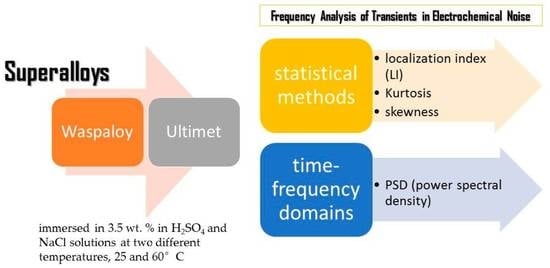
Frequency Analysis of Transients in Electrochemical Noise of Superalloys Waspaloy and Ultimet
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
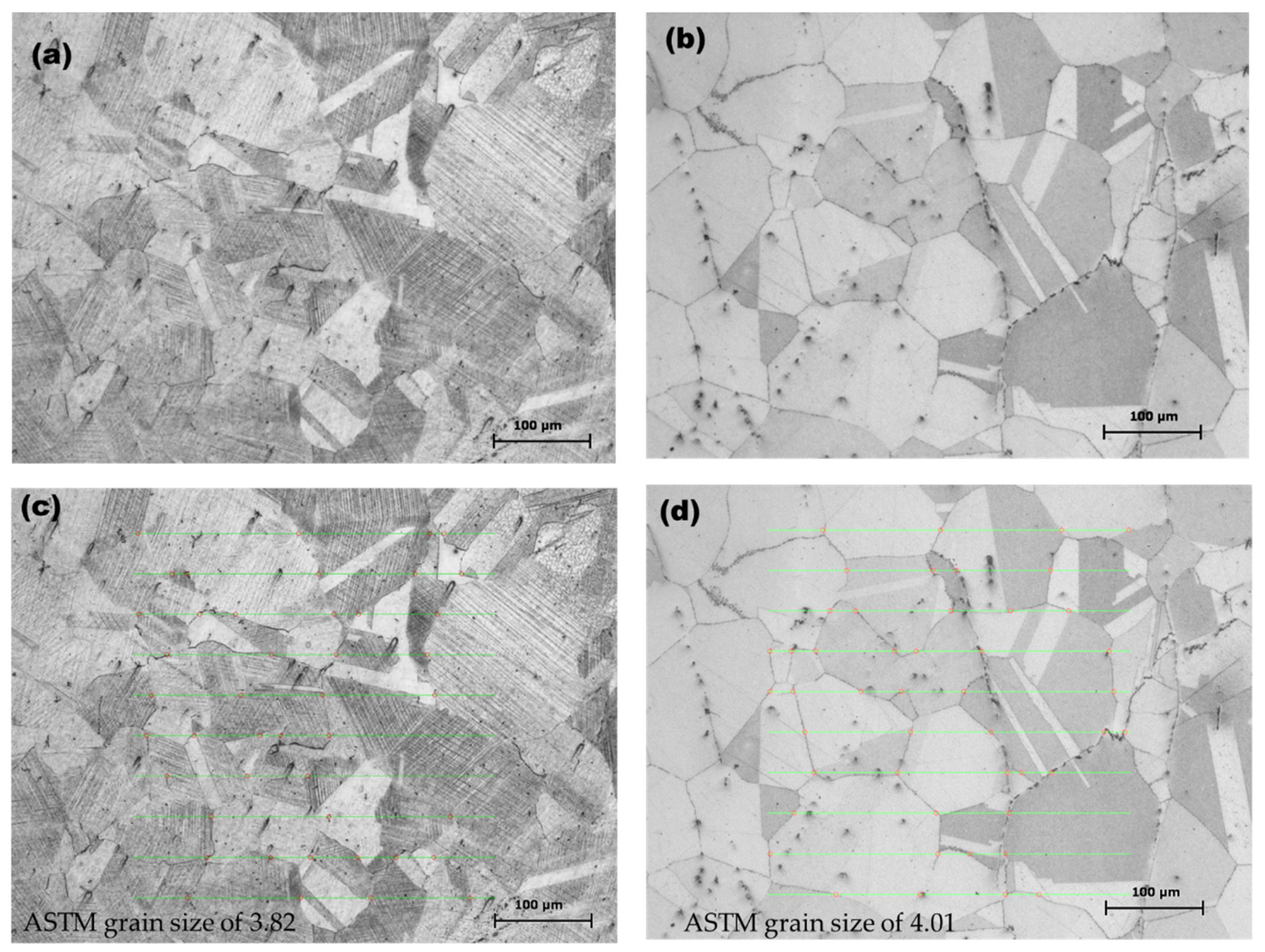
2.2. Microstructural Characterization
