Synthesis and Characterization of Core–Shell Magnetic Nanoparticles NiFe2O4@Au
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Nickel Ferrite Nanoparticles
2.3. Gold Shell Formation
2.4. Characterization/Analysis
3. Results
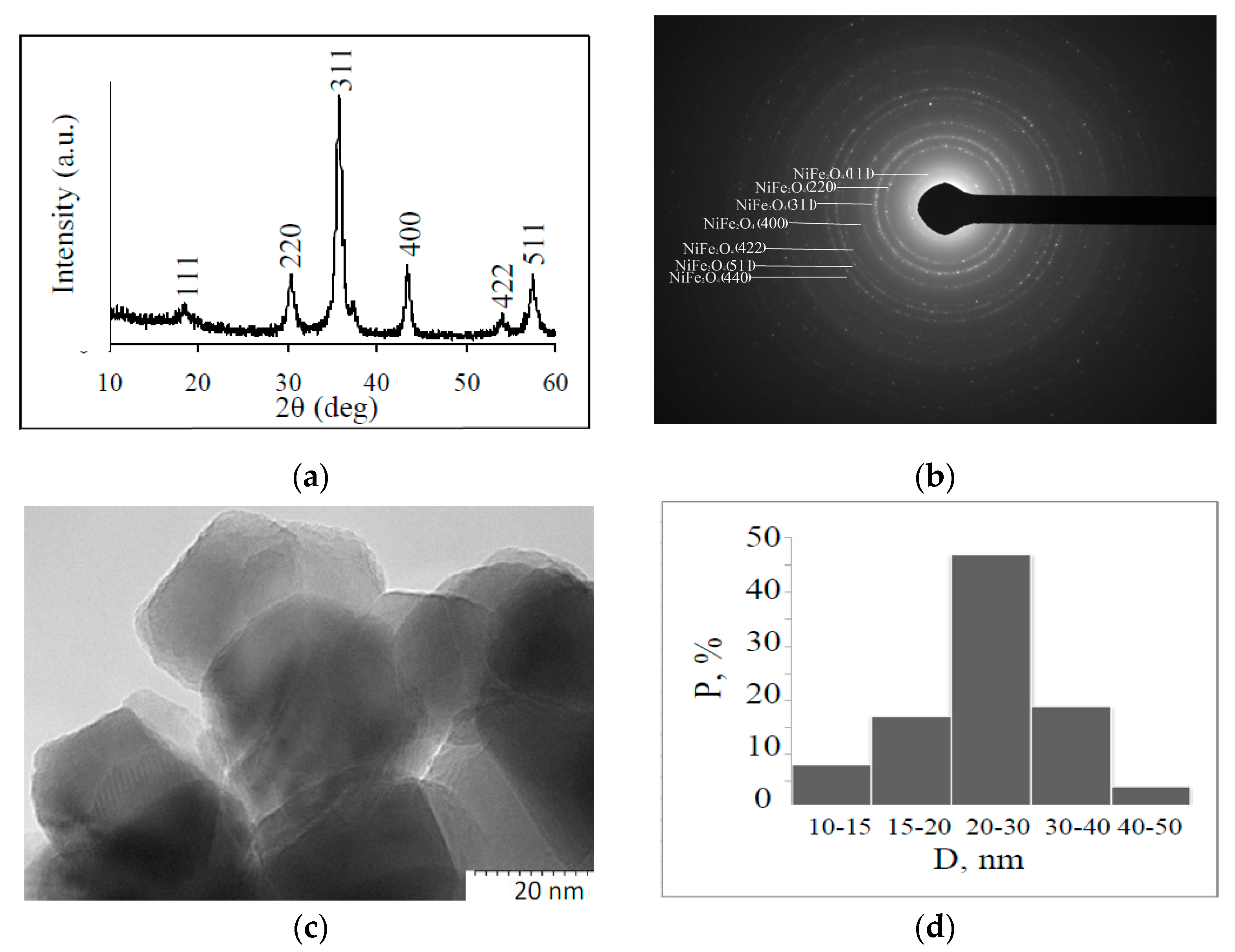
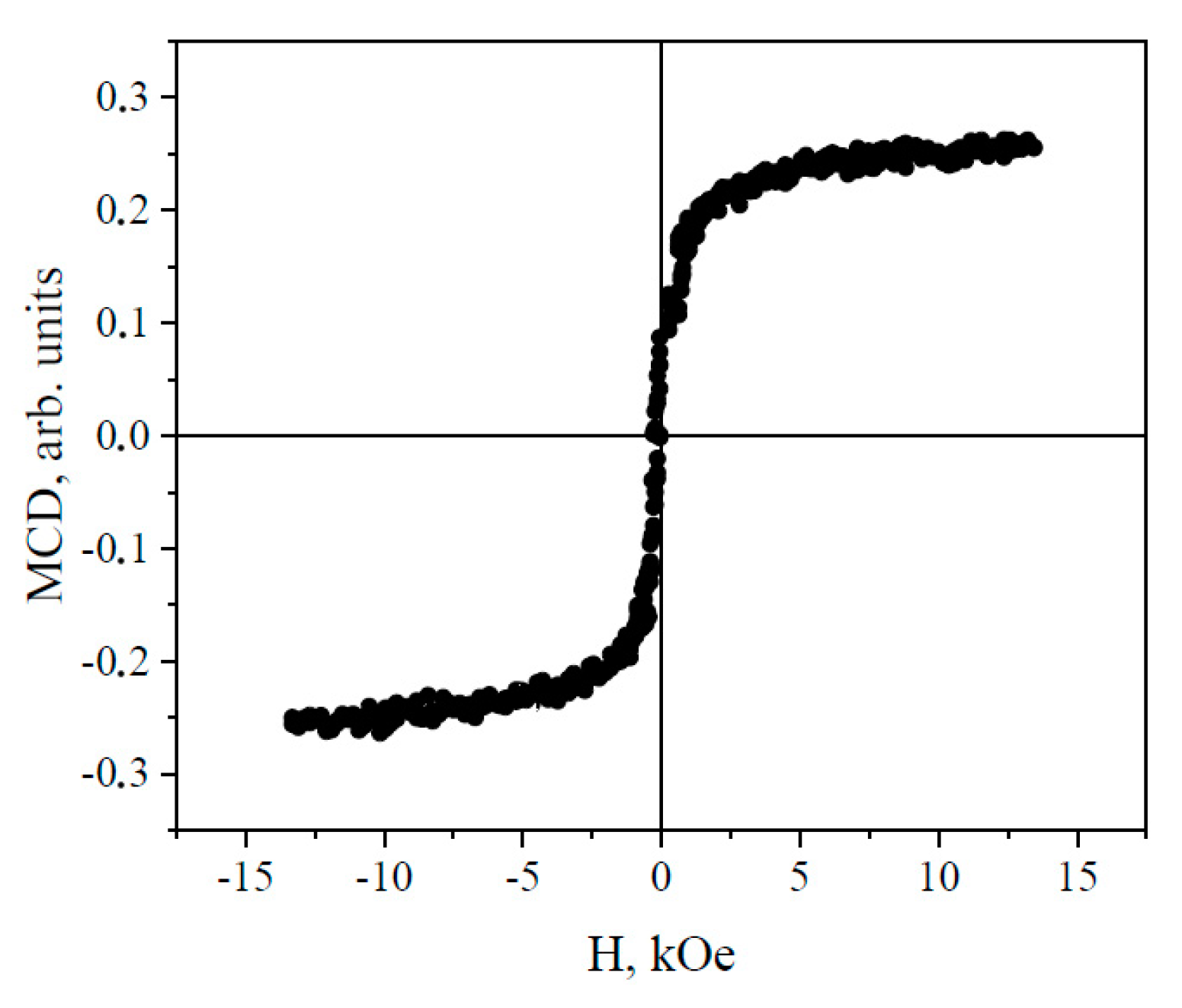
3.1. Fabrication and Characterization of Nickel Ferrite Nanoparticles
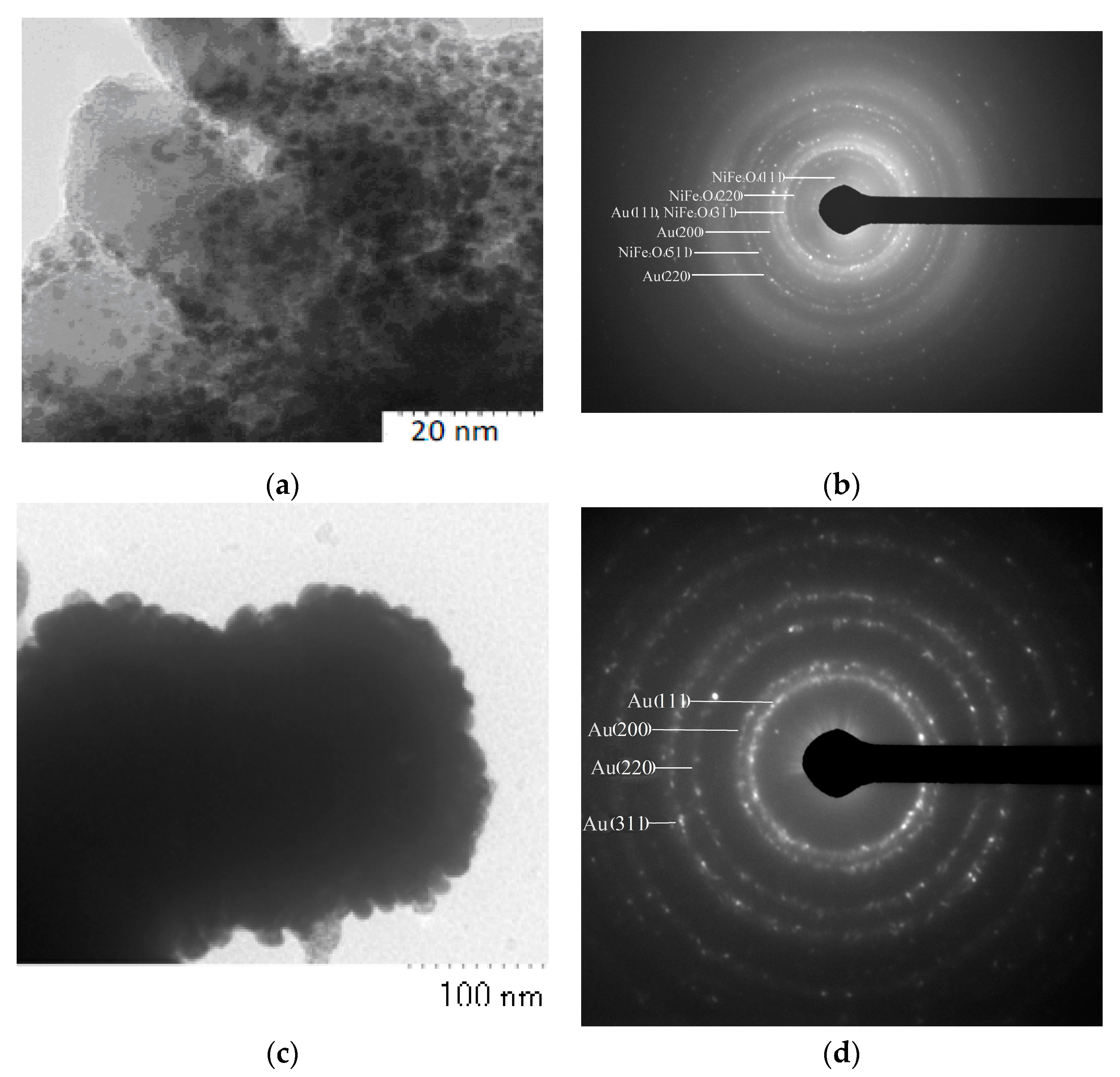
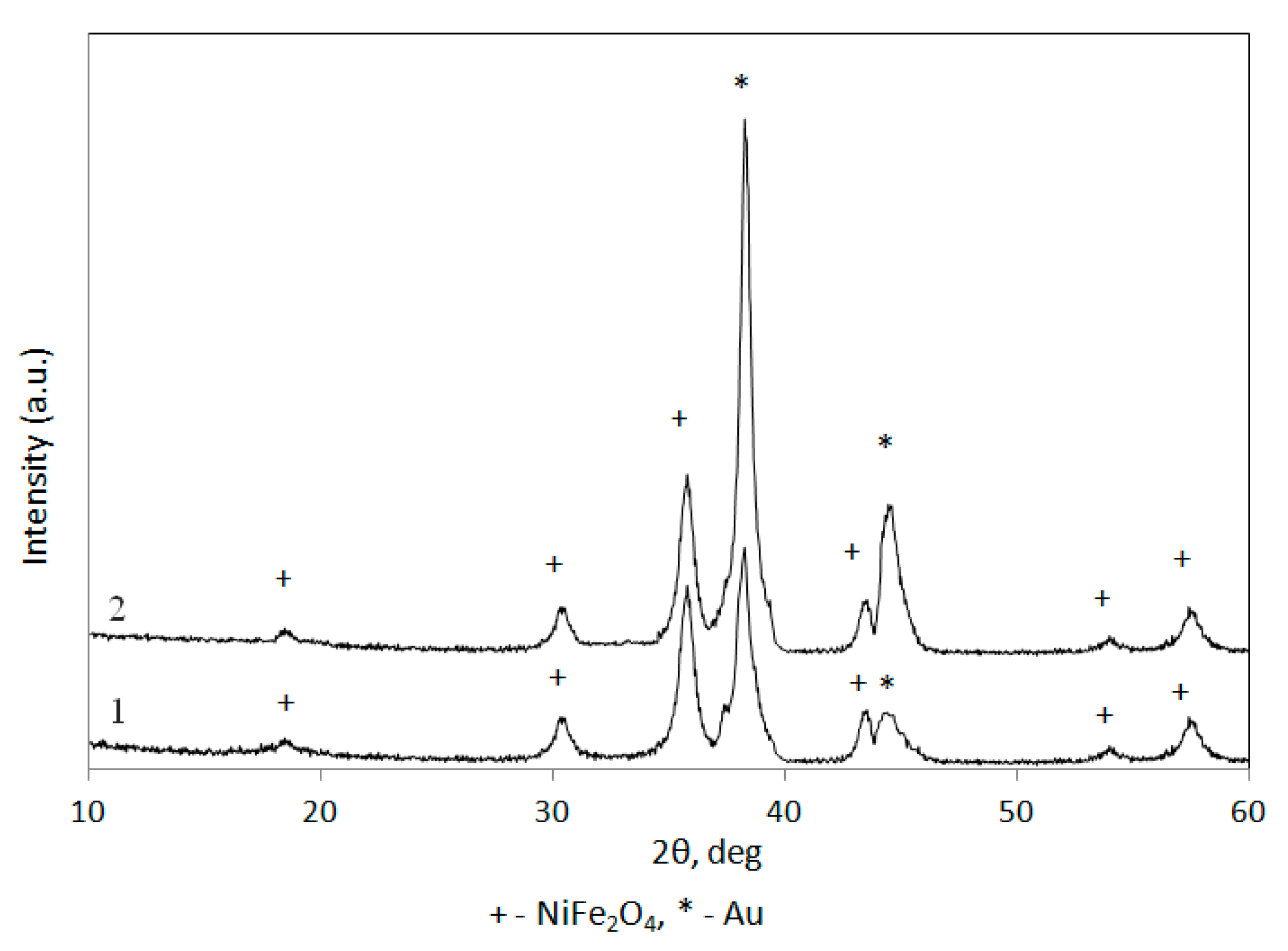
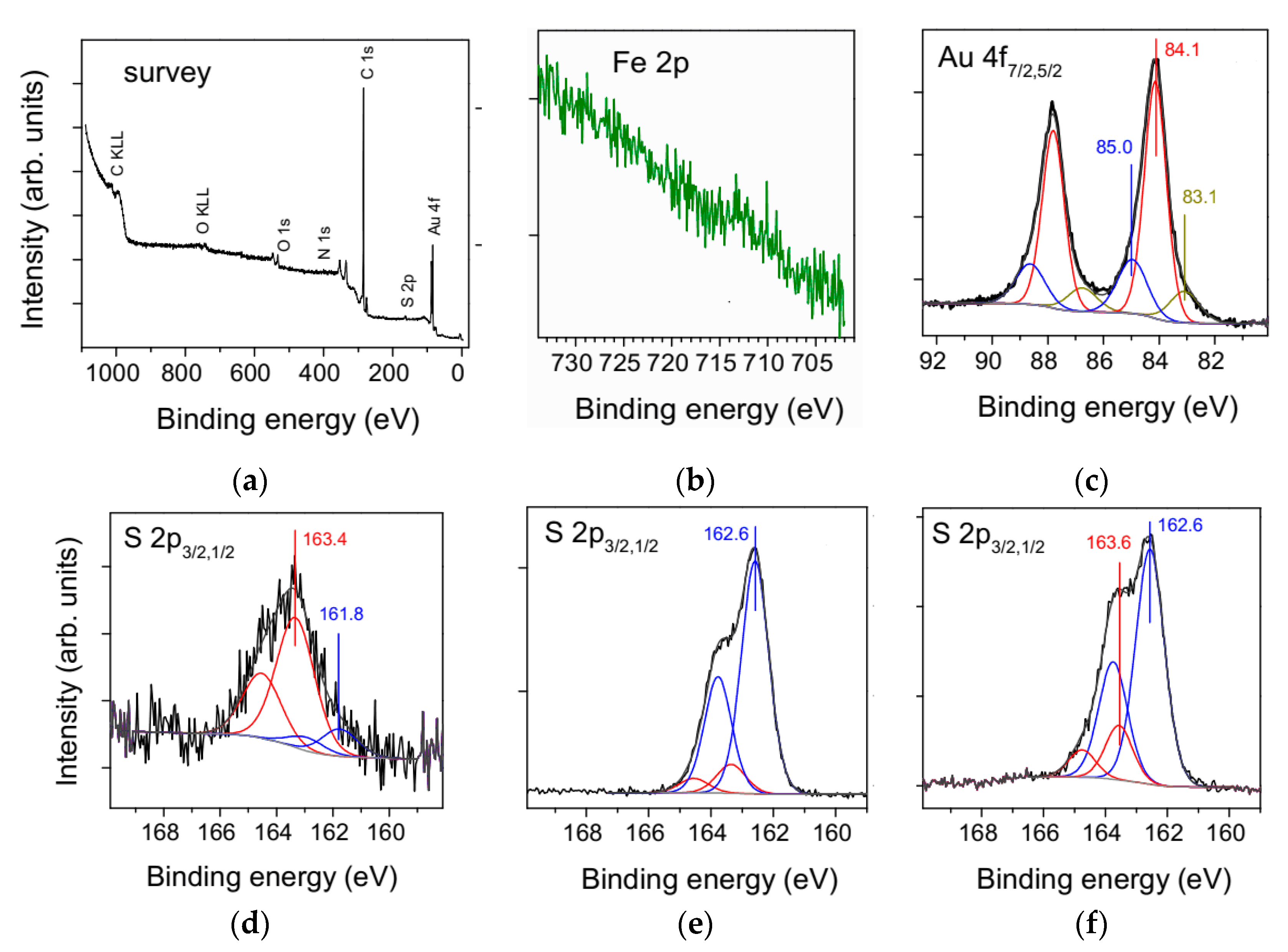
3.2. Fabrication and Characterization of NiFe2O4@Au
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, D.; Deng, F.; Liu, D.; He, B.; He, B.; Tang, X.; Zhang, Q. The appliances and prospects of aurum nanomaterials in biodiagnostics, imaging, drug delivery and combination therapy. J. Pharm. Sci. 2018, 13, 143–153. [Google Scholar] [CrossRef]
- Long, F.; Li, W.; Chen, W.; Liu, D.; Chen, Y.; Zhou, R.; Li, P. Amperometric Biosensor Based on Cu2O@Au Nanocomposites for the Detection of Galectin-1 via Lactose-Galectin Interactions. Nanotechnology 2019, 30, 485706. [Google Scholar] [CrossRef]
- Jaque, D.; Martinez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martin Rodriguez, E.; Garcia Sole, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Balanov, V.A.; Kiseleva, A.P.; Krivoshapkina, E.F.; Kashtanov, E.A.; Gimaev, R.R.; Zverev, V.I.; Krivoshapkin, P.V. Synthesis of (Mn(1−x)Znx)Fe2O4 nanoparticles for magnetocaloric applications. J. Sol-Gel Sci. Technol. 2020, 1–6. [Google Scholar] [CrossRef]
