Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Cobalt Ferrite Nanoparticles
2.3. Synthesis of Hybrid CoFe2O4/Au Nanoparticles
2.4. Nanoparticle Characterization
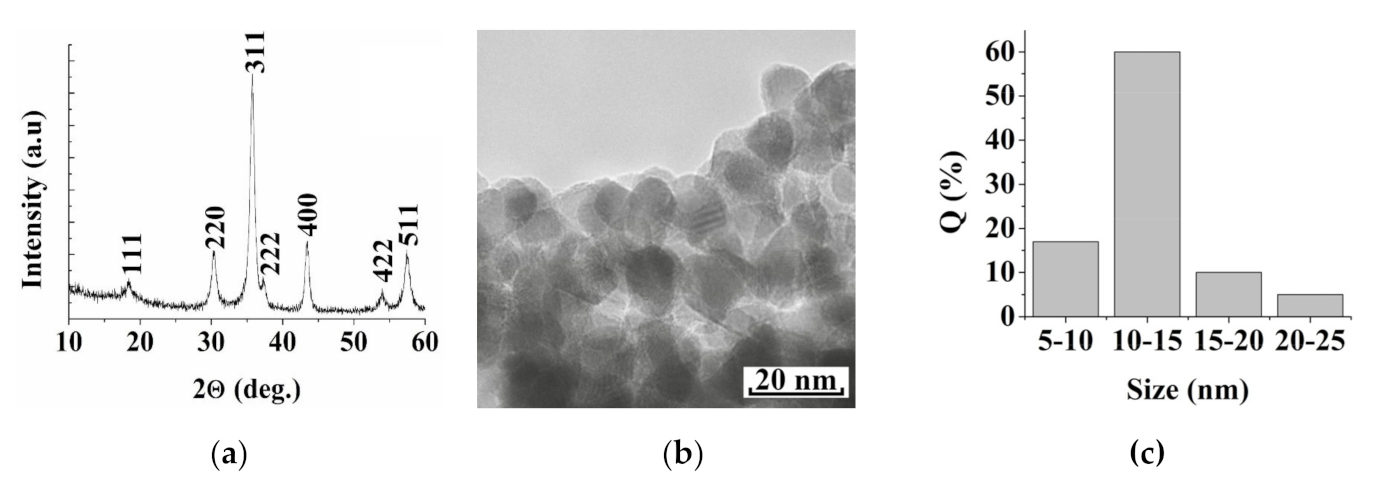
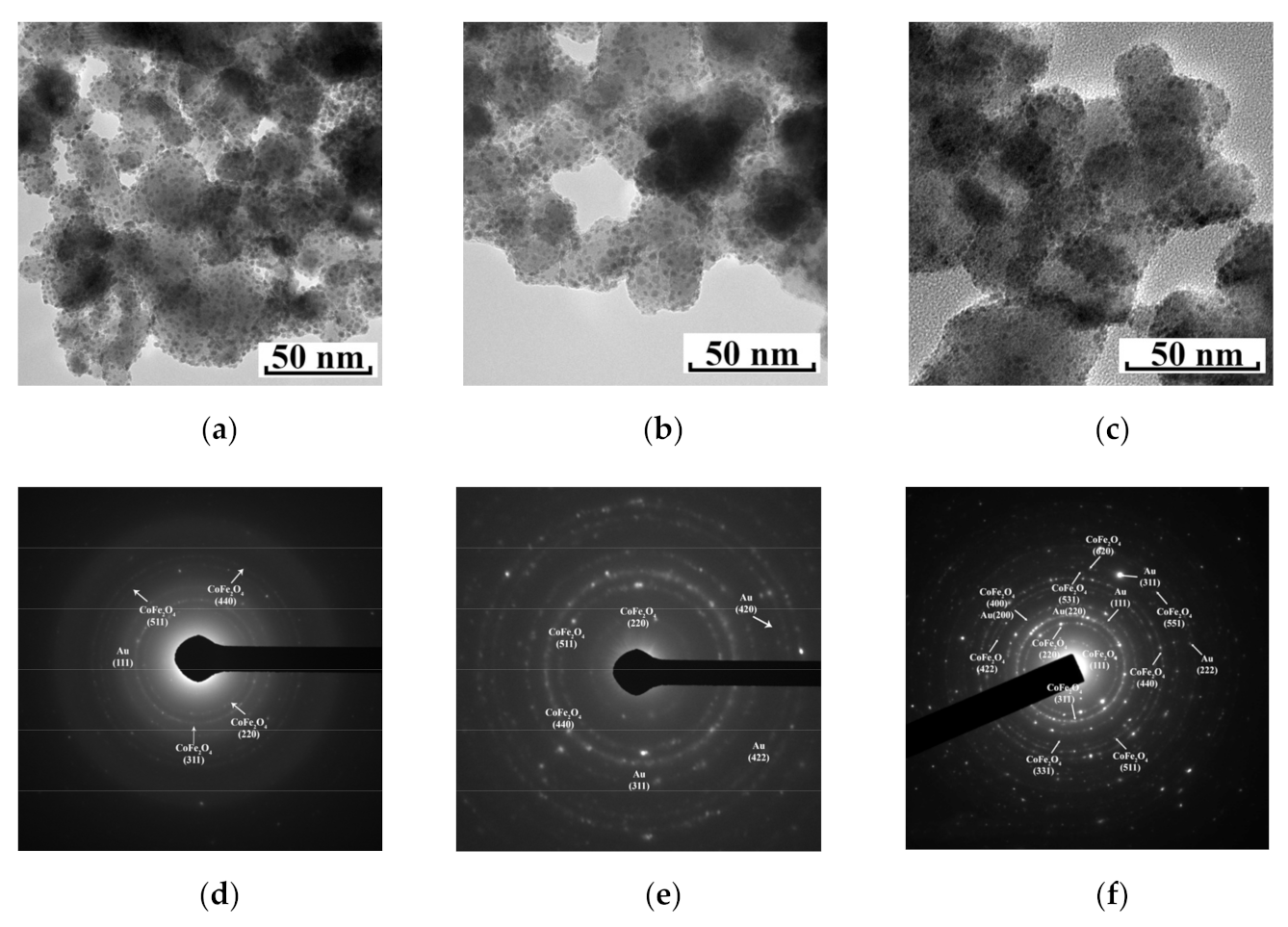
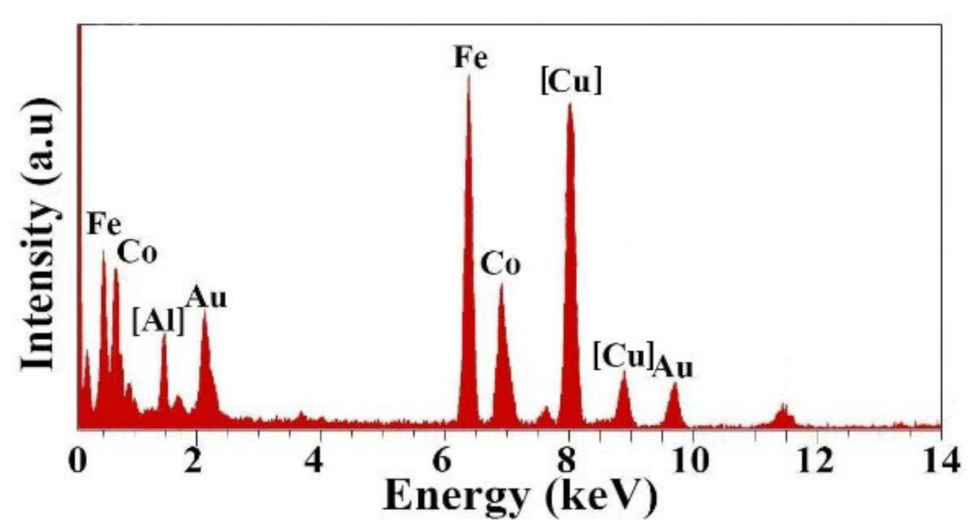
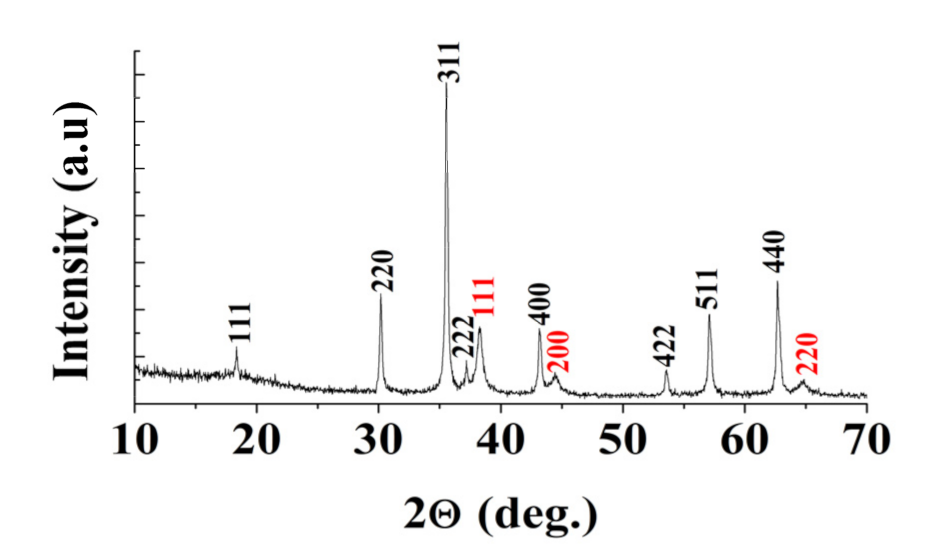
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, L.M.; Alvarez, V.A. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering 2019, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, J.; Li, Y.; Wei, Z.; Cao, G.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Porous Carbon Protected Magnetite and Silver Hybrid Nanoparticles: Morphological Control, Recyclable Catalysts, and Multicolor Cell Imaging. ACS Appl. Mater. Interfaces 2013, 5, 9446–9453. [Google Scholar] [CrossRef]
- Lu, L.; Hao, Q.; Lei, W.; Xia, X.; Liu, P.; Sun, D.; Wang, X.; Yang, X. Well-Combined Magnetically Separable Hybrid Cobalt Ferrite/Nitrogen-Doped Graphene as Efficient Catalyst with Superior Performance for Oxygen Reduction Reaction. Small 2015, 11, 5833–5843. [Google Scholar] [CrossRef] [PubMed]
- Saire-Saire, S.; Barbosa, E.C.M.; Garcia, D.; Andrade, L.H.; Garcia-Segura, S.; Camargo, P.H.C.; Alarcon, H. Green synthesis of Au decorated CoFe2O4 nanoparticles for catalytic reduction of 4-nitrophenol and dimethylphenylsilane oxidation. RSC Adv. 2019, 9, 22116–22123. [Google Scholar] [CrossRef]
- Silvestri, A.; Mondini, S.; Mareli, S.; Prifferi, V.; Faciola, L.; Ponti, A.; Ferretti, A.M.; Poloto, L. Synthesis of water dispersible and catalytically active gold-decorated cobalt ferrite nanoparticles. Langmuir 2016, 32, 7117–7126. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.V.; Nalench, Y.A.; Myrovali, E.; Garanina, A.S.; Grebennikov, I.S.; Gifer, P.K.; Abakumov, M.A.; Spasova, M.; Angelakeris, M.; Savchenko, A.G.; et al. Size-selected Fe3O4–Au hybrid nanoparticles for improved magnetism-based theranostics. Beilstein J. Nanotechnol. 2018, 9, 2684–2699. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.H.; Shin, T.H.; Cheon, J. Theranostic Magnetic Nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Reichel, V.E.; Matuszak, J.; Bente, K.; Heil, T.; Kraupner, A.; Dutz, S.; Cicha, I.; Faivre, D. Magnetite-Arginine Nanoparticles as a Multifunctional Biomedical Tool. Nanomaterials 2020, 10, 2014. [Google Scholar] [CrossRef]
- Mohapatra, S.; Rout, S.R.; Das, R.K.; Nayak, S.; Ghosh, S.K. Highly Hydrophilic Luminescent Magnetic Mesoporous Carbon Nanospheres for Controlled Release of Anticancer Drug and Multimodal Imaging. Langmuir 2016, 32, 1611–1620. [Google Scholar] [CrossRef]
- Jat, S.K.; Selvaraj, D.; Muthiah, R.; Bhattacharjee, R.R. A Self-Releasing Magnetic Nanomaterial for Sustained Release of Doxorubicin and Its Anticancer Cell Activity. ChemistrySelect 2018, 3, 13123–13131. [Google Scholar] [CrossRef]