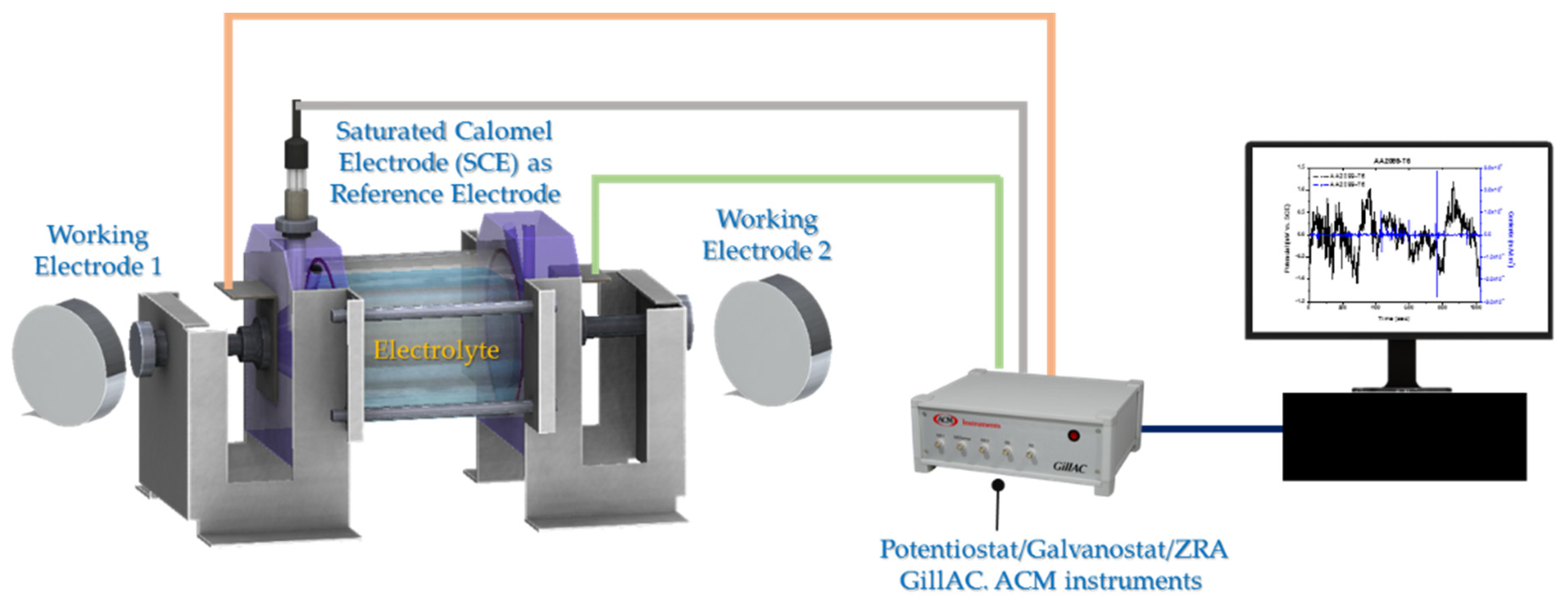
2.3. Electrochemical Techniques
3. Results
3.1. OM Microstructural Analysis
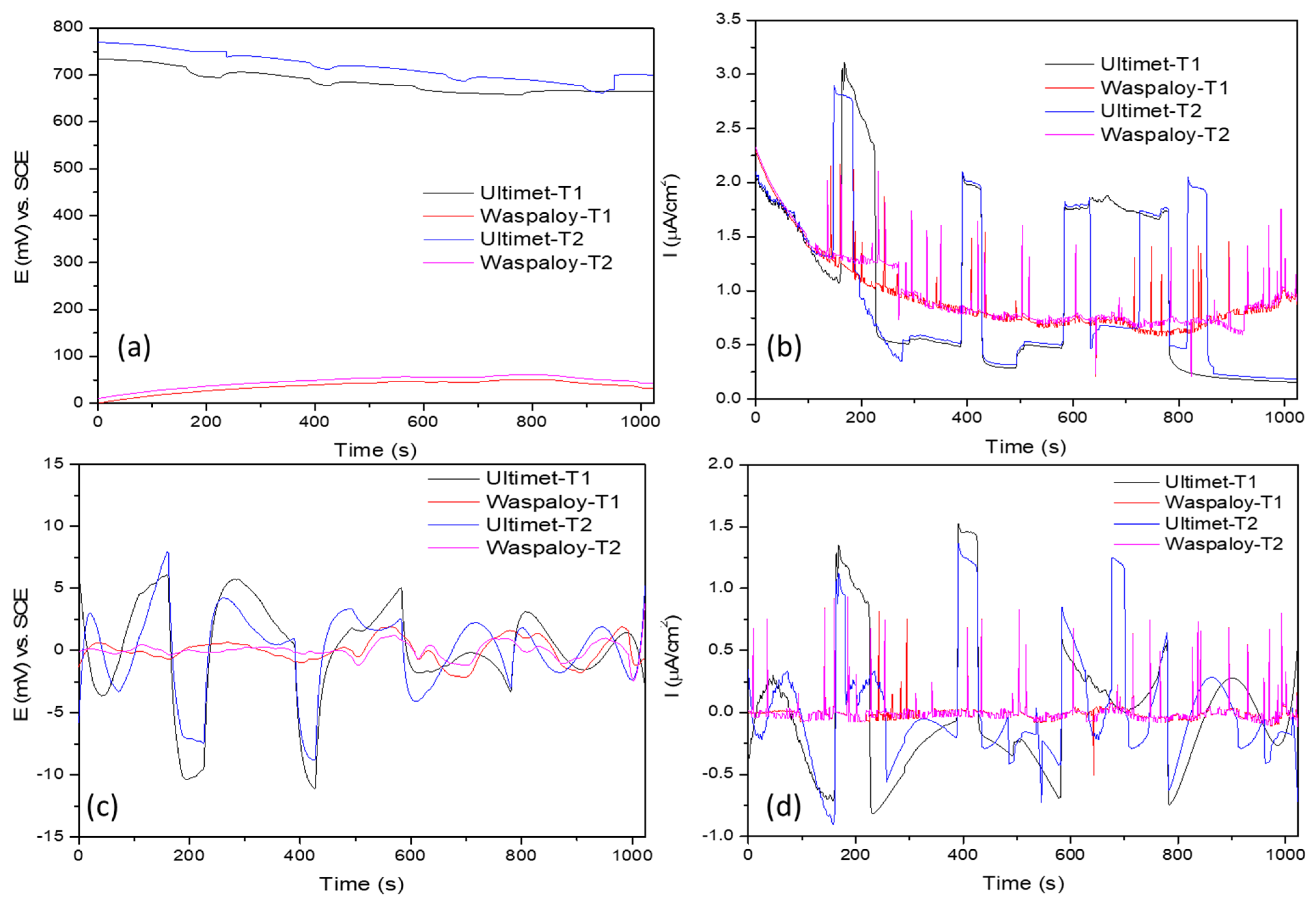
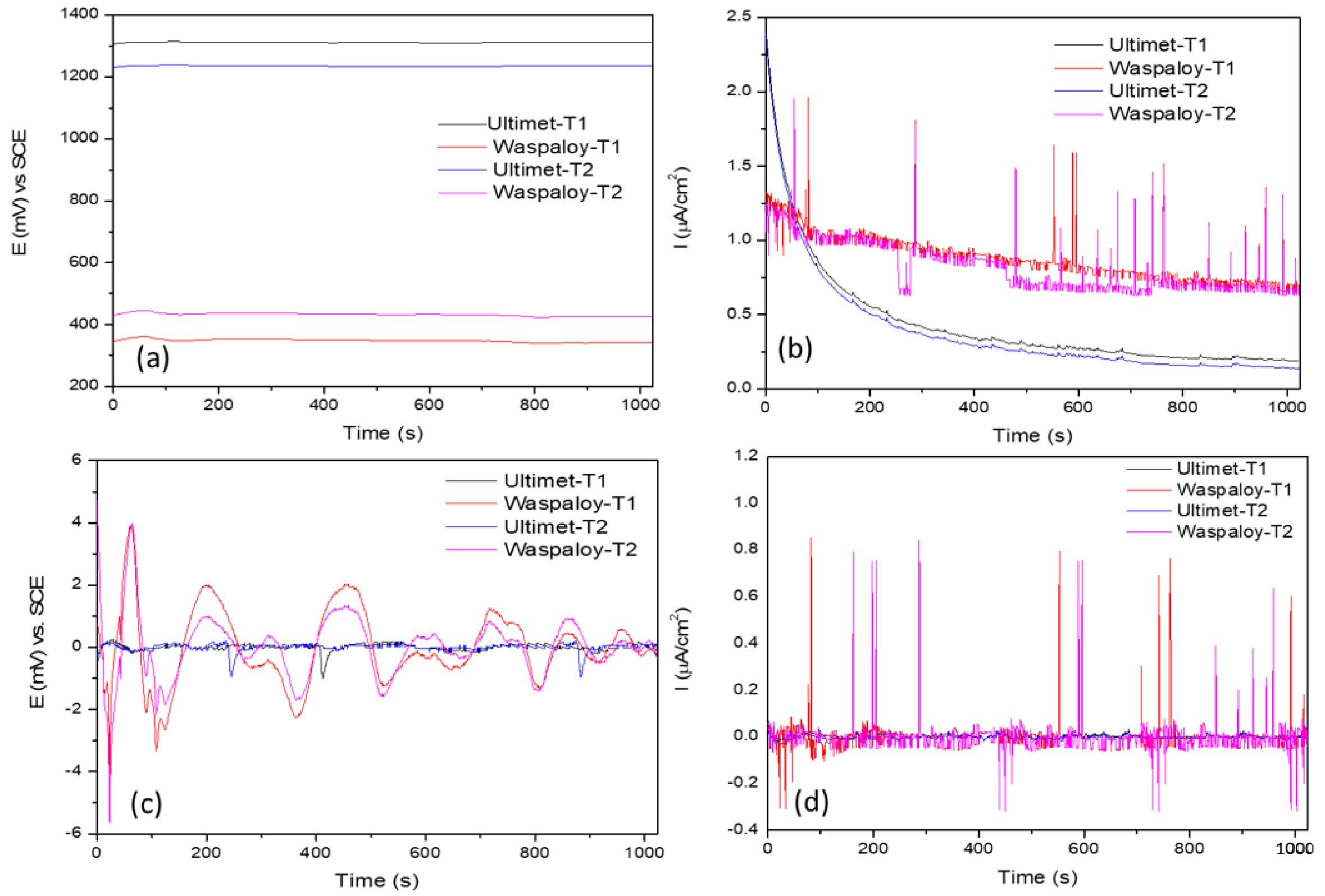
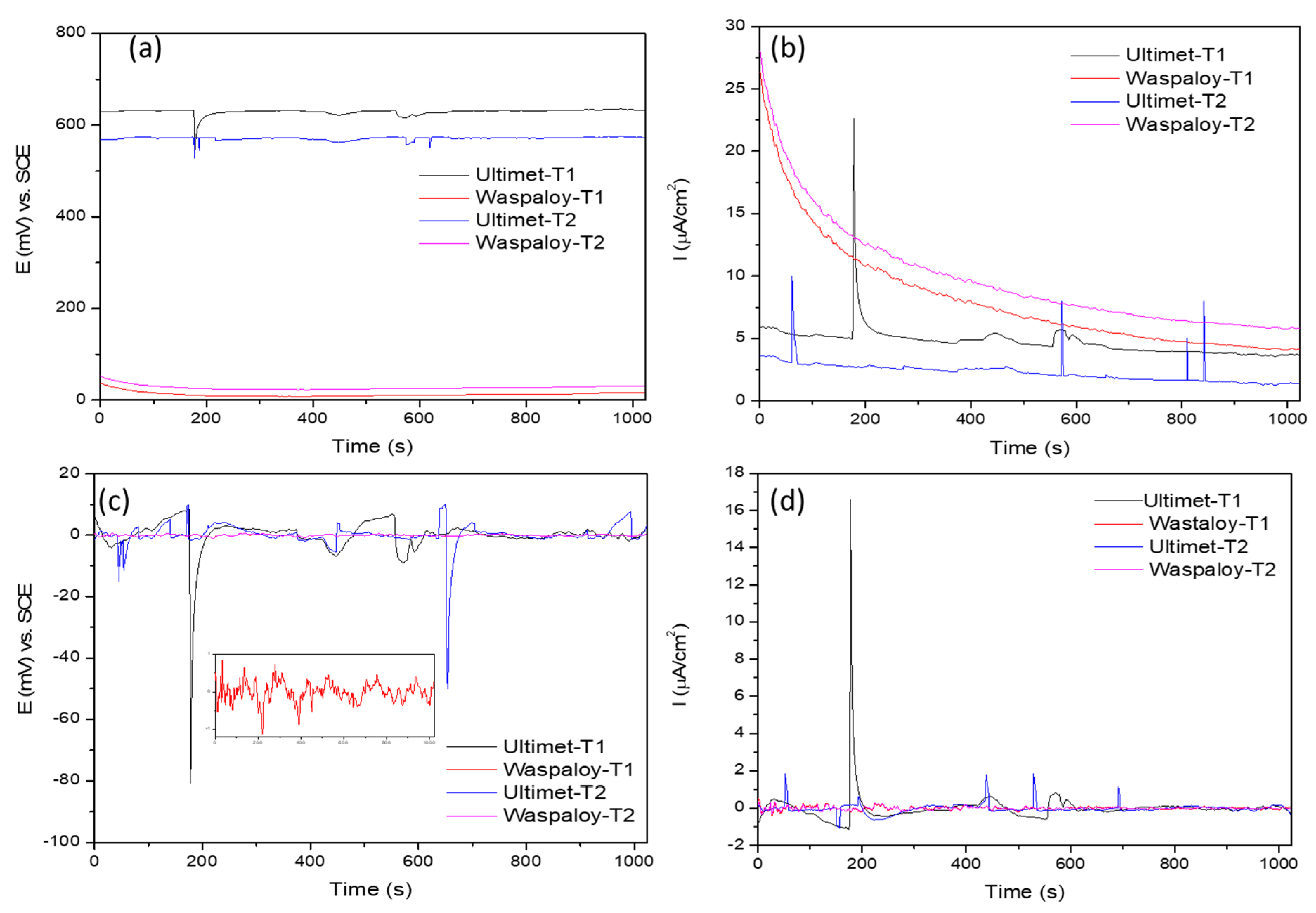
3.2. Electrochemical Noise (EN)