- Maaz, K.; Karim, S.; Mumtaz, A.; Hasanain, S.K.; Liu, J.; Duan, J.L. Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route. J. Magn. Magn. Mater. 2009, 321, 1838–1842. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Khan, M.A.M.; Alrokayan, S.A. Comparative cytotoxic response of nickel ferrite nanoparticles in human liver HepG2 and breast MFC-7 cancer cells. Chemosphere 2015, 135, 278–288. [Google Scholar] [CrossRef]
- An, P.; Zuo, F.; Li, X.; Wu, Y.; Zhang, J.; Zheng, Z.; Ding, X.; Peng, Y. A bio-inspired polydopamine approach to preparation of gold-coated Fe3O4 core-shell nanoparticles: Synthesis, characterization and mechanism. NANO 2013, 8, 1350061. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.; Sun, S. Dumbbell-like functional Au−Fe3O4 Nanoparticles for Target-Specific Platin Delivery. J. Am. Chem. Soc. 2009, 131, 4216–4217. [Google Scholar] [CrossRef]
- Billen, A.; de Cattelle, A.; Jochum, J.K.; Van Bael, M.J.; Billen, J.; Seo, J.W.; Brullot, W.; Koeckelberghs, G.; Verbiest, T. Novel synthesis of superparamagnetic plasmonic core-shell iron oxide-gold nanoparticles. Physica B 2019, 560, 85–90. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, J.; Wang, Z.; Wang, L.; An, Y.; Lin, M.; Gao, Z.; Zhang, D. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef]
- Stoeva, S.I.; Huo, F.; Lee, J.-S.; Mirkin, C.A. Three-Layer Composite Magnetic Nanoparticle Probes for DNA. J. Am. Chem. Soc. 2005, 127, 15362–15363. [Google Scholar] [CrossRef]