- Shima; Damodaran, P. Mesoporous Magnetite Nanoclusters as Efficient Nanocarriers for Paclitaxel Delivery. ChemistrySelect 2020, 5, 9261–9268. [Google Scholar] [CrossRef]
- Oh, Y.; Moorthy, M.S.; Manivasagan, P.; Bharathiraja, S.; Oh, J. Magnetic hyperthermia and pH-responsive effective drug delivery to the sub-cellular level of human breast cancer cells by modified CoFe2O4 nanoparticles. Biochimie 2017, 133, 7–19. [Google Scholar] [CrossRef]
- Mohapatra, S.; Rout, S.R.; Maiti, S.; Maiti, T.K.; Panda, A.B. Monodisperse mesoporous cobalt ferrite nanoparticles: Synthesis and application in targeted delivery of antitumor drugs. J. Mater. Chem. 2011, 21, 9185–9193. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, D.; Yin, Z.; Chi, X.; Wang, X.; Gao, J. Magnetite nanoparticles as smart carriers to manipulate the cytotoxicity of anticancer drugs: Magnetic control and pH-responsive release. J. Mater. Chem. 2012, 22, 15717–15725. [Google Scholar] [CrossRef]
- Ebadi, M.; Buskaran, K.; Bullo, S.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Synthesis and Cytotoxicity Study of Magnetite Nanoparticles Coated with Polyethylene Glycol and Sorafenib–Zinc/Aluminium Layered Double Hydroxide. Polymers 2020, 12, 2716. [Google Scholar] [CrossRef]
- Odio, O.F.; Lartundo-Rojas, L.; Santiago-Jacinto, P.; Martínez, R.; Reguera, E. Sorption of Gold by Naked and Thiol-Capped Magnetite Nanoparticles: An XPS Approach. J. Phys. Chem. C 2014, 118, 2776–2791. [Google Scholar] [CrossRef]
- Bui, T.Q.; Ngo, H.T.M.; Tran, H.T. Surface-protective assistance of ultrasound in synthesis of superparamagnetic magnetite nanoparticles and in preparation of mono-core magnetite-silica nanocomposites. J. Sci. Adv. Mater. Devices 2018, 3, 323–330. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Khan, M.M.; Alrokayan, S.A. Comparative cytotoxic response of nickel ferrite nanoparticles in human liver HepG2 and breast MFC-7 cancer cells. Chemosphere 2015, 135, 278–288. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting Unique Alignment of Cobalt Ferrite Nanoparticles, Mild Hyperthermia, and Controlled Intrinsic Cobalt Toxicity for Cancer Therapy. Adv. Mater. 2020, 32, e2003712. [Google Scholar] [CrossRef]
- Yu, Z.; Grasso, M.F.; Cui, X.; Silva, R.N.; Zhang, P. Sensitive and Label-Free SERS Detection of Single-Stranded DNA Assisted by Silver Nanoparticles and Gold-Coated Magnetic Nanoparticles. ACS Appl. Bio Mater. 2020, 3, 2626–2632. [Google Scholar] [CrossRef]
- Lone, S.A.; Sanyal, P.; Das, P.; Sadhu, K.K. Citrate Stabilized Au-FexOy Nanocomposites for Variable Exchange Bias, Catalytic Properties and Reversible Interaction with Doxorubicin. ChemistrySelect 2019, 4, 8237–8245. [Google Scholar] [CrossRef]
