Effects of Gas Pressure during Electron Beam Energy Deposition in the EBM Additive Manufacturing Process
Abstract
1. Introduction
2. Motivation
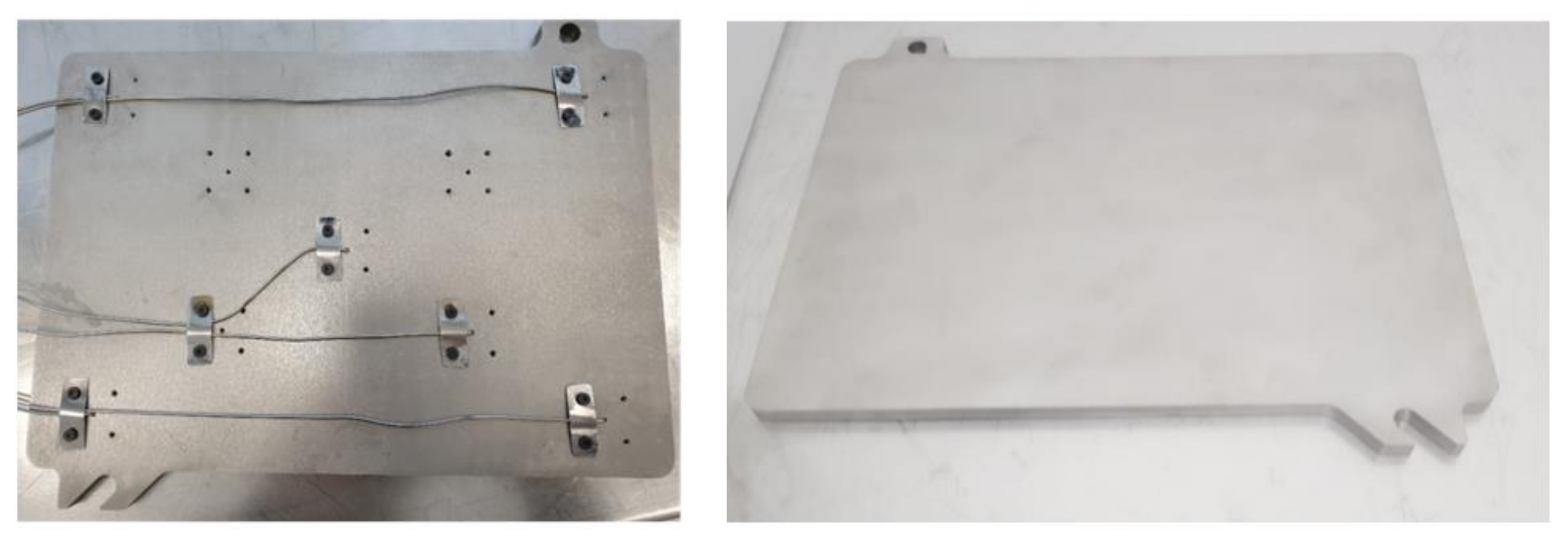
3. Methods