3.2.1. Statistical Analysis
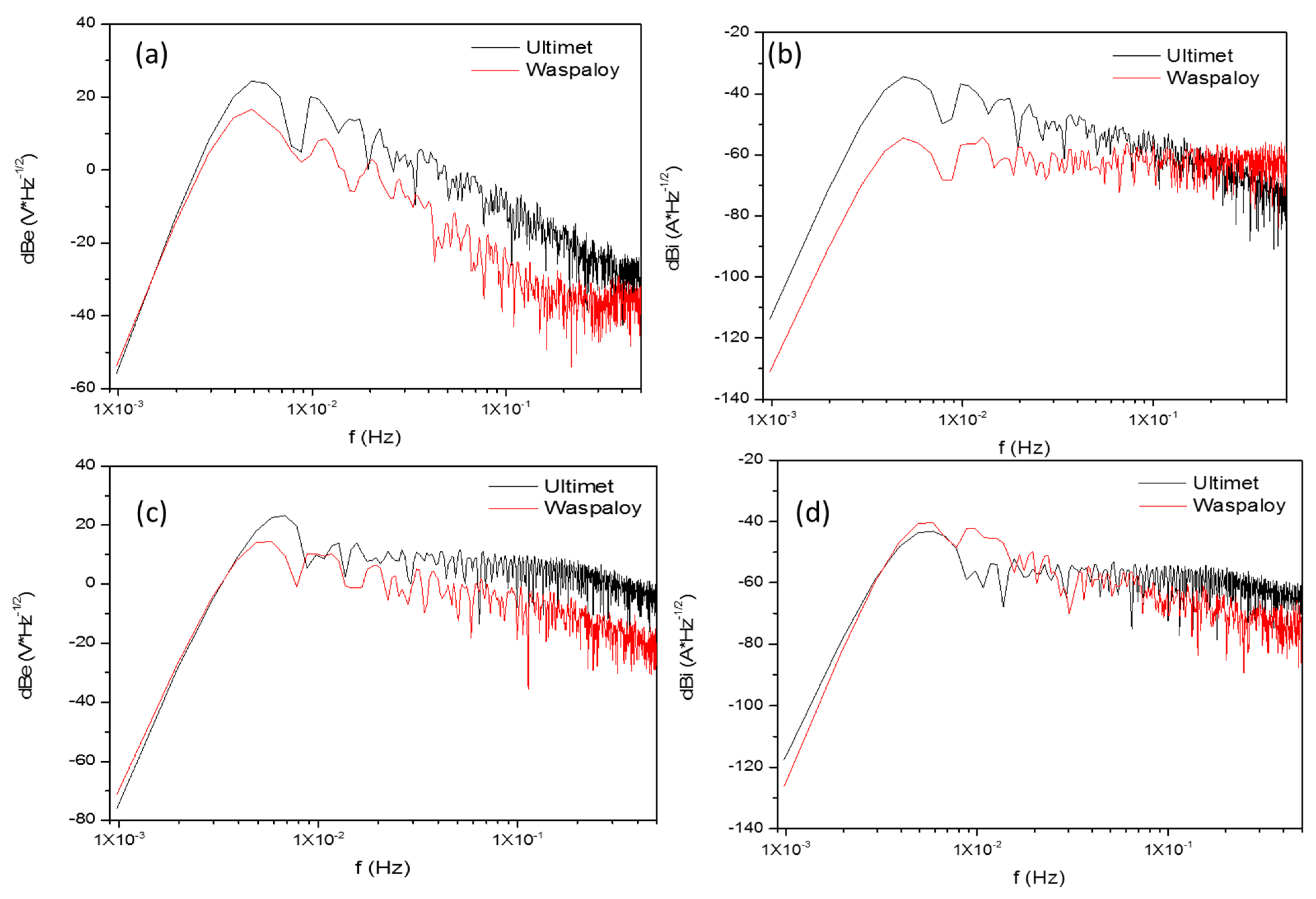
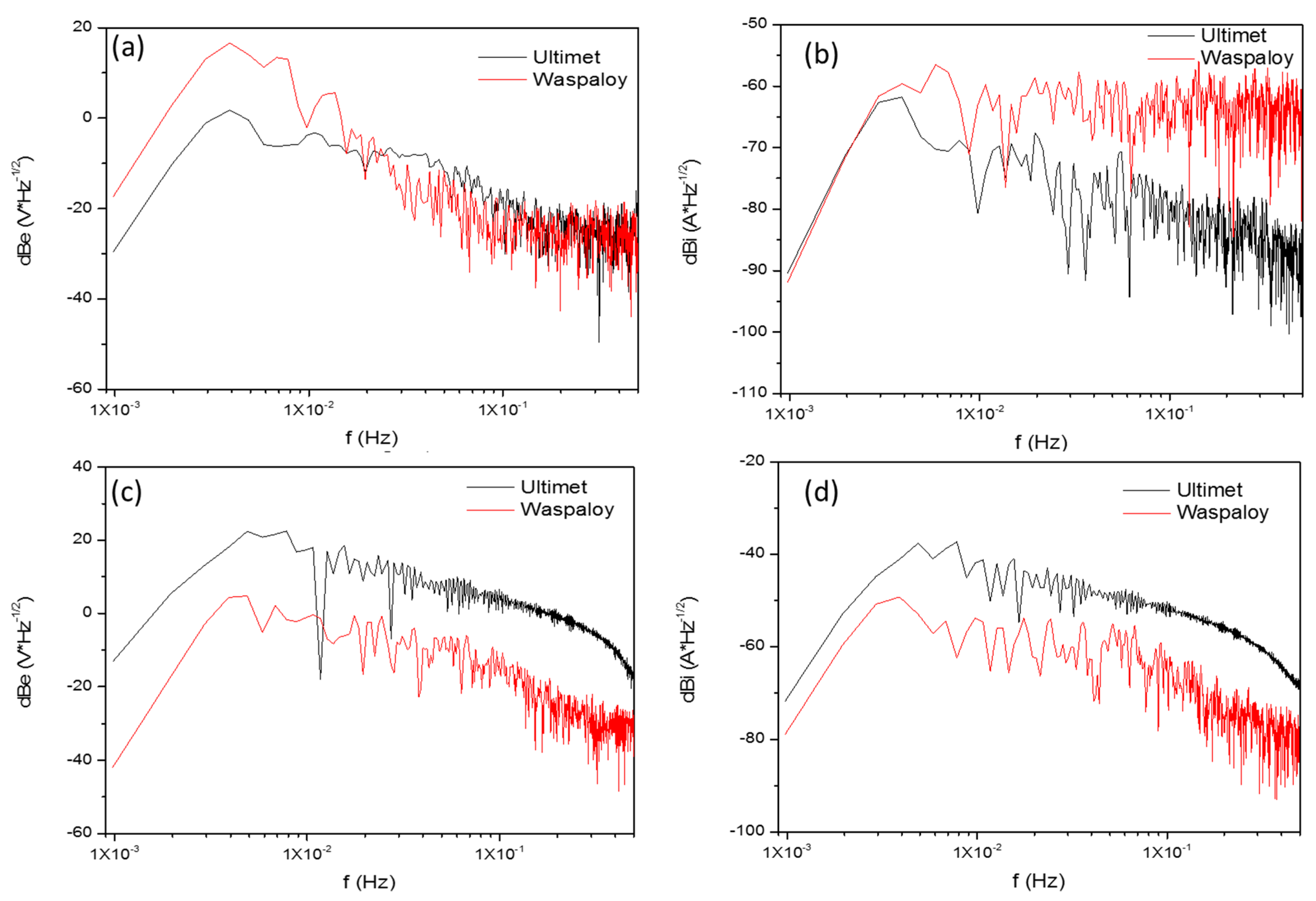
3.2.2. Power Spectral Density Analysis
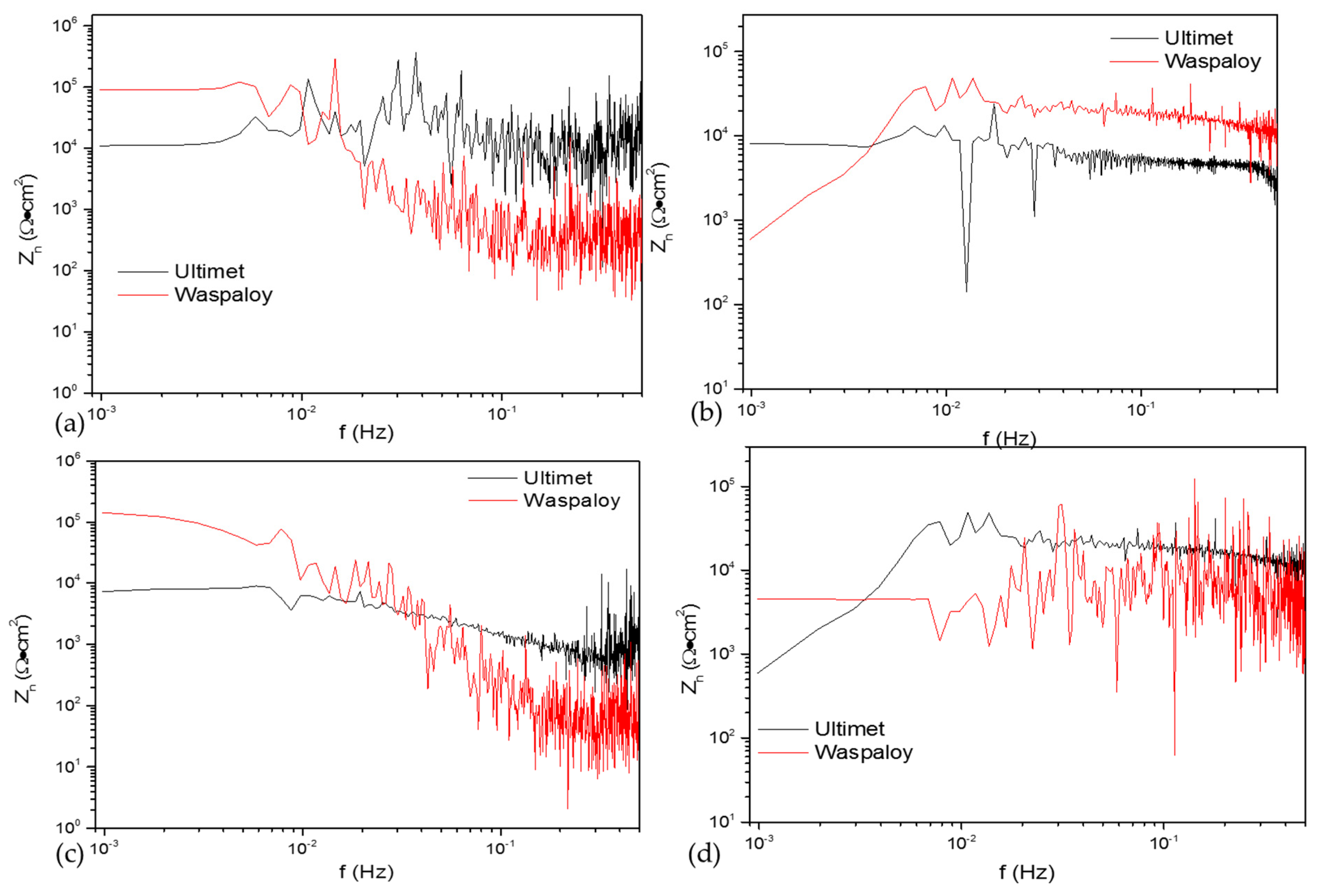
3.2.3. Noise Impedance (Zn)
4. Discussion
5. Conclusions
- Results indicated that, after trend removal, EN signals conserved transients and fluctuation behavior and gave practical corrosion information for removing DC. The standard deviation of ECN and the noise resistance have direct relationships with the corrosion rate.
