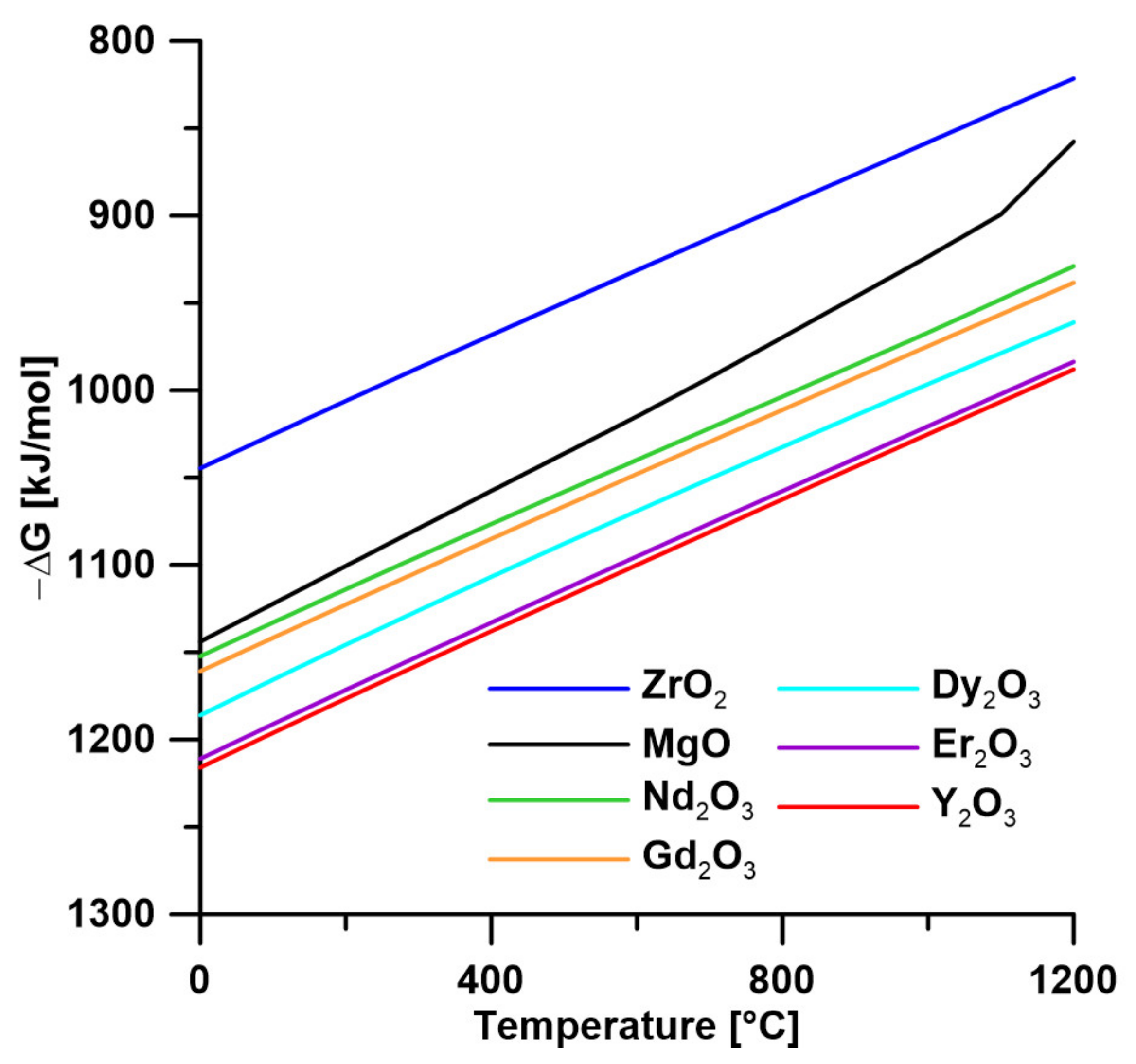
| Alloy | Tig (°C) | Oxide | PBR | The Solubility Limit of the Element (wt %) | ΔG (J, at 700°C) * |
|---|---|---|---|---|---|
| Mg-0.55Y | 721 | Y2O3 | 1.13 | 3.4 | −133 |
| Mg-0.48Gd | 707 | Gd2O3 | 1.29 | 4.53 | −56 |
| Mg-1.46Dy | 726 | Dy2O3 | 1.26 | 4.83 | −87 |
| Mg-1.42Er | 762 | Er2O3 | 1.20 | 6.91 | −125 |
| Mg | 504 | MgO | 0.81 |
| Sample | Composition (wt %) | Impurities (ppm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mg | Y | Nd | Dy | Gd | Zr | Fe | Ni | Cu | |
| WE43 Ingot | Bal. | 3.6 | 2.2 | 0.3 | 0.3 | 0.4 | 16 | 5 | 23 |
| WE43 Powder | Bal. | 4.0 | 2.2 | 0.4 | 0.4 | 0.5 | 32 | 13 | 29 |
| Material | Y | Nd | Gd | Dy | Zr | Sum | Grain Size [µm] |
|---|---|---|---|---|---|---|---|
| As-cast (dark) | 2.1 ± 0.3 | 1.0 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | 4.0 | 78 |
| As-cast (bright) | 5.5 ± 0.4 | 2.5 ± 0.3 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 9.3 | 78 |
| T4 | 3.8 ± 0.2 | 2.2 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 7.1 | 138 |
| Ex | 2.8 ± 0.3 | 1.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 5.1 | 3.3 |
| T4Ex * | 4.3 ± 0.2 | 2.2 ± 0.2 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 7.8 | 2.6 |
| PEx * | 4.2 ± 0.2 | 2.2 ± 0.3 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.9 ± 0.1 | 8.1 | 1.8 |