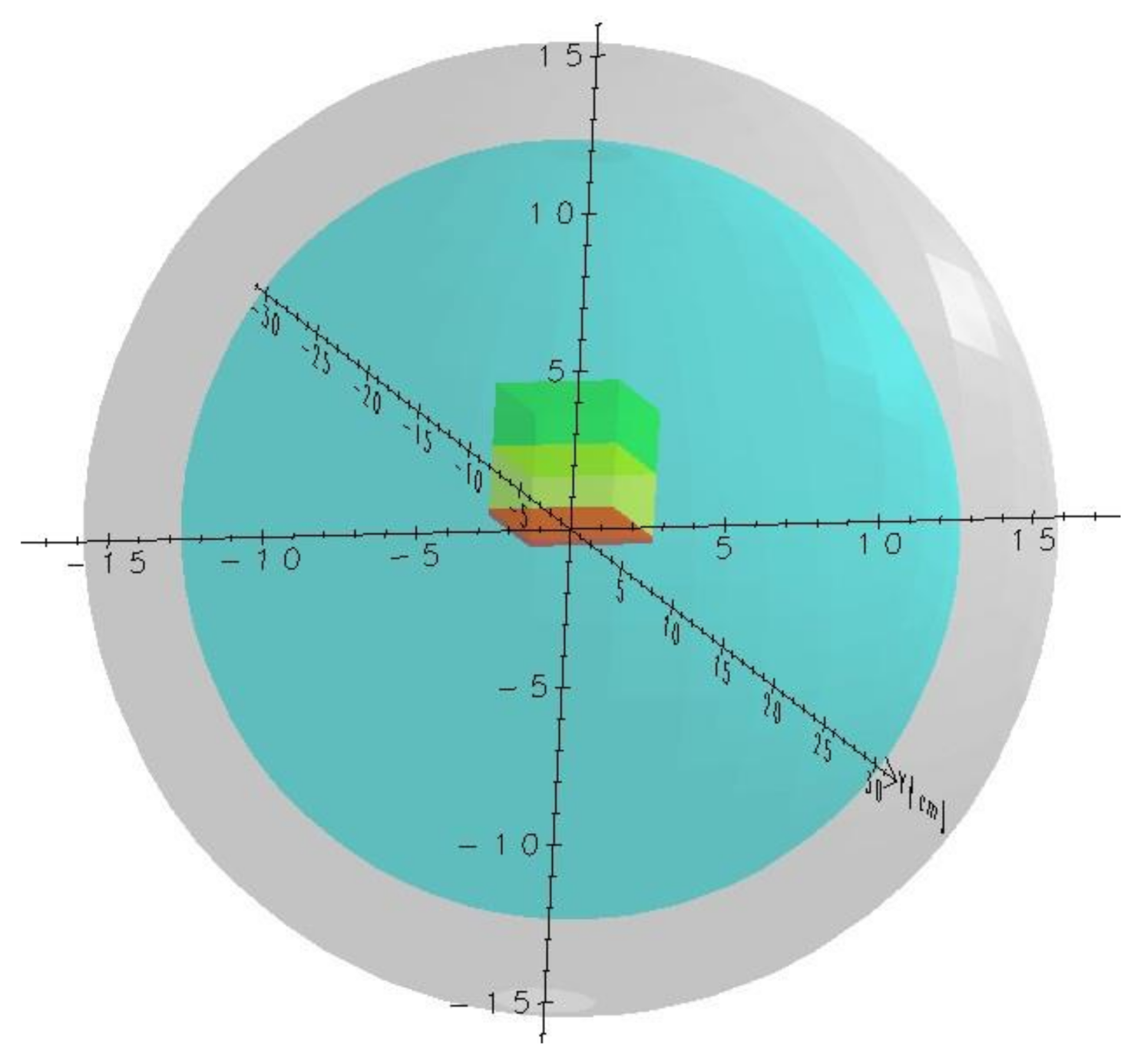
Preliminary Assumptions for MC Simulations
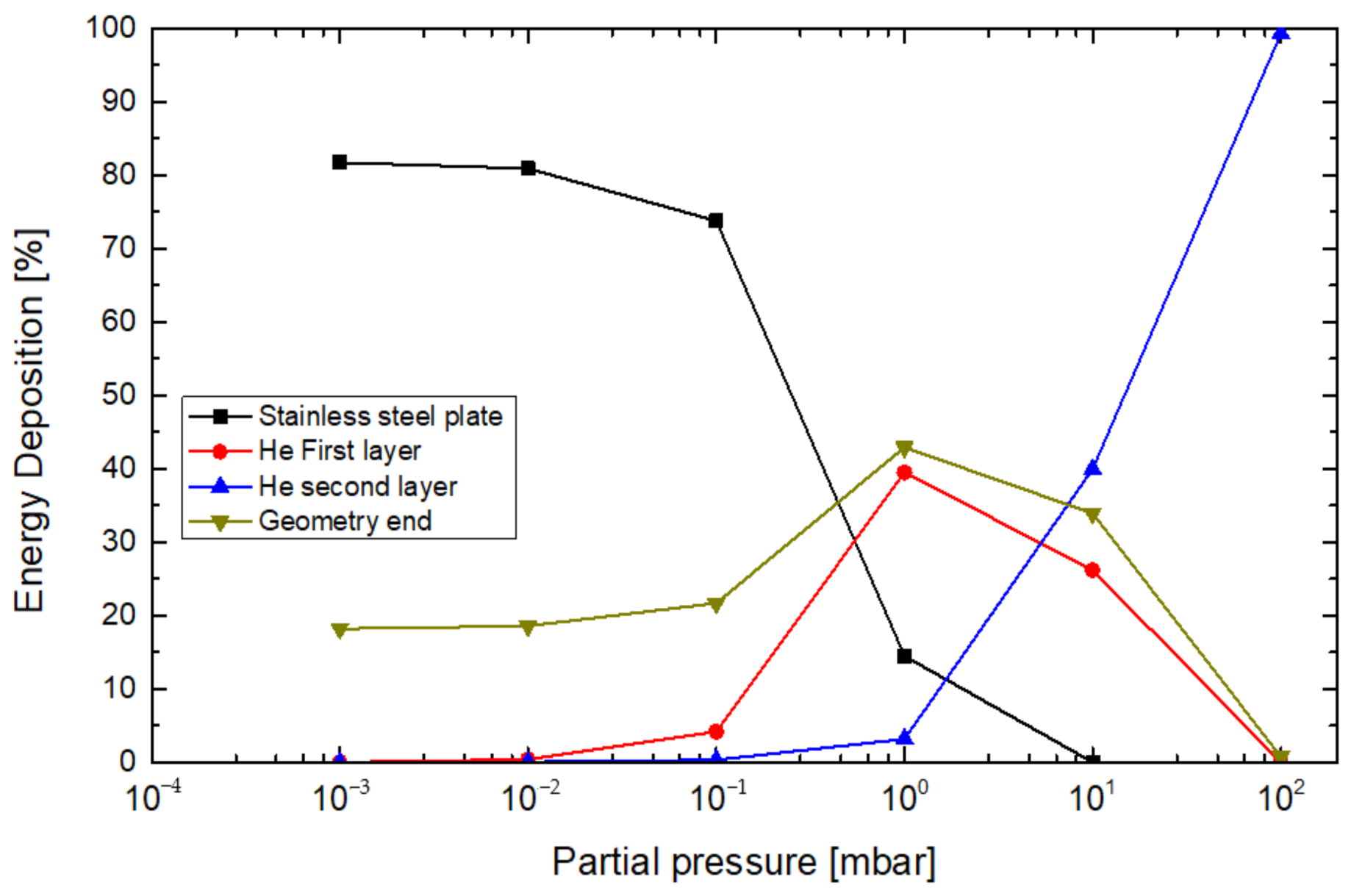
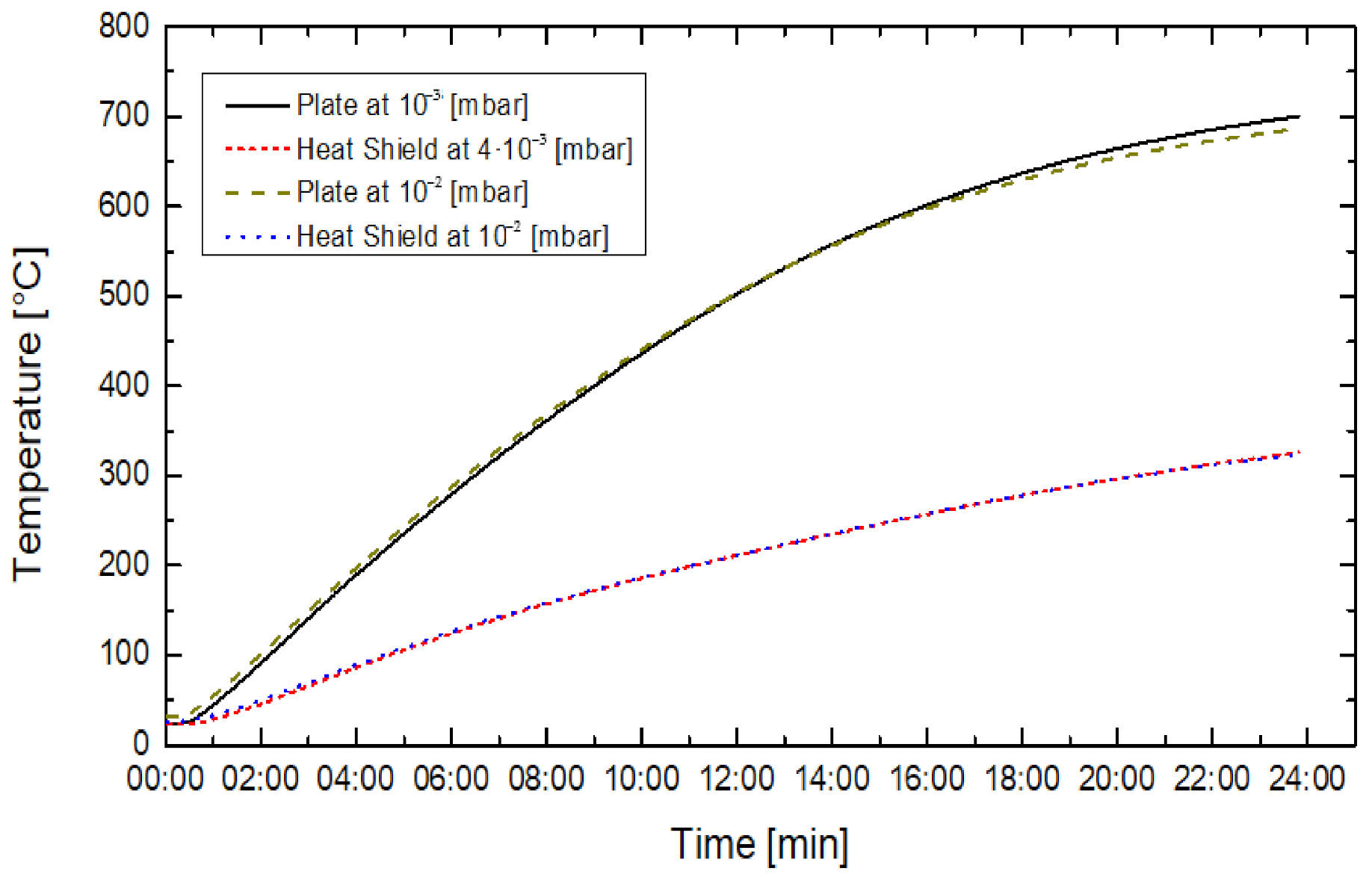
4. Results
5. Discussion
6. Conclusions
- Increasing the gas pressure by one order of magnitude does not affect the process. It did not significantly reduce (less than 1%) the energy deposition efficiency.
- When increasing gas pressure, the heat shield temperature did not change substantially, meaning that it did not influence the thermal conductivity, despite the slight increase of gas presence in the chamber.