- Statistical analysis (time-domain) and PSD (frequency domain) results showed that NaCl Ultimet presents higher corrosion resistance at 60 °C than at 25 °C.
- In NaCl at 25 °C, Ultimet and Waspaloy presented localized corrosion, and at 60 °C mixed. Furthermore, in H2SO4 at 60 °C, Waspaloy presented uniform corrosion by LI evaluation parameter. Skewness showed the same result.
- When skewness is negative, it indicates a significant predominance of cathodic transients.
- EN results show that Rn and Ψ0 parameters should be considered as a counterpart to calculate the corrosion resistance of materials.
- For this type of superalloy, LI and skewness are more practical to determine corrosion than PSD slope.
- High kurtosis values are associated with a chaotic system.
- The discordance of statistical results could be related to developing a different corrosion process on the surface. When LI indicates mixed corrosion and skewness uniform corrosion, it suggests that localized and uniform corrosion occurs on the surface, but uniform corrosion is the predominant system.
- To reduce uncertainty created by a standard error in the calculation of kurtosis and skewness, it is necessary to increase the number of data acquired for EN characterization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys, 1st ed.; Springer: Cham, Switzerland, 2020; pp. 129–189. [Google Scholar]
- Mouritz, P.A. Introduction to Aerospace Materials; Woodhead Publishing: Cambridge, UK, 2012; pp. 202–223. [Google Scholar]
- Osoba, L.O.; Oladoye, A.M.; Ogbonna, V.E. Corrosion evaluation of superalloys Haynes 282 and Inconel 718 in Hydrichloric acid. J. Alloys Compd. 2019, 5, 376–384. [Google Scholar] [CrossRef]
- Reed, C.R. The Superalloys Fundamentals and Applications; Cambridge University Press: Edinburg, UK, 2006. [Google Scholar]
- ASM International. Heat-Resistant Materials; ASM International, ASM Specialty Handbook; ASM International: Almere, The Netherlands, 1997; p. 255. [Google Scholar]
- Simon, H.; Thoma, M. Attack on superalloys by chemical and electrolytic processes. Aircr. Eng. Aerosp. Tec. 1981, 53, 10. [Google Scholar] [CrossRef]
- Geddes, B.; León, H.; Huang, X. Superalloys Alloying and Performance; ASM International: Materials Park Ohio, OH, USA, 2010. [Google Scholar]
- Akandea, I.G.; Oluwolea, O.O.; Fayomib, O.S.I.; Odunlamic, O.A. Overview of mechanical, microstructural, oxidation properties and high-temperature applications of superalloys. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Donachie, J.M.; Donachie, J.S. Superalloys a Technical Guide; ASM International: Materials Park Ohio, OH, USA, 2002. [Google Scholar]
- Sims, C.T.; Stoloff, N.S.; Hagel, W.S. Superalloys: Genesis and Character, Superalloys II; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Maebashi, T.; Doi, M. Coarsening behaviours of coherent γ′ and γ precipitates in elastically constrained Ni–Al–Ti alloys. Mater. Sci. Eng. A 2004, 373, 72–79. [Google Scholar] [CrossRef]
- Murata, Y.; Suga, K.; Yukawa, N. Effect of transition elements on the properties of MC carbides in IN-100 nickel-based superalloy. J. Mater. Sci. 1986, 21, 3653–3660. [Google Scholar] [CrossRef]
- Beltran, A.M.; Sims, C.T.; Stoloff, N.S.; Hagel, W.C. Superalloys II; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical polarization. I. A theoretical analysis of the shape of the polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Pellegrini-Cervantes, M.J.; Almeraya-Calderon, F.; Borunda-Terrazas, A.; Bautista-Margulis, R.G.; Chacón-Nava, J.G.; Fajardo-San-Miguel, G.; Almaral-Sanchez, J.L.; Barrios-Durstewitz, C.; Martinez-Villafañe, A. Corrosion Resistance, Porosity and Strength of lended Portland Cement Mortar Containing Rice Husk Ash and Nano-SiO2. Int. J. Electrochem. Sci. 2013, 8, 10697–10710. [Google Scholar]
- Volmer, M.; Weber, A. Keimbildung in nbersättigten Gebilden. Z. Phys. Chem. 1959, 119, 277–3013. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in heterogeneous equilibria, II. The kinetic interpretation of the Nernst theory of electromotive force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in heterogeneous equilibria, I. Conditions at the boundary surface of crystalline solids and liquids, and the application of statistical mechanics. Trans. Faraday Soc. 1924, 19, 659–665. [Google Scholar] [CrossRef]
- Macdonald, D.D. Review of mechanistic analysis by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 1509–1525. [Google Scholar] [CrossRef]
- Estupiñán-López, H.F.; Almeraya-Calderón, F.; Bautista Margulis, G.R.; Baltazar Zamora, M.A.; Martínez-Villafañe, A.; Uruchurtu, C.J.; Gaona-Tiburcio, C. Transient Analysis of Electrochemical Noise for 316 and Duplex 2205 Stainless Steels Under Pitting Corrosion. Int. J. Electrochem. Sci. 2011, 6, 1785–1796. [Google Scholar]
- Mehdipour, M.; Naderi, R.; Markhali, B.P. Electrochemical study of effect of the concentration of azole derivatives on corrosion behavior of stainless steel in H2SO4. Prog. Org. Coat. 2014, 77, 1761–1767. [Google Scholar] [CrossRef]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.W.; Buchheit, G. Electrochemical Techniques in Corrosion Science and Engineering; Taylor & Francis: Boca Raton, FL, USA, 2002; pp. 54–123. [Google Scholar]
- Kearns, J.R.; Eden, D.A.; Yaffe, M.R.; Fahey, J.V.; Reichert, D.L.; Silverman, D.C. ASTM Standardization of Electrochemical Noise Measurement. In Electrochemical Noise Measurement for Corrosion Applications; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: Russell, OH, USA, 1996; pp. 446–471. [Google Scholar]
- Botana, P.J.; Bárcena, M.M.; Villero, Á.A. Ruido Electroquímico: Métodos de Análisis; Septem Ediciones: Cadiz, Spain, 2002; pp. 50–70. [Google Scholar]
- Gaona-Tiburcio, C.; Aguilar, L.M.R.; Zambrano-Robledo, P.; Estupiñán-López, F.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Castillo-González, E.; Almeraya-Calderón, F. Electrochemical Noise Analysis of Nickel Based Superalloys in Acid Solutions. Int. J. Electrochem. Sci. 2014, 9, 523–533. [Google Scholar]