- Blanco-Esqueda, I.G.; Ortega-Zarzosa, G.; Martínez, J.R.; Guerrero, A.L. Preparation and Characterization of Nickel Ferrite-SiO2/Ag Core/Shell Nanocomposites. Adv. Mater. Sci. Eng. 2015, 2015, 678739. [Google Scholar] [CrossRef]
- Smith, M.K.; Mc Keague, M.; Rosa, C.D. Synthesis, transfer, and characterization of core-shell gold-coated magnetic nanoparticles. MethodsX 2019, 6, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Ahmandi, N.; Poursalehi, R.; Kirilyuk, A.; Moravvej-Farshi, M.K. Effect of gold plasmonic shell on nonlinear optical characteristics and structure of iron based nanoparticles. Appl. Surf. Sci. 2019, 479, 114–118. [Google Scholar] [CrossRef]
- Mikalauskaite, A.; Kondrotas, R.; Niaura, G.; Jagminas, A. Gold-Coated Cobalt Ferrite Nanoparticles via Methionine-Induced Reduction. J. Phys. Chem. 2015, 119, 17398–17407. [Google Scholar] [CrossRef]
- Jagminas, A.; Mažeika, K.; Kondrotas, R.; Kurtinaitienė, M.; Jagminienė, A.; Mikalauskaite, A. Functionalization of cobalt ferrite nanoparticles by a vitamin C-assisted covering with gold. Nanomater. Nanotechno. 2014, 4, 4–9. [Google Scholar] [CrossRef]
- Lu, Z.-R.; Kopeckova, P.; Wu, Z.; Kopecek, J. Synthesis of semitelechelic poly[N-(2-hydroxypropyl)metacrylamide] by radical polymerization in the presence of alkyl mercaptans. Macromol. Chem. Phys. 1999, 200, 2022–2030. [Google Scholar] [CrossRef]