- Hence, the option of reducing volatility for elements such as chromium or aluminum is promising, especially since new pre-alloyed materials, such as stainless steel, are fresh candidates for EBM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Beam Current [mA] | Beam Velocity [mm/s] | Focus Offset [mA] | Line Offset [mm] | Line Order | Hatch Direction | No. of Repetitions |
|---|---|---|---|---|---|---|
| 33 | 42000 | 44 | 0.4 | 20 | unidirectional | 3 |
| Mn [%] | Si [%] | Ni [%] | Cr [%] | Mo [%] | P [%] | |
|---|---|---|---|---|---|---|
| Powder | 1.67 | 0.72 | 12.34 | 18.28 | 2.68 | 0.024 |
| As Build | 1.57 | 0.71 | 12.46 | 18.40 | 2.72 | 0.024 |
References
- Zhang, L.; Liu, Y.; Li, S.; Hao, Y. Additive Manufacturing of Titanium Alloys by Electron Beam Melting: A Review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- Körner, C. Additive manufacturing of metallic components by selective electron beam melting—A review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef]
- Gong, X.; Anderson, T.; Chou, K. Review on powder-based electron beam additive manufacturing technology. Manuf. Rev. 2014, 1, 2. [Google Scholar] [CrossRef]
- Gaytan, S.M.; Murr, L.E.; Medina, F.; Martinez, E.; Lopez, M.I.; Wicker, R.B. Advanced metal powder based manufacturing of complex components by electron beam melting. Mater. Technol. Futur. Light Mater. 2009, 24, 180–190. [Google Scholar] [CrossRef]
- Cordero, Z.C.; Meyer, H.M.; Nandwana, P.; Dehoff, R.R. Powder bed charging during electron-beam additive manufacturing. Acta Mater. 2017, 124, 437–445. [Google Scholar] [CrossRef]
- Milberg, J.; Sigl, M. Electron beam sintering of metal powder. Prod. Eng. 2008, 2, 117–122. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies, 2nd ed.; Springer: London, UK, 2014. [Google Scholar] [CrossRef]
- Pauzon, C.; Hryha, E.; Forêt, P.; Nyborg, L. Effect of argon and nitrogen atmospheres on the properties of stainless steel 316L parts produced by laser-powder bed fusion. Mater. Des. 2019, 179. [Google Scholar] [CrossRef]
- Calleja, A.; Tabernero, I.; Ealo, J.A.; Campa, F.J.; Lamikiz, A.; de Lacalle, L.N.L. Feed rate calculation algorithm for the homogeneous material deposition of blisk blades by 5-axis laser cladding. Int. J. Adv. Manuf. Technol. 2014, 74, 1219–1228. [Google Scholar] [CrossRef]
- Schwerdtfeger, J.; Körner, C. Selective electron beam melting of Ti–48Al–2Nb–2Cr: Microstructure and aluminium loss. Intermetallics 2014, 49, 29–35. [Google Scholar] [CrossRef]
- Semiatin, S.; Ivanchenko, V.; Ivasishin, O. Diffusion models for evaporation losses during electron-beam melting of alpha/beta-titanium alloys. Metall. Mater. Trans. B 2004, 35, 235–245. [Google Scholar] [CrossRef]
- Sarychev, V.; Nevskii, S.; Konovalov, S.; Granovskii, A.; Ivanov, Y.; Gromov, V. Model of nanostructure formation in Al-Si alloy at electron beam treatment. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components–process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Sarangan, A. Physical and Chemical Vapor Deposition. In Nanofabrication—Principles to Laboratory Practice, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 315. [Google Scholar] [CrossRef]
- Juechter, V.; Scharowsky, T.; Singer, R.F.; Körner, C. Processing window and evaporation phenomena for Ti–6Al–4V produced by selective electron beam melting. Acta Mater. 2014, 76, 252–258. [Google Scholar] [CrossRef]