- Ma, C.; Song, S.; Gao, Z.; Wang, J.; Hu, W.; Behnamian, Y.; Xia, D.H. Electrochemical noise monitoring of the atmospheric corrosion of steels: Identifying corrosion form using wavelet analysis. Corros. Eng. Sci. Technol. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Behnamian, Y.; Gao, Z.; Wu, Z.; Qin, Z.; Xia, D.H. Measuring atmospheric corrosion with electrochemical noise: A review of contemporary methods. Measurement 2019, 138, 54–79. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.; Behnamian, Y.; Hu, W.; Cheng, F.; Luo, J.L.; Huet, F. Review—Electrochemical Noise Applied in Corrosion Science: Theoretical and Mathematical Models towards Quantitative Analysis. J. Electrochem. Soc. 2020, 167, 081507. [Google Scholar] [CrossRef]
- Contreras, A.; Salazar, M.; Carmona, A.; Galván-Martínez, R. Electrochemical Noise for Detection of Stress Corrosion Cracking of Low Carbon Steel Exposed to Synthetic Soil Solution. Mater. Res. 2017, 20, 1–10. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; de Garza-Montes, O.N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Monticelli, C. Evaluation of Corrosion Inhibitors by Electrochemical Noise Analysis. J. Electrochem. Soc. 1992, 139, 706. [Google Scholar] [CrossRef]
- Park, C.J.; Kwon, H.S. Electrochemical noise analysis of localized corrosion of duplex stainless steel aged at 475 °C. Mater. Chem. Phys. 2005, 91, 355–360. [Google Scholar] [CrossRef]
- Suresh, G.U.; Kamachi, M.S. Electrochemical Noise Analysis of Pitting Corrosion of Type 304L Stainless Steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Barceinas-Sánchez, J.D.O.; Poblano-Salas, C.A.; Pedraza-Basulto, G.K.; Nieves-Mendoza, D.; Zambrano-Robledo, P.C.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Corrosion Behavior of AISI 409Nb Stainless Steel Manufactured by Powder Metallurgy Exposed in H2SO4 and NaCl Solutions. Int. J. Electrochem. Sci. 2013, 8, 564–577. [Google Scholar]
- Nagiub, A.M. Electrochemical Noise Analysis for Different Green Corrosion Inhibitors for Copper Exposed to Chloride Media. Port. Electrochim. Acta 2017, 35, 201–210. [Google Scholar] [CrossRef]
- Dawson, D.L. Electrochemical Noise Measurement: The definitive In-Situ Technique for Corrosion Applications? In Electrochemical Noise Measurement for Corrosion Applications STP 1277; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: Russell, OH, USA, 1996; pp. 3–39. [Google Scholar]
- Cottis, R.; Turgoose, S.; Mendoza-Flores, J. The Effects of Solution Resistance on Electrochemical Noise Resistance Measurements: A Theorical Analysis. In Electrochemical Noise Measurement for Corrosion Applications STP 1277; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: Russell, OH, USA, 1996; pp. 93–100. [Google Scholar]
- ASTM E3-95. Standard Practice for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- ASTM E407-07. Standard Practice for Microetching Metals and Alloys; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM G199-09. Standard Guide for Electrochemical Noise Measurement; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Vander, V.F.G. ASM Handbook Volume 9: Metallography and Microstructures; ASM International: Materials Park Ohio, OH, USA, 2004; ISBN 978-0-87170-706-2. [Google Scholar]
- Jiang, L.; Liaw, K.P.; Brooks, R.C.; Somieski, B.; Klarstrom, L.D. Nondestructive evaluation of fatigue damage in ULTIMET® superalloy. Mater. Sci. Eng. A 2001, 313, 153–159. [Google Scholar] [CrossRef]
- ASTM E112-13. Standard Test Methods for Determining Average Grain Size; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Osório, R.W.; Cheung, N.; Spinelli, J.E.; Goulart, P.R.; Garcia, A. The effects of a eutectic modifier on microstructure and surface corrosion behavior of Al-Si hypoeutectic alloys. J. Solid State Electrochem. 2007, 11, 1421–1427. [Google Scholar] [CrossRef]
- Donelan, P. Modelling microstructural and mechanical properties of ferritic ductile cast iron. Mater. Sci. Technol. 2000, 16, 261–269. [Google Scholar] [CrossRef]
- Petch, J.N. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Brogdon, L.M.; Rosenberger, H.A. Evaluation of the Influence of Grain Structure on the Fatigue Variability of Waspaloy. Superalloys 2008, 583–588. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Estupiñán, F.; Zambrano, R.P.; Martínez-Villafañe, A.; Borunda, T.A.; Colás, O.R.; Gaona-Tiburcio, C. Análisis de los transitorios de ruido electroquímico para aceros inoxidables 316 y -DUPLEX 2205 en NaCl y FeCl. Rev. Metal. 2012, 4, 147–156. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Shao, Y.; Meng, G.; Wang, F. In-situ study of the formation process of stannate conversion coatings on AZ91D magnesium alloy using electrochemical noise. Corros. Sci. 2010, 52, 892–900. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Basharnavaz, H.; Bezaatpour, A. A Schiff base compound as effective corrosion inhibitor for magnesium in acidic media. Mater. Chem. Phys. 2013, 138, 794–802. [Google Scholar] [CrossRef]
- Homborg, A.M.; Tinga, T.; Zhang, X.; Van Westing, E.P.M.; Ferrari, G.M.; Wit, J.H.W.; Mol, J.M.W. A Critical Appraisal of the Interpretation of Electrochemical Noise for Corrosion Studies. Corrosion 2017, 70, 971–987. [Google Scholar] [CrossRef]
- Eden, D.A.; John, D.G.; Dawson, J.L. “Corrosion Monitoring” International Patent WO 87/07022 (World Intellectual Property Organization, Nov. 19, 1997). Available online: https://patentimages.storage.googleapis.com/19/ca/d4/c180ce2c0b9dfe/WO1987007022A1.pdf (accessed on 15 March 2021).
- Corral-Higuera, R.; Arredondo-Rea, P.; Neri-Flores, M.A.; Gómez-Soberón, J.M.; Almaral-Sánchez, J.L.; Castorena-González, J.C.; Almeraya-Calderón, F. Chloride ion penetrability and Corrosion Behavior of Steel in Concrete with Sustainability Characteristics. Int. J. Electrochem. Sci. 2011, 6, 958–970. [Google Scholar]
- Mansfeld, F.; Sun, Z. Technical Note: Localization Index Obtained from Electrochemical Noise Analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]
- Reid, S.A.; Eden, D.A. Assessment of Corrosion. US9264824B1; UK, 24 July 2001. Available online: http://www.khdesign.co.uk/Patents/US6264824.Eden%20AI.pdf (accessed on 15 March 2021).