- Félix, L.L.; Sanz, B.; Sebastián, V.; Torres, T.E.; Sousa, M.H.; Coaquira, J.A.H.; Ibarra, M.R.; Goya, G.F. Gold-decorated magnetic nanoparticles design for hyperthermia applications and as a potential platform for their surface-functionalization. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Khan, A.U.; Ullah, S.; Yuan, Q.; Ali, S.; Ahmad, A.; Khan, Z.U.H.; Rahman, A.U. In situ fabrication of Au–CoFe2O4: An efficient catalyst for soot oxidation. Appl. Nanosci. 2020, 10, 3901–3910. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, A.; Guo, H.; Chen, X.; Gan, Z.; Tao, W.; Zhang, M.; Wu, R.; Li, Z. Controlled synthesis of Au–Fe3O4 hybrid hollow spheres with excellent SERS activity and catalytic properties. Dalton Trans. 2014, 43, 7998–8006. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Ren, G.; Fu, S.; Chai, F.; Jiang, J.; Qu, F. Fabrication of Fe3O4@Au hollow spheres with recyclable and efficient catalytic properties. New J. Chem. 2015, 40, 818–824. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Ren, X.; Tan, L.; Huang, Z.; Tang, F. One-pot gradient solvothermal synthesis of Au–Fe3O4 hybrid nanopar-ticles for magnetically recyclable catalytic applications. J. Mater. Chem. A 2013, 1, 10513. [Google Scholar] [CrossRef]
- Xie, L.; Qian, W.; Yang, S.; Sun, J.; Gong, T. A Facile and Green Synthetic Route for Preparation of Heterostructure Fe3O4@Au Nanocomposites. MATEC Web Conf. EDP Sci. 2017, 88, 02001. [Google Scholar] [CrossRef]
- Li, Z.H.; Bai, J.H.; Zhang, X.; Lv, J.M.; Fan, C.S.; Zhao, Y.M.; Wu, Z.L.; Xu, H.J. Facile synthesis of Au nanoparticle-coated Fe3O4 magnetic composite nanospheres and their application in SERS detection of malachite green. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118532. [Google Scholar] [CrossRef]
- Liua, X.; Yanga, X.; Lic, K.; Liua, H.; Xiaoa, R.; Wanga, W.; Wanga, C.; Wang, S. Fe3O4@Au SERS tags-based lateral flow assay for simultaneous detection of serum amyloid A and C-reactive protein in unprocessed blood sample. Sens. Actuators B Chem. 2020, 320, 128350. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Kou, Q.; Liu, Y.; Han, D.; Wang, D.; Sun, Y.; Zhang, Y.; Wang, Y.; Lu, Z.; et al. Enhanced Catalytic Reduction of 4-Nitrophenol Driven by Fe3O4-Au Magnetic Nanocomposite Interface Engineering: From Facile Preparation to Recyclable Application. Nanomaterials 2018, 8, 353. [Google Scholar] [CrossRef]
- Kheradmand, E.; Poursalehi, R.; Delavari, H. Optical and magnetic properties of iron-enriched Fe/FexOy@Au magnetoplas-monic nanostructures. Appl. Nanosci. 2020, 10, 1083–1094. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, P.J.; Chen, Y.C. Multifunctional Fe3O4@Au Nanoeggs as Photothermal Agents for Selective Killing of Nosocomial and Antibiotic-Resistant Bacteria. Small 2009, 5, 51–56. [Google Scholar] [CrossRef]