- Silvestri, A.; Mondini, S.; Marelli, M.; Pifferi, V.; Falciola, L.; Ponti, A.; Polito, L. Synthesis of Water Dispersible and Catalytically Active Gold-Decorated Cobalt Ferrite Nanoparticles. Langmuir 2016, 32, 7117–7126. [Google Scholar] [CrossRef]
- Zeng, J.; Gong, M.; Wang, D.; Li, M.; Xu, W.; Li, Z.; Li, S.; Zhang, D.; Yan, Z.; Yin, Y. Direct synthesis of water-dispersible magnetic/plasmonic hetero-nanostructures for multi-modality biomedical imaging. Nano Lett. 2019, 19, 3011–3019. [Google Scholar] [CrossRef]
- Kumal, R.R.; Abu-Laban, M.; Hamal, P.; Kruger, B.; Smith, H.T.; Hayes, D.J.; Haber, L.H. Near-infrared photothermal release of SiRNA from the surface of colloidal gold-silver-gold core-shell-shell nanoparticles studied with second-harmonic generation. J. Phys. Chem. 2018, 122, 19699–19704. [Google Scholar] [CrossRef]
- Tschulik, K.; Ngamchuea, K.; Ziegler, C.; Beier, M.G.; Damm, C.; Eychmueller, A.; Compton, R.G. Core–Shell Nanoparticles: Characterizing Multifunctional Materials beyond Imaging—Distinguishing and Quantifying Perfect and Broken Shells. Adv. Funct. Mater. 2015, 25, 5149–5158. [Google Scholar] [CrossRef]
- Chauhan, L.; Shukla, A.K.; Sreenivas, K. Dielectric and magnetic properties of Nickel ferrite ceramics using crystalline powders derived from DL alanine fuel in sol–gel auto-combustion. Ceram. Int. 2015, 41, 8341–8351. [Google Scholar] [CrossRef]
- Nabiyouni, G.; Jafari Fesharaki, M.; Mozafari, M.; Amighian, J. Characterization and Magnetic Properties of Nickel Ferrite Nanoparticles Prepared by Ball Milling Technique. Chin. Phys. Lett. 2010, 27, 126401. [Google Scholar] [CrossRef]