- Klassen, A.; Forster, V.E.; Juechter, V.; Körner, C. Numerical simulation of multi-component evaporation during selective electron beam melting of TiAl. J. Mater. Process. Tech. 2017, 247, 280–288. [Google Scholar] [CrossRef]
- Rouquette, S.; Guo, J.; le Masson, P. Estimation of the parameters of a Gaussian heat source by the Levenberg–Marquardt method: Application to the electron beam welding. Int. J. Therm. Sci. 2007, 46, 128–138. [Google Scholar] [CrossRef]
- Yan, W.; Ge, W.; Smith, J.; Lin, S.; Kafka, O.L.; Lin, F.; Liu, W.K. Multi-scale modeling of electron beam melting of functionally graded materials. Acta Mater. 2016, 115, 403–412. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okumura, Y.; Ichimura, M.; Nakamura, H.; Honda, K.; Shibahara, M.; Hirano, T.; Yamada, H.; Tanaka, R.; Sakai, I.; et al. Surface Modification and Adhesion Mechanism of Isotactic Polypropylene with Low-Energy Electron-Beam Treatments. Langmuir 2020, 36, 10846–10852. [Google Scholar] [CrossRef]
- Drouin, D.; Couture, A.R.; Joly, D.; Tastet, X.; Aimez, V.; Gauvin, R. CASINO V2.42—A Fast and Easy-to-use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users. Scanning 2007, 29, 92–101. [Google Scholar] [CrossRef]
- Galati, M.; Iuliano, L. A literature review of powder-based electron beam melting focusing on numerical simulations. Addit. Manuf. 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Yan, W.; Smith, J.; Ge, W.; Lin, F.; Liu, W. Multiscale modeling of electron beam and substrate interaction: A new heat source model. Comput. Mech. 2015, 56, 265–276. [Google Scholar] [CrossRef]
- Hirayama, H.; Namito, Y.; Nelson, W.R.; Bielajew, A.F.; Wilderman, S.J.; Michigan, U. The EGS5 Code System (No. SLAC-R-730); Department of Energy Stanford University: Stanford, CA, USA, 2005. [Google Scholar] [CrossRef]
- Berger, M.J.; Seltzer, S.M. Stopping Powers and Ranges of Electrons and Positions; NIST Information Services Office: Washington, DC, USA, 1982. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/IR/nbsir82-2550.pdf (accessed on 7 April 2021).
- Berger, M.J.; Inokuti, M.; Anderson, H.H.; Bichsel, H.; Dennis, J.A.; Powers, D.; Seltzer, S.M.; Turner, J.E. Report 37. J. Int. Comm. Radiat. Units Meas. 1984, os19. [Google Scholar] [CrossRef]
- Rogers, D.W.O. Fifty years of Monte Carlo simulations for medical physics. Phys. Med. Biol. 2006, 51, R287–R301. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.B. Monte Carlo Calculation of the Penetration and Diffusion of Fast Charged Particles. Methods Comput. Phys. 1963, 1, 135–215. [Google Scholar]
- Bielajew, A.F.; Wilderman, S.J. Innovative electron transport methods in EGS5. In Proceedings of the Second International Workshop on EGS (KEK-PROC--2000-20), Tsukuba, Japan, 8–12 August 2000; Ban, S., Ed.; High Energy Accelerator Research Organization: Tsukuba, Japan, 2000. RN:33008052. [Google Scholar]
- Siegbahn, K. Alpha-, Beta- and Gamma-Ray Spectroscopy; Elsevier B.V.: Amsterdam, The Netherlands, 1965; Volume 1. [Google Scholar] [CrossRef]
- Messel, H.; Crawford, D.F. Electron–Photon Shower Distribution Function, 1st ed.; Pergamon Press London: London, UK, 1970. [Google Scholar]
- Møller, C. Passage of hard beta rays through matter. Ann. Phys. 1932, 406, 531–585. [Google Scholar] [CrossRef]
- Jenkins, R.; de Vries, J.L. Practical X-Ray Spectrometry, 2nd ed.; Springer: New York, NY, USA, 1969. [Google Scholar] [CrossRef]