- El-Sayed, H.M. Evidence on the presence of Ruderman–Kittel–Kasuya–Yosida (RKKY) interaction in CoFe2O4@Au nano structure. Superlattices Microstruct. 2016, 91, 98–104. [Google Scholar] [CrossRef]
- Ghorbani, M.; Mahmoodzadeh, F.; Nezhad-Mokhtari, P.; Hamishehkar, H. A novel polymeric micelle-decorated Fe3O4/Au core–shell nanoparticle for pH and reduction-responsive intracellular co-delivery of doxorubicin and 6-mercaptopurine. New J. Chem. 2018, 42, 18038–18049. [Google Scholar] [CrossRef]
- Mikalauskaitė, A.; Kondrotas, R.; Niaura, G.; Jagminas, A. Gold-Coated Cobalt Ferrite Nanoparticles via Methionine-Induced Reduction. J. Phys. Chem. C 2015, 119, 17398–17407. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; An, Y.L.; Lin, M.; Gao, Z.; Zhang, J. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef]
- Jagminas, A.; Mažeika, K.; Kondrotas, R.; Kurtinaitienė, M.; Jagminienė, A.; Mikalauskaitė, A. Functionalization of Cobalt Ferrite Nanoparticles by a Vitamin C-Assisted Covering with Gold. Nanomater. Nanotechnol. 2014, 4, 11. [Google Scholar] [CrossRef]
- Zeng, J.; Gong, M.; Wang, D.; Li, M.; Xu, W.; Li, Z.; Li, S.; Zhang, D.; Yan, Z.; Yin, Y. Direct Synthesis of Water-Dispersible Magnetic/Plasmonic Heteronanostructures for Multimodality Biomedical Imaging. Nano Lett. 2019, 19, 3011–3018. [Google Scholar] [CrossRef]
- Evsevskaya, N.; Pikurova, E.; Saikova, S.V.; Nemtsev, I.V. Effect of the Deposition Conditions on the Anion Resin Exchange Precipitation of Indium(III) Hydroxide. ACS Omega 2020, 5, 4542–4547. [Google Scholar] [CrossRef]
- Saikova, S.V.; Kirshneva, E.A.; Panteleeva, M.V.; Pikurova, E.V.; Evsevskaya, N.P. Production of Gadolinium Iron Garnet by Anion Resin Exchange Precipitation. Russ. J. Inorg. Chem. 2019, 64, 1191–1198. [Google Scholar] [CrossRef]
- Saikova, S.V.; Trofimova, T.V.; Pavlikov, A.Y.; Samoilo, A.S. Effect of Polysaccharide Additions on the Anion-Exchange Deposition of Cobalt Ferrite Nanoparticles. Russ. J. Inorg. Chem. 2020, 65, 291–298. [Google Scholar] [CrossRef]
- Saykova, D.; Saikova, S.; Mikhlin, Y.; Panteleeva, M.; Ivantsov, R.; Belova, E. Synthesis and Characterization of Core–Shell Magnetic Nanoparticles NiFe2O4@Au. Metals 2020, 10, 1075. [Google Scholar] [CrossRef]
- Velikanov, D.A. Vibration Magnetic Meter. RF Patent for the Invention RU2341810 (C1); Bulletin No. 35; Federal Service for Intellectual Property, Patents and Trademarks: Moscow, Russia, 2008.
- Velikanov, D.A. Vibrating Magnetometer. RF Patent for the Invention RU2339965 (C1); Bulletin No. 33; Federal Service for Intellectual Property, Patents and Trademarks: Moscow, Russia, 2008.