- He, X.; Zhong, W.; Au, C.-T.; Du, Y. Size dependence of the magnetic properties of Ni nanoparticles prepared by thermal decomposition method. Nanoscale Res. Lett. 2013, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Ivantsov, R.; Evsevskaya, N.; Saikova, S.; Linok, E.; Yurkin, G.; Edelman, I. Synthesis and characterization of Dy3Fe5O12 nanoparticles fabricated with the anion resin exchange precipitation method. Mater. Sci. Eng. B 2017, 226, 171–176. [Google Scholar] [CrossRef]
- Saikova, S.V.; Trofimova, T.V.; Pavlikov, A.Y.; Samoilo, A.S. Effect of Polysaccharide Additions on the Anion-Exchange Deposition of Cobalt Ferrite Nanoparticles. Russ. J. Inorg. Chem. 2020, 65, 291–298. [Google Scholar] [CrossRef]
- Trofimova, T.V.; Saikova, S.V.; Panteleeva, M.V.; Pashkov, G.L.; Bondarenko, G.N. Anion-Exchange Synthesis of Copper Ferrite Powders. Glass Ceram. 2018, 75, 74–79. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Peptide methionine sulfoxide reductase A (MsrA): Direct electrochemical oxidation on carbon electrodes. Bioelectrochem 2013, 89, 11–18. [Google Scholar] [CrossRef]
- Khoury, R.A.; Ranasinghe, J.C.; Dikkumbura, A.S.; Hamal, P.; Kumal, R.R.; Karam, T.E.; Smith, H.T.; Haber, L.H. Monitoring the Seed-Mediated Growth of Gold Nanoparticles Using in Situ Second Harmonic Generation and Extinction Spectroscopy. J. Phys. Chem. 2018, 122, 24400–24406. [Google Scholar] [CrossRef]