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Cottis, R. Interpretation of Electrochemical Noise Data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Coakley, J.; Vorontsov, V.A.; Littlell, K.C.; Heenan, R.K.; Ohnuma, G.; Jones, N.G.; Dye, D. Nanoprecipitation in a beta-titanium alloy. J. Alloy. Compd. 2015, 623, 146. [Google Scholar] [CrossRef]
- Bertocci, U.; Huet, F. Noise Analysis Applied to Electrochemical Systems. Corrosion 1995, 51, 131–144. [Google Scholar] [CrossRef]
- Lee, C.C.; Mansfeld, F. Analysis of electrochemical noise data for a passive system in the frequency domain. Corr. Sci. 1998, 40, 959–962. [Google Scholar] [CrossRef]
- Legat, A.; Dolecek, V. Corrosion Monitoring System Based on Measurement and Analysis of electrochemical Noise. Corrosion 1995, 51, 295–300. [Google Scholar] [CrossRef]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An integrated approach in the time, frequency and time-frequency domain for the identification of corrosion using electrochemical noise. Electrochim. Acta 2016, 222, 627–640. [Google Scholar] [CrossRef]
- Bertucci, U.; Gabrielli, C.; Huet, F.; Keddam, M.; Rousseau, P. Noise Resistance Applied to Corrosion Measurements: II. Experimental Tests. J. Electrochim. Soc. 1997, 144, 37. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.-Z.; Behnamian, Y. Detection of corrosion degradation using electrochemical noise (EN): Review of signal processing methods for identifying corrosion forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Cottis, R.A.; Turgoose, S. Electrochemical Impedance and Noise, Corrosion Testing Made Easy; Syrett, B.C., Ed.; NACE International: Houston, TX, USA, 1999; Volume 7. [Google Scholar]
- Galvan-Martinez, R.; Orozco-Cruz, R.; Torres-Sanchez, R.; Martinez, E.A. Corrosion study of the X52 steel immersed in seawater with a corrosion inhibitor using a rotating cylinder electrode. Mater. Corros. 2010, 61, 872–876. [Google Scholar] [CrossRef]
- Sakairi, M.; Sasaki, R.; Kaneko, A.; Seki, Y.; Nagasawa, D. Evaluation of metal cation effects on galvanic corrosion behavior of the A5052 aluminum alloy in low chloride ion containing solution by electrochemical noise impedance. Electrochim. Acta 2014, 131, 123. [Google Scholar] [CrossRef]
- Lentka, L.; Smulko, J. Methods of trend removal in electrochemical noise data-overview. Measurement 2019, 131, 569–581. [Google Scholar] [CrossRef]
- Jing, L.; Brooks, C.R.; Liaw, P.K.; Wang, H.; Rawn, C.L.; Klastrom, D.L. High-frequency metal fatigue: The high-cycle fatigue behavior of ULTIMET® alloy. Mater. Eng. 2001, A314, 162. [Google Scholar] [CrossRef]
- Keyvani, M.; Garcin, T.; Militzer, M.; Fabregue, D. Laser ultrasonic measurement of recrystallization and grain growth in an L605 cobalt superalloy. Mater. Charact. 2020, 167, 110465. [Google Scholar] [CrossRef]
- Kelekanjeri, V.S.K.G.; Moss, L.K.; Gerhardt, R.A.; Ilavsky, J. Quantification of the coarsening kinetics of γ’ precipitates in Waspaloy microstructures with different pri-or homogenizing treatments. Act. Mater. 2009, 57, 4658. [Google Scholar] [CrossRef]
- Hussein, S.I.S.; Segal, J.; McCartney, D.G.; Pashby, I.R. Microstructure formation inWaspaloy multilayer builds following direct metal deposition with laser and wire. Mater. Sci. Eng. A 2008, 497, 260–269. [Google Scholar] [CrossRef]
- Charpagne, M.; Franchet, J.M.; Bozzolo, N. Overgrown grains appearing during sub-solvus heat treatment in a polycrystalline γ-γ’ Nickel-based superalloy. Mater. Des. 2018, 144, 353–360. [Google Scholar] [CrossRef]
- Long, H.; Mao, S.; Liu, Y.; Zhang, Z.; Han, X. Microstructural and compositional design of Ni-based single crystalline superalloys—A review. J. Alloys Compd. 2018, 743, 203–220. [Google Scholar] [CrossRef]
- Martínez-Villafañe, A.; Almeraya-Calderón, M.F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform. 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Human, A.M.; Roebuck, B.; Exner, H.E. Electrochemical polarisation and corrosion behaviour of cobalt and Co(W,C) alloys in 1 N sulphuric acid. Mater. Sci. Eng. 1998, A241, 202–210. [Google Scholar] [CrossRef]
- Kup, A.N.; Tiras, E.; Karahan, H.I.; Aylikci, V.; Eskil, M.; Cengiz, E. Alloying effect on K to L shell vacancy transfer probabilities in Zn-Co Alloys and Al-Ni-Mo superalloys. J. Chem. Phys. 2010, 377, 100–108. [Google Scholar]
- Ting, C.; Hendrik, J.; Jing, X.; Qiuhong, L.; Jeffrey, H.; Xingbo, L. Influence of surface modifications on pitting corrosion behavior of nickel-base alloy 718. Part 2: Effect of aging treatment. Corr. Sci. 2014, 78, 151–161. [Google Scholar] [CrossRef]
- Razavi, S.R. Laser beam welding of Waspaloy: Characterization and corrosion behavior evaluation. Opt. Laser Technol. 2016, 82, 113–120. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Li, Y.; Wang, F. The electrochemical corrosion behavior of K38G nanocrystalline thin film in 3.5% NaCl solution. Thin Solid Films 2011, 519, 4781–4787. [Google Scholar] [CrossRef]
- Zhang, B.; Xiu, M.; Tan, T.Y.; Wei, J.; Wang, P. Pitting Corrosion of SLM Inconel 718 sample under surface and heat treatments. Appl. Surf. Sci. 2019, 490, 556–567. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, C.; Sun, J. Electrochemical corrosion behavior of the cobalt modified aluminide coating in 3.5 wt% NaCl Solutions. Prog. Nat. Sci. 2018, 28, 85–89. [Google Scholar] [CrossRef]
- Klein, L.; Virtanen, S. Electrochemical characterization of novel γ/γ’—Strengthened Co-base superalloys. Electrochim. Acta 2012, 76, 275–281. [Google Scholar] [CrossRef]
- Eden, D.A. Electrochemical Noise—The First Two Octaves. In NACE International Corrosion/98; NACE International: San Diego, FL, USA, 1998; pp. 1–31. [Google Scholar]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado, E.M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef] [PubMed]