- Balakrishnan, G.; Barnett, G.V.; Kar, S.R.; Das, T.K. Detection and identification of the vibrational markers for the quantification of methionine oxidation in therapeutic proteins. Anal. Chem. 2018, 90, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.A.; Ranasinghe, J.C.; Dikkumbura, A.S.; Hamal, P.; Kumal, R.R.; Karam, T.E.; Smith, H.T.; Haber, L.H. Monitoring the Seed-Mediated Growth of Gold Nanoparticles Using in Situ Second Harmonic Generation and Extinction Spectroscopy. J. Phys. Chem. C 2018, 122, 24400–24406. [Google Scholar] [CrossRef]
- Kim, G.; Weiss, S.J.; Levine, R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 901–905. [Google Scholar] [CrossRef]
- Angelova, P.; Solel, E.; Parvari, G.; Turchanin, A.; Botoshansky, M.; Gölzhäuser, A.; Keinan, E. Chemisorbed Monolayers of Corannulene Penta-Thioethers on Gold. Langmuir 2013, 29, 2217–2223. [Google Scholar] [CrossRef]
- Chen, K.; Li, H.; Xu, Y.; Liu, K.; Li, H.; Xu, X.; Qiu, X.; Liu, M. Untying thioether bond structures enabled by “voltage-scissors” for stable room temperature sodium–sulfur batteries. Nanoscale 2019, 11, 5967–5973. [Google Scholar] [CrossRef]
- Bergès, J.; De Oliveira, P.; Fourré, I.; Houée-Levin, C. The One-Electron Reduction Potential of Methionine-Containing Peptides Depends on the Sequence. J. Phys. Chem. B 2012, 116, 9352–9362. [Google Scholar] [CrossRef]
- Stein, C.R.; Bezerra, M.T.S.; Holanda, G.H.A.; André-Filho, J.; Morais, P.C. Structural and magnetic properties of cobalt ferrite nanoparticles synthesized by co-precipitation at increasing temperatures. AIP Adv. 2018, 8, 056303. [Google Scholar] [CrossRef]
- Ammar, S.; Helfen, A.; Jouini, N.; Fiévet, F.; Rosenman, I.; Villain, F.; Molinié, P.; Danot, M. Magnetic properties of ultrafine cobalt ferrite particles synthesized by hydrolysis in a polyol medium. J. Mater. Chem. 2001, 11, 186–192. [Google Scholar] [CrossRef]
- Labchir, N.; Hannour, A.; Hssi, A.A.; Vincent, D.; Chatelon, J.; Dufeu, D.; Ihlal, A.; Sajieddine, M. Microwave response of coplanar waveguide based on electrodeposited CoFe2O4 nanowires. J. Magn. Magn. Mater. 2020, 510, 166952. [Google Scholar] [CrossRef]
- Grigorova, M.; Blythe, H.; Blaskov, V.; Rusanov, V.; Petkov, V.; Masheva, V.; Nihtianova, D.; Martinez, L.; Muñoz, J.; Mikhov, M. Magnetic properties and Mössbauer spectra of nanosized CoFe2O4 powders. J. Magn. Magn. Mater. 1998, 183, 163–172. [Google Scholar] [CrossRef]