- Levin, C.S.; Hofiman, C.; Ali, T.A.; Kelly, A.T.; Morosan, E.; Nordlander, P.; Whitmire, K.H.; Halas, N.J. Magnetic-Plasmonic Core-Shell Nanoparticles. ACS Nano. 2009, 3, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Weiss, S.; Levine, R. Methionine Oxidation and Reduction in Proteins. Biochim. Biophys. Acta 2014, 1840, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Glišić, B.; Djuran, M.; Stanić, Z.; Rajković, S. Oxidation of methionine residue in Gly-Met dipeptide induced by [Au(en)Cl2]+ and influence ofthe chelated ligand on the rate of this redox process. Gold Bull. 2014, 47, 33–40. [Google Scholar] [CrossRef]
- Angelova, P.; Solel, E.; Parvari, G.; Turchanin, A.; Botoshansky, M.; Gölzhäuser, A.; Keinan, E. Chemisorbed Monolayers of Corannulene Penta-Thioethers on Gold. Langmuir 2013, 29, 2217–2223. [Google Scholar] [CrossRef]
- Chen, K.; Li, H.J.W.; Xu, Y.; Liu, K.; Li, H.; Xu, X.; Qiu, X.; Liu, M. Untying Thioether Bond Structure Enabled by “Voltage-Scissors” for Stable Room Temperature Sodium-Sulfur Batteries. Nanoscale 2019, 11, 5967–5973. [Google Scholar] [CrossRef]
- Bergès, J.; de Oliveira, P.; Fourré, I.; Houée-Levin, C. The One-Electron Reduction Potential of Methionine-Containing Peptides Depends on the Sequence. J. Phys. Chem. B 2012, 116, 9352–9362. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Nasluzov, V.A.; Romanchenko, A.S.; Shor, A.M.; Pal’yanova, G.A. XPS and DFT studies of the electronic structures of AgAuS and Ag3AuS2. J. Alloys Compd. 2014, 617, 314–321. [Google Scholar] [CrossRef]