- Geller, M.; Orion, I.; Golan, E.; Castro, G.R.; Rubio-Zuazo, J.; Tiferet, E. Electron inelastic mean free path in carbon and polycarbonate using a newly developed wide spectrum measurement method. J. Electron Spectros. Relat. Phenomena 2018, 229, 85–93. [Google Scholar] [CrossRef]
- Givon, A.; Tiferet, E.; Orion, I. Algorithm for evaluating layer thickness based on electron average energy shift analysis. Nucl. Inst. Methods Phys. Res. B 2012, 288, 23–27. [Google Scholar] [CrossRef]
- Alcock, C.B.; Itkin, V.P.; Horrigan, M.K. Vapour Pressure Equations for the Metallic Elements: 298–2500K. Can. Metall. Q. 1984, 23, 309–313. [Google Scholar] [CrossRef]
- Kim, C.S. Thermophysical Properties of Stainless Steels No. ANL-75-55; Argonne National Lab: Lemont, IL, USA, 1975. [CrossRef]
- Falk-Windisch, H.; Svensson, J.E.; Froitzheim, J. The effect of temperature on chromium vaporization and oxide scale growth on interconnect steels for Solid Oxide Fuel Cells. J. Power Sources 2015, 287, 25–35. [Google Scholar] [CrossRef]
- Jurisch, M.; Wenz, T.; Kirchner, A.; Kloeden, B.; Weissgaerber, T.; Kieback, B. Processing of FeCrV-steels by Selective Electron Beam Melting (SEBM). In Proceedings of the EURO PM 2019 Congress & Exhibition, Maastricht, The Netherlands, 13–16 October 2019. [Google Scholar]
- Zhong, Y.; Rännar, L.E.; Liu, L.; Koptyug, A.; Wikman, S.; Olsen, J.; Cui, D.; Shen, Z. Additive manufacturing of 316L stainless steel by electron beam melting for nuclear fusion applications. J. Nucl. Mater. 2017, 486, 234–245. [Google Scholar] [CrossRef]


| Energy Deposition [%] | ||||||
|---|---|---|---|---|---|---|
| Region | Transport Change | Discarded Below Cutoff | Discarded Below AE or AP | Discarded Geometry end | XRF | Summary |
| SS304L plate | 0.724 | 0 | 8.79 × 10−2 | 0 | 4.4 × 10−3 | 0.817 |
| Out | 0 | 0 | 0 | 0.181 | 0 | 0.181 |
| Sum | 0.724 | 0 | 8.79 × 10−2 | 0.181 | 4.4 × 10−3 | 0.998 |
| Beam Current [mA] | Beam Velocity [mm/s] | Focus Offset [mA] | Line Offset [mm] | Line Order | Hatch Direction | Duration [min] |
|---|---|---|---|---|---|---|
| 44 | 35,000 | 90 | 1.2 | 20 | unidirectional | 105 |
| Region | Plate | Heat Shield |
|---|---|---|
| Temperature Difference—∆T [°C] | 15 | 3 |
| Temperature Diversion [%] | 2.1 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damri, E.; Tiferet, E.; Braun, D.; Ganor, Y.I.; Chonin, M.; Orion, I. Effects of Gas Pressure during Electron Beam Energy Deposition in the EBM Additive Manufacturing Process. Metals 2021, 11, 601. https://doi.org/10.3390/met11040601
Damri E, Tiferet E, Braun D, Ganor YI, Chonin M, Orion I. Effects of Gas Pressure during Electron Beam Energy Deposition in the EBM Additive Manufacturing Process. Metals. 2021; 11(4):601. https://doi.org/10.3390/met11040601
Chicago/Turabian StyleDamri, Elroei, Eitan Tiferet, Dor Braun, Yaron Itay Ganor, Michael Chonin, and Itzhak Orion. 2021. "Effects of Gas Pressure during Electron Beam Energy Deposition in the EBM Additive Manufacturing Process" Metals 11, no. 4: 601. https://doi.org/10.3390/met11040601
APA StyleDamri, E., Tiferet, E., Braun, D., Ganor, Y. I., Chonin, M., & Orion, I. (2021). Effects of Gas Pressure during Electron Beam Energy Deposition in the EBM Additive Manufacturing Process. Metals, 11(4), 601. https://doi.org/10.3390/met11040601