- Ghobadifard, M.; Farhadi, S.; Mohebbi, S. Sonocatalytic performance of magnetic flower-like CoFe2O4 nanoparticles prepared from a heterometallic oxo-centered trinuclear complex under microwave irradiation. Polyhedron 2018, 155, 66–76. [Google Scholar] [CrossRef]
- Peddis, D.; Yaacoub, N.; Ferretti, M.; Martinelli, A.; Piccaluga, G.; Musinu, A.; Cannas, C.; Navarra, G.; Greneche, J.M.; Fiorani, D. Cationic distribution and spin canting in CoFe2O4 nanoparticles. J. Physics Condens. Matter 2011, 23, 426004. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tang, F.Y.; Chen, S.Y.; Chen, Y.M.; Chiang, E.P.I. Glycine-N Methyltransferase Expression in HepG2 Cells Is Involved in Methyl Group Homeostasis by Regulating Transmethylation Kinetics and DNA Methylation. J. Nutr. 2011, 141, 777–782. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Engelen, M.P.K.J.; Simbo, S.Y.; Have, G.A.M.T.; Thaden, J.J.; Cynober, L.; Deutz, N.E.P. Metabolic consequences of supplemented methionine in a clinical context. J. Nutr. 2020, 150, 2538S–2547S. [Google Scholar] [CrossRef]
- Elango, R. Methionine Nutrition and Metabolism: Insights from Animal Studies to Inform Human Nutrition. J. Nutr. 2020, 150, 2518S–2523S. [Google Scholar] [CrossRef]
- Borges, P.T.; Brissos, V.; Hernandez, G.; Masgrau, L.; Lucas, M.F.; Monza, E.; Frazão, C.; Cordeiro, T.; Martins, L.O. The Methionine-Rich Loop of Multicopper Oxidase McoA follows Open-To-Close Transitions with a Role in Enzyme Catalysis. ACS Catal. 2020, 10, 7162–7176. [Google Scholar] [CrossRef]
- Biggs, G.S.; Klein, O.J.; Boss, S.R.; Barker, P.D. Unlocking the Full Evolutionary Potential of Artificial Metalloenzymes through Direct Metal-Protein Coordination: A review of recent advances for catalyst development. Johns. Matthey Technol. Rev. 2020, 64, 407–418. [Google Scholar] [CrossRef]
- Glišić, B.Đ.; Djuran, M.I.; Stanić, Z.D.; Rajković, S. Oxidation of methionine residue in Gly-Met dipeptide induced by [Au(en)Cl2]+ and influence of the chelated ligand on the rate of this redox process. Gold Bull. 2014, 47, 33–40. [Google Scholar] [CrossRef]
- Vujačić, A.V.; Savić, J.Z.; Sovilj, S.P.; Szécsényi, K.M.; Todorović, N.; Petković, M.Ž.; Vasić, V.M. Mechanism of complex formation between [AuCl4]− and L-methionine. Polyhedron 2009, 28, 593–599. [Google Scholar] [CrossRef]