- Palyanova, G.; Mikhlin, Y.; Zinina, V.; Kokh, K.; Seryotkin, Y.; Zhuravkova, T. New gold chalcogenides in the Au-Te-Se-S system. J. Phys. Chem. Solids. 2020, 136, 109276. [Google Scholar] [CrossRef]
- Radnik, J.; Mohr, C.; Claus, P. On the origin of binding energy shifts of core levels of supported gold nanoparticles and dependence of pretreatment and material synthesis. Phys. Chem. Chem. Phys. 2003, 5, 172–177. [Google Scholar] [CrossRef]
- Arrii, S.; Morfin, F.; Renouprez, A.J.; Rousset, J.L. Oxidation of CO on Gold Supported Catalysts Prepared by Laser Vaporization: Direct Evidence of Support Contribution. J. Am. Chem. Soc. 2004, 126, 1199–1205. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A 2011, 391, 367–376. [Google Scholar] [CrossRef]
- Li, P.; Ma, R.; Zhou, Y.; Chen, Y.; Liu, Q.; Peng, G.; Liang, Z.; Wang, J. Spinel nickel ferrite nanoparticles strongly cross-linked with multiwalled carbon nanotubes as a bi-efficient electrocatalyst for oxygen reduction and oxygen evolution. RSC Adv. 2015, 5, 73834–73841. [Google Scholar] [CrossRef]
- Zvezdin, A.K.; Kotov, V.A. Modern Magnetooptics and Magnetooptical Materials; IOP Publishing: Bristol, UK; Philadelphia, PA, USA, 1997; p. 404. [Google Scholar]
- Himcinschi, C.; Vrejolu, I.; Salvan, G.; Fronk, M.; Talkenberger, A.; Zahn, D.; Rafaja, D.; Kortus, J. Optical and magneto-optical study of nickel and cobalt ferrite epitaxial thin films and submicron structures. J. Appl. Phys. 2013, 113, 084101. [Google Scholar] [CrossRef]