- Galván-Martínez, R.; Cabrera-de la Cruz, D.; Contreras, A.; Orozco-Cruz, R. A novel experimental arrangement for corrosión study of X60 pipeline steel weldments at turbulent flow conditions. Corros. Eng. Sci. Technol. 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Bertocci, U.; Gabrielli, C.; Huet, F.; Keddam, M. Noise Resistance Applied to Corrosion Measurements: I. Theorical Analysis. J. Electrochem. Soc. 1997, 144, 31. [Google Scholar] [CrossRef]

| Superalloys | Elements | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Fe | Co | Mo | Si | C | W | Al | Ti | Zr | |
| Ultimet | 9.2 | 25.5 | 3 | 54.1 | 4.8 | 0.31 | 0.06 | 2.1 | -- | -- | -- |
| Waspaloy | 55.3 | 18.8 | 2.1 | 12.5 | 4.2 | 0.75 | 0.07 | -- | 1.4 | 3.4 | 0.01 |
| Corrosion Type | LI |
|---|---|
| Localized | 1.0—0.1 |
| Mixt | 0.1—0.01 |
| Uniform | 0.01—0.001 |
| Corrosion Type | Potential | Current | ||
|---|---|---|---|---|
| Skewness | Kurtosis | Skewness | Kurtosis | |
| Uniform | <±1 | <3 | <±1 | <3 |
| Pitting | <−2 | >>3 | >±2 | >>3 |
| Transgranular (SCC) | 4 | 20 | −4 | 20 |
| Intergranular (SCC #1) | −6.6 | 18 to 114 | 1.5 to 3.2 | 6.4 to 15.6 |
| Intergranular (SCC #2) | −2 to −6 | 5 to 45 | 3 to 6 | 10 to 60 |
| Solution | T (°C) | Materials | Test | Rn | icorr (mA/cm2) | LI | Corrosion Type | Kurtosis | Corrosion Type | Skew (I) | Corrosion Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (ohm) | (I) | ||||||||||
| NaCl | 25 | Ultimet | 1 | 7.58 × 103 ± 379 | 3.43 × 10−3 ± 1.7 × 10−4 | 0.4 | Localized | 4.1 | Pitting | 1.04 | Pitting |
| 2 | 7.12 × 103 ± 356 | 3.41 × 10−3 ± 1.7 × 10−4 | 0.36 | Localized | 3.9 | Pitting | 1.09 | Pitting | |||
| 60 | 1 | 2.12 × 104 ± 1060 | 1.22 × 10−3 ± 6.1 × 10−5 | 0.02 | Mix | 29.4 | Pitting | 2.52 | Pitting | ||
| 2 | 2.02 × 104 ± 1010 | 1.19 × 10−3 ± 6.0 × 10−5 | 0.02 | Mix | 27.5 | Pitting | 2.68 | Pitting | |||
| 25 | Waspaloy | 1 | 8.64 × 103 ± 432 | 3.01 × 10−3 ± 1.5 × 10−4 | 0.11 | Localized | 38.43 | Pitting | 5.47 | Pitting | |
| 2 | 8.71 × 103 ± 435 | 3.09 × 10−3 ± 1.5 × 10−4 | 0.23 | Localized | 39.56 | Pitting | 5.51 | Pitting | |||
| 60 | 1 | 4.67 × 103 ± 233 | 5.56 × 10-3 ± 2.8 × 10−4 | 0.02 | Mix | 3.71 | Pitting | −0.49 | Uniform | ||
| 2 | 4.03 × 103 ± 201 | 5.65 × 10−3 ± 2.8 × 10−4 | 0.02 | Mix | 3.61 | Pitting | −0.52 | Uniform | |||
| H2SO4 | 25 | Ultimet | 1 | 1.28 × 104 ± 640 | 2.02 × 10−3 ± 1.0 × 10−4 | 0.02 | Mix | 9.41 | Pitting | 1.01 | Pitting |
| 2 | 1.16 × 104 ± 580 | 2.00 × 10−3 ± 1.0 × 10−4 | 0.020 | Mix | 9.49 | Pitting | 1.09 | Pitting | |||
| 60 | 1 | 6.44 × 103 ± 322 | 4.04 × 10−3 ± 2.0 × 10−4 | 0.17 | Localized | 181.91 | Pitting | 11.32 | Pitting | ||
| 2 | 6.58 × 103 ± 329 | 4.02 × 10−3 ± 2.0 × 10−4 | 0.18 | Localized | 175.53 | Pitting | 11.01 | Pitting | |||
| 25 | Waspaloy | 1 | 1.40 × 104 ± 700 | 1.84 × 10−3 ± 9.2 × 10−5 | 0.1 | Mix | 61.82 | Pitting | 6.83 | Pitting | |
| 2 | 1.34 × 104 ± 670 | 1.91 × 10−3 ± 9.6 × 10−5 | 0.09 | Mix | 61.63 | Pitting | 6.71 | Pitting | |||
| 60 | 1 | 2.83 × 103 ± 141 | 9.18 × 10−3 ± 4.6 × 10−4 | 0.01 | Uniform | 6.05 | Pitting | 0.32 | Uniform | ||
| 2 | 2.95 × 103 ± 147 | 9.21 × 10−3 ± 4.6 × 10−4 | 0.01 | Uniform | 6.01 | Pitting | 0.29 | Uniform |
| Corrosion Type | dB (V)·Decade−1 | dB (A)·Decade−1 | ||
|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | |
| Uniform | 0 | −7 | 0 | −7 |
| Pitting | −20 | −25 | −7 | −14 |
| Passive | −15 | −25 | −1 | 1 |
| Alloys | Terms | Ψ0 (dBi) | Β (dB (V)) | B (dB (A)) |
|---|---|---|---|---|
| Ultimet | NaCl, 25 °C | −72.2 | −22.9 | −15.6 |
| Waspaloy | −91.3 | −20.4 | 1.5 | |
| Ultimet | NaCl, 60 °C | −78.7 | −6.1 | −4.0 |
| Waspaloy | −82.3 | −13.0 | −11.4 | |
| Ultimet | H2SO4, 25 °C | −71.3 | −10.4 | −7.5 |
| Waspaloy | −71.7 | −11.5 | 1.4 | |
| Ultimet | H2SO4, 60 °C | −52.9 | −14.2 | −11.4 |
| Waspaloy | −59.7 | −15.2 | −13.3 |
| Alloys | Terms | Ψ0 (dBi) | Zn (Ω·cm2) |
|---|---|---|---|
| Ultimet | NaCl, 25 °C | −72.2 | 8.1 × 104 |
| Waspaloy | −91.3 | 1.2 × 105 | |
| Ultimet | NaCl, 60 °C | −78.7 | 1.9 × 103 |
| Waspaloy | −82.3 | 4.5 × 103 | |
| Ultimet | H2SO4, 25 °C | −71.3 | 1.1 × 104 |
| Waspaloy | −71.7 | 9.2 × 104 | |
| Ultimet | H2SO4, 60 °C | −52.9 | 7.9 × 103 |
| Waspaloy | −59.7 | 1.9 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; Estupinán-López, F.; López-León, L.D.; Chacón-Nava, J.; Almeraya-Calderón, F. Frequency Analysis of Transients in Electrochemical Noise of Superalloys Waspaloy and Ultimet. Metals 2021, 11, 702. https://doi.org/10.3390/met11050702
Jáquez-Muñoz JM, Gaona-Tiburcio C, Cabral-Miramontes J, Nieves-Mendoza D, Maldonado-Bandala E, Olguín-Coca J, Estupinán-López F, López-León LD, Chacón-Nava J, Almeraya-Calderón F. Frequency Analysis of Transients in Electrochemical Noise of Superalloys Waspaloy and Ultimet. Metals. 2021; 11(5):702. https://doi.org/10.3390/met11050702
Chicago/Turabian StyleJáquez-Muñoz, Jesús Manuel, Citlalli Gaona-Tiburcio, Jose Cabral-Miramontes, Demetrio Nieves-Mendoza, Erick Maldonado-Bandala, Javier Olguín-Coca, Francisco Estupinán-López, Luis Daimir López-León, José Chacón-Nava, and Facundo Almeraya-Calderón. 2021. "Frequency Analysis of Transients in Electrochemical Noise of Superalloys Waspaloy and Ultimet" Metals 11, no. 5: 702. https://doi.org/10.3390/met11050702
APA StyleJáquez-Muñoz, J. M., Gaona-Tiburcio, C., Cabral-Miramontes, J., Nieves-Mendoza, D., Maldonado-Bandala, E., Olguín-Coca, J., Estupinán-López, F., López-León, L. D., Chacón-Nava, J., & Almeraya-Calderón, F. (2021). Frequency Analysis of Transients in Electrochemical Noise of Superalloys Waspaloy and Ultimet. Metals, 11(5), 702. https://doi.org/10.3390/met11050702