- Natile, G.; Bordignon, E.; Cattalini, L. Chloroauric acid as oxidant Stereospecific oxidation of methionine to methionine sulfoxide. Inorg. Chem. 1976, 15, 246–248. [Google Scholar] [CrossRef]
- Scuderi, D.; Bergè, J.; de Oliveira, P.; Houée-Levin, C. Methionine one-electron oxidation: Coherent contributions from radiolysis, IRMPD spectroscopy, DFT calculations and electrochemistry. Radiat. Phys. Chem. 2016, 128, 103–111. [Google Scholar] [CrossRef]
- Asmus, K.D. Stabilization of oxidized sulfur centers in organic sulfides. Radical cations and odd-electron sulfur-sulfur bonds. Accounts Chem. Res. 1979, 12, 436–442. [Google Scholar] [CrossRef]
- Asmus, K.D. Heteroatom-centered free radicals some selected contributions by radiation chemistry. Solid State NMR Polym. 2001, 87, 341–393. [Google Scholar] [CrossRef]
- Archirel, P.; Houée-Lévin, C.; Marignier, J.L. Oxidation of Two Inverse Dipeptides, Methionine–Valine and Valine–Methionine: A Joint Experimental and Computational Study. J. Phys. Chem. B 2019, 123, 9087–9097. [Google Scholar] [CrossRef]
- Fourré, I.; Bergès, J.; Houée-Levin, C. Structural and Topological Studies of Methionine Radical Cations in Dipeptides: Electron Sharing in Two-Center Three-Electron Bonds. J. Phys. Chem. A 2010, 114, 7359–7368. [Google Scholar] [CrossRef]
- Lam, E.; Hrapovic, S.; Majid, E.; Chong, J.H.; Luong, J.H.T. Catalysis using gold nanoparticles decorated on nanocrystalline cellulose. Nanoscale 2012, 4, 997–1002. [Google Scholar] [CrossRef]
- Kannan, A.; Rajakumar, P. Synthesis and catalytic application of glycodendrimers decorated with gold nanoparticles: Reduction of 4-nitrophenol. RSC Adv. 2015, 5, 46908–46915. [Google Scholar] [CrossRef]
- Nghiem, T.H.L.; Le, T.N.; Do, T.H.; Vu, T.T.D.; Do, Q.H.; Tran, H.N. Preparation and characterization of silica–gold core–shell nanoparticles. J. Nanopart. Res. 2013, 15, 2091. [Google Scholar] [CrossRef]
- Goon, I.Y.; Lai, L.M.H.; Lim, M.; Munroe, P.; Gooding, J.J.; Amal, R. Fabrication and Dispersion of Gold-Shell-Protected Magnetite Nanoparticles: Systematic Control Using Polyethyleneimine. Chem. Mater. 2009, 21, 673–681. [Google Scholar] [CrossRef]