- Verde, E.; Landi, G.; Carriao, M.; Drummond, I.; Gomes, J.; Vieira, E.; Sousa, M.; Bakuzis, A. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. AIP Adv. 2012, 2, 032120. [Google Scholar] [CrossRef]


| Sample | Relative Concentrations (At. %) | ||
|---|---|---|---|
| S | N | Au | |
| Methionine | 3.9 | 3.7 | - |
| Methionine + HAuCl4 | 6.8 | 4.9 | 0.6 |
| NiFe2O4@Au | 14.0 | 0 | 47.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saykova, D.; Saikova, S.; Mikhlin, Y.; Panteleeva, M.; Ivantsov, R.; Belova, E. Synthesis and Characterization of Core–Shell Magnetic Nanoparticles NiFe2O4@Au. Metals 2020, 10, 1075. https://doi.org/10.3390/met10081075
Saykova D, Saikova S, Mikhlin Y, Panteleeva M, Ivantsov R, Belova E. Synthesis and Characterization of Core–Shell Magnetic Nanoparticles NiFe2O4@Au. Metals. 2020; 10(8):1075. https://doi.org/10.3390/met10081075
Chicago/Turabian StyleSaykova, Diana, Svetlana Saikova, Yuri Mikhlin, Marina Panteleeva, Ruslan Ivantsov, and Elena Belova. 2020. "Synthesis and Characterization of Core–Shell Magnetic Nanoparticles NiFe2O4@Au" Metals 10, no. 8: 1075. https://doi.org/10.3390/met10081075
APA StyleSaykova, D., Saikova, S., Mikhlin, Y., Panteleeva, M., Ivantsov, R., & Belova, E. (2020). Synthesis and Characterization of Core–Shell Magnetic Nanoparticles NiFe2O4@Au. Metals, 10(8), 1075. https://doi.org/10.3390/met10081075