| SPS * | 4.0 ± 0.1 | 2.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.1 | 8.2 | 5.9 |
| As-cast | T4 | Ex | T4+Ex | P+Ex | SPS |
|---|---|---|---|---|---|
| 1.78 ± 0.07 | 0.82 ± 0.24 | 0.63 ± 0.05 | 0.53 ± 0.32 | 0.58 ± 0.11 | 3.15 ± 0.27 |
| As-cast | T4 | Ex | T4+Ex | P+Ex | SPS |
|---|---|---|---|---|---|
| 855 ± 36 | 1082 ± 11 | 1000 ± 17 | 1024 ± 6 | 936 ± 6 | 994 ± 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvorský, D.; Kubásek, J.; Hosová, K.; Čavojský, M.; Vojtěch, D. Microstructure, Mechanical, Corrosion, and Ignition Properties of WE43 Alloy Prepared by Different Processes. Metals 2021, 11, 728. https://doi.org/10.3390/met11050728
Dvorský D, Kubásek J, Hosová K, Čavojský M, Vojtěch D. Microstructure, Mechanical, Corrosion, and Ignition Properties of WE43 Alloy Prepared by Different Processes. Metals. 2021; 11(5):728. https://doi.org/10.3390/met11050728
Chicago/Turabian StyleDvorský, Drahomír, Jiří Kubásek, Klára Hosová, Miroslav Čavojský, and Dalibor Vojtěch. 2021. "Microstructure, Mechanical, Corrosion, and Ignition Properties of WE43 Alloy Prepared by Different Processes" Metals 11, no. 5: 728. https://doi.org/10.3390/met11050728
APA StyleDvorský, D., Kubásek, J., Hosová, K., Čavojský, M., & Vojtěch, D. (2021). Microstructure, Mechanical, Corrosion, and Ignition Properties of WE43 Alloy Prepared by Different Processes. Metals, 11(5), 728. https://doi.org/10.3390/met11050728