- Martínez-Banderas, A.I.; Aires, A.; Quintanilla, M.; Holguín-Lerma, J.A.; Lozano-Pedraza, C.; Teran, F.J.; Moreno, J.A.; Perez, J.E.; Ooi, B.S.; Ravasi, T.; et al. Iron-Based Core–Shell Nanowires for Combinatorial Drug Delivery and Photothermal and Magnetic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 43976–43988. [Google Scholar] [CrossRef]

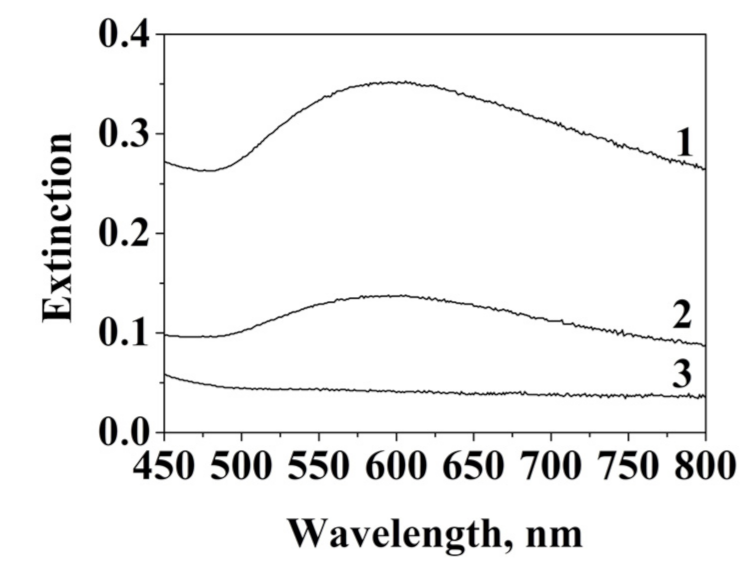
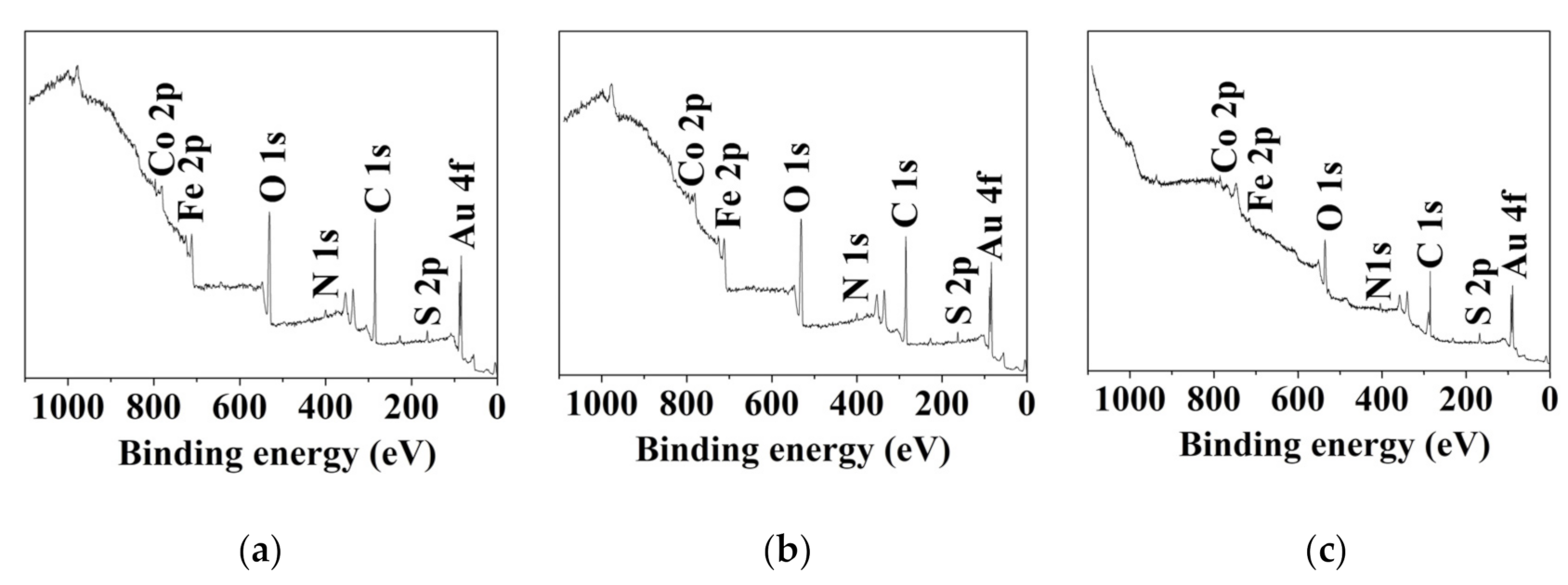
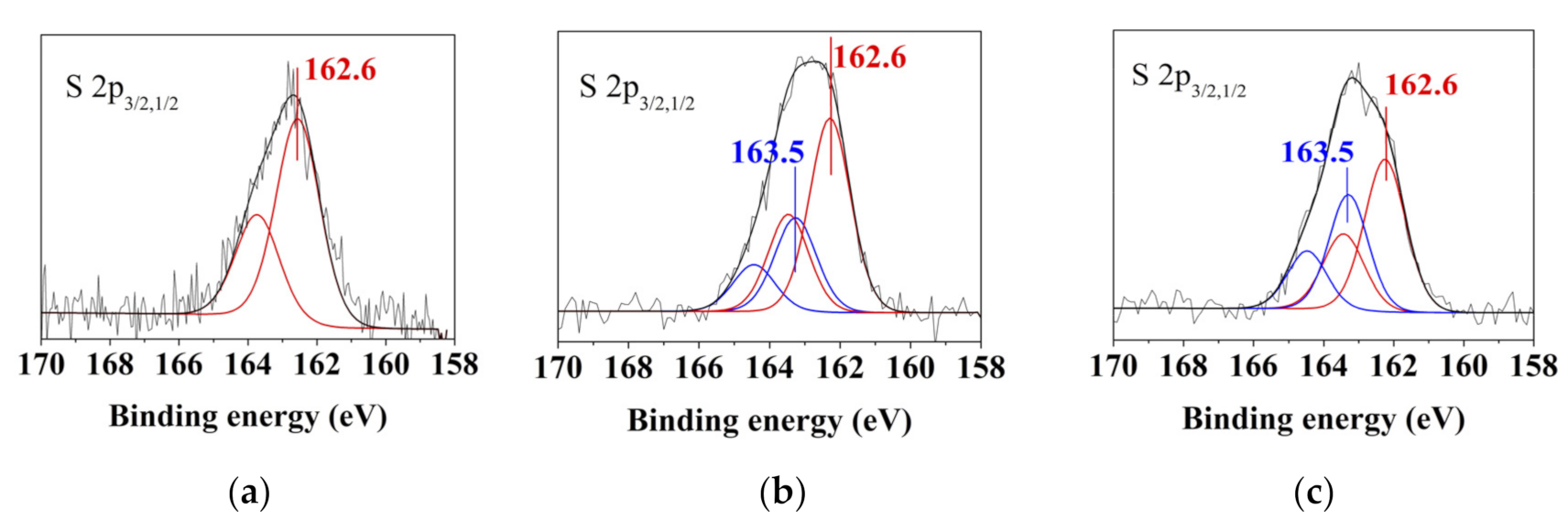
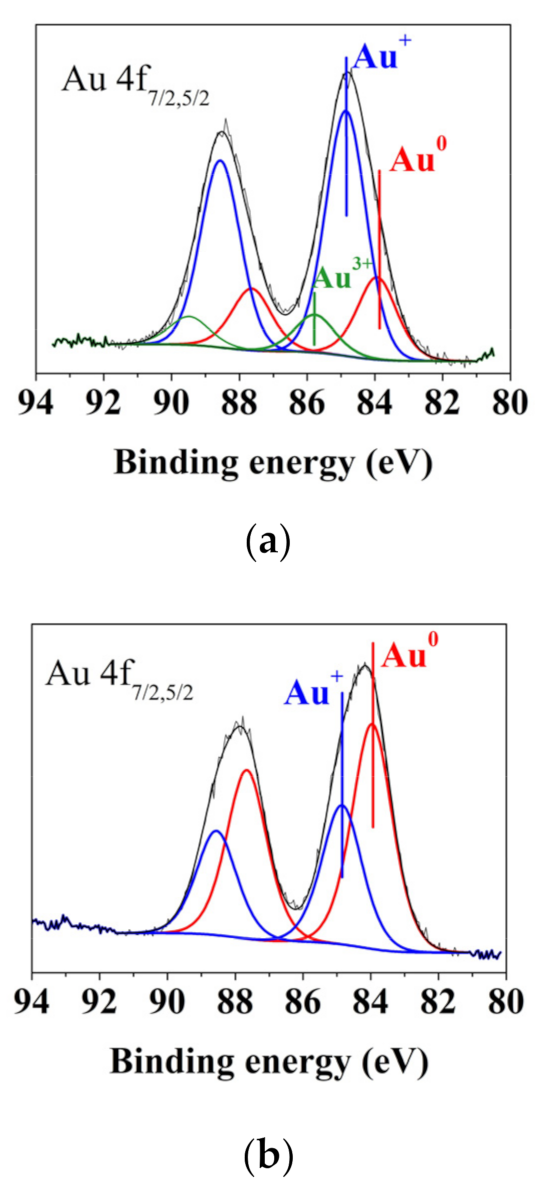
| Element | Concentration, at. % | ||
|---|---|---|---|
| Number of Gold Deposition Cycles | |||
| 1 | 6 | 10 | |
| Au | 13.1 | 9.6 | 8.7 |
| N | 9.8 | 6.8 | 6.6 |
| O | 61.8 | 61.6 | 69.0 |
| Fe | 2.0 | 6.4 | 3.4 |
| Co | 3.3 | 3.8 | 3.2 |
| S | 10.0 | 9.8 | 9.1 |
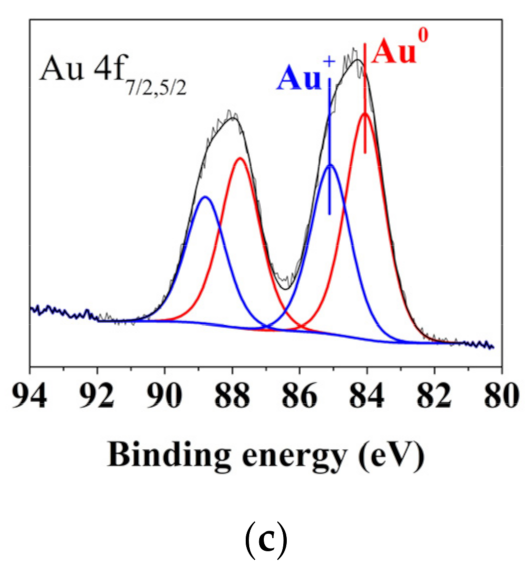
| The Number of Gold Deposition Cycles | Au(0) | Au(I) | Au(III) | |||
|---|---|---|---|---|---|---|
| % | BE (eV) | % | BE (eV) | % | BE (eV) | |
| 1 | 14.6 | 84.0 | 78.2 | 85.0 ± 0.1 | 7.2 | 85.8 |
| 6 | 76.8 | 83.9 | 23.2 | 85.0 ± 0.1 | - | - |
| 10 | 76.6 | 84.0 | 23.4 | 85.0 ± 0.1 | - | - |
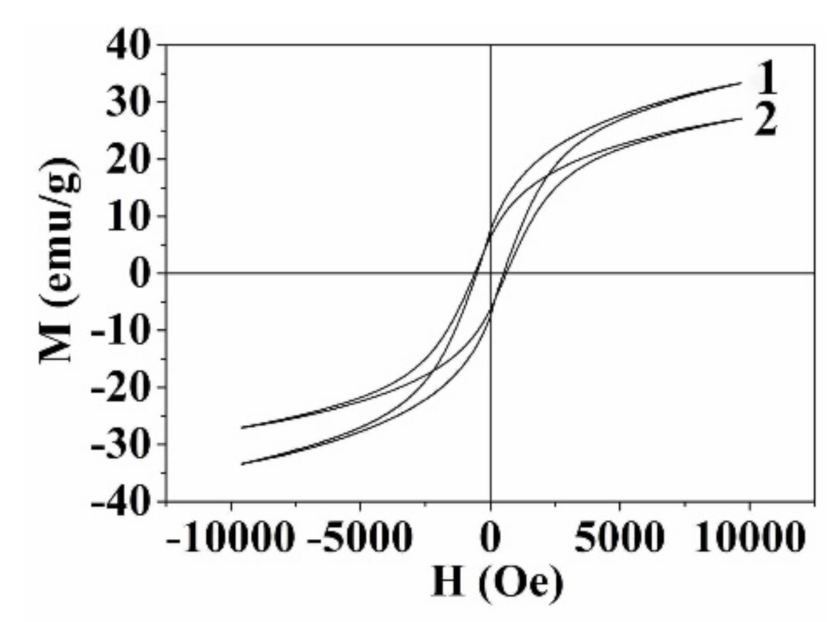
| Sample | Ms, emu/g | Mr, emu/g | Hc, Oe |
|---|---|---|---|
| CoFe2O4 | 33.21 | 7.55 | 510 |
| CoFe2O4/Au | 27.04 | 6.31 | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikova, S.; Pavlikov, A.; Trofimova, T.; Mikhlin, Y.; Karpov, D.; Asanova, A.; Grigoriev, Y.; Volochaev, M.; Samoilo, A.; Zharkov, S.; et al. Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization. Metals 2021, 11, 705. https://doi.org/10.3390/met11050705
Saikova S, Pavlikov A, Trofimova T, Mikhlin Y, Karpov D, Asanova A, Grigoriev Y, Volochaev M, Samoilo A, Zharkov S, et al. Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization. Metals. 2021; 11(5):705. https://doi.org/10.3390/met11050705
Chicago/Turabian StyleSaikova, Svetlana, Alexander Pavlikov, Tatyana Trofimova, Yuri Mikhlin, Denis Karpov, Anastasiya Asanova, Yuri Grigoriev, Mikhail Volochaev, Alexander Samoilo, Sergey Zharkov, and et al. 2021. "Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization" Metals 11, no. 5: 705. https://doi.org/10.3390/met11050705
APA StyleSaikova, S., Pavlikov, A., Trofimova, T., Mikhlin, Y., Karpov, D., Asanova, A., Grigoriev, Y., Volochaev, M., Samoilo, A., Zharkov, S., & Velikanov, D. (2021). Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization. Metals, 11(5), 705. https://doi.org/10.3390/met11050705









