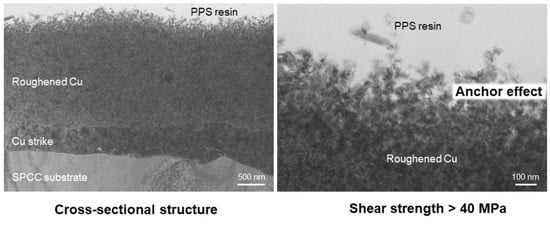
Fabrication of Roughened Electrodeposited Copper Coating on Steel for Dissimilar Joining of Steel and Thermoplastic Resin
Abstract
1. Introduction
2. Materials and Methods
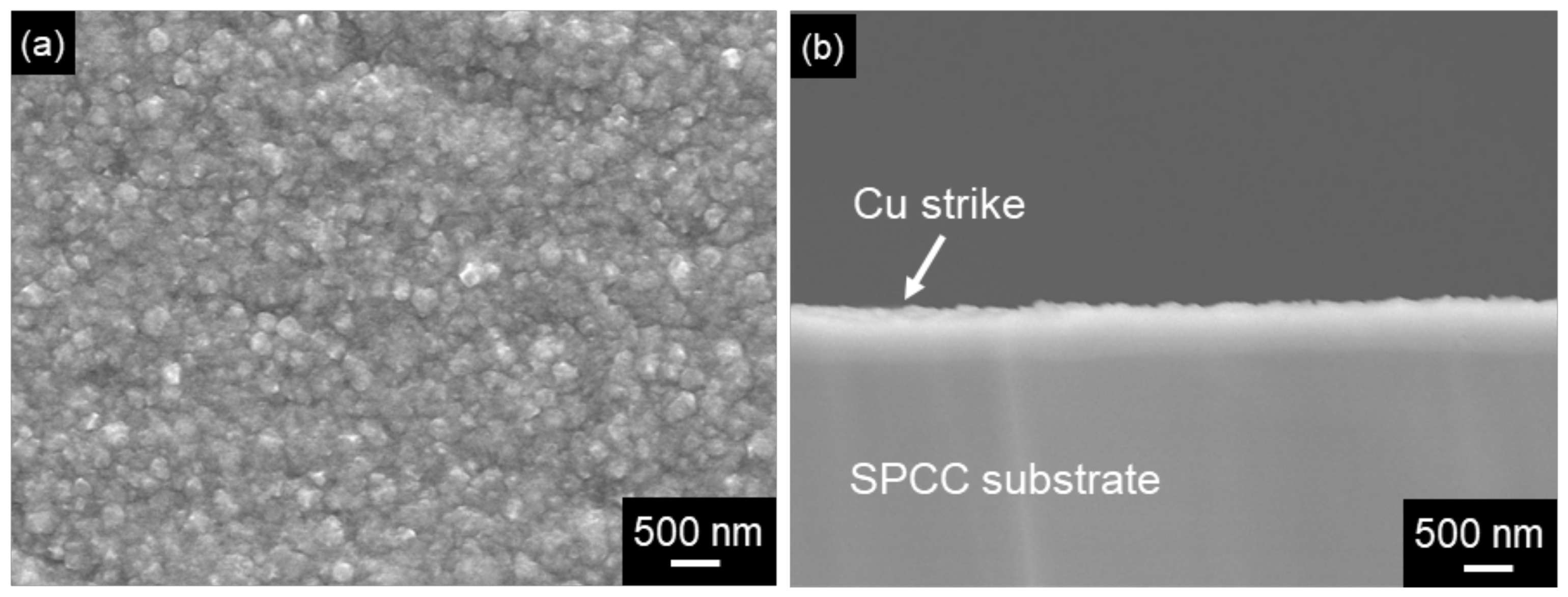
2.1. Fabrication of Roughened Electrodeposited Copper Films on Steel Substrates
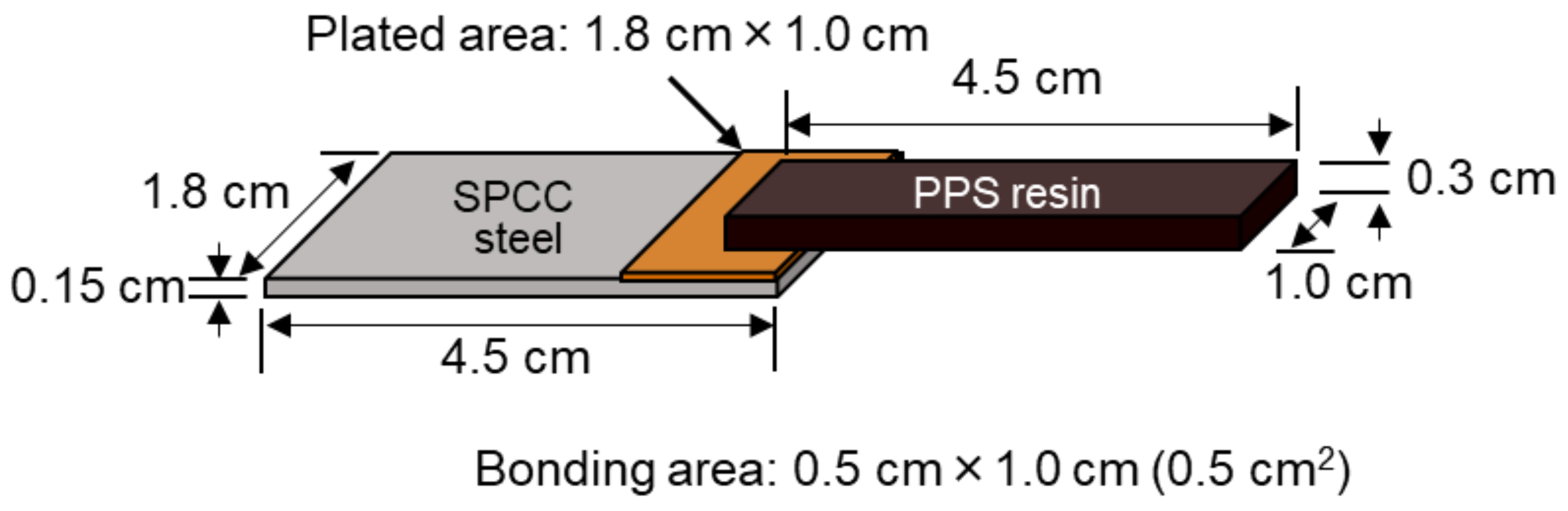
2.2. Preparation of Bonded Samples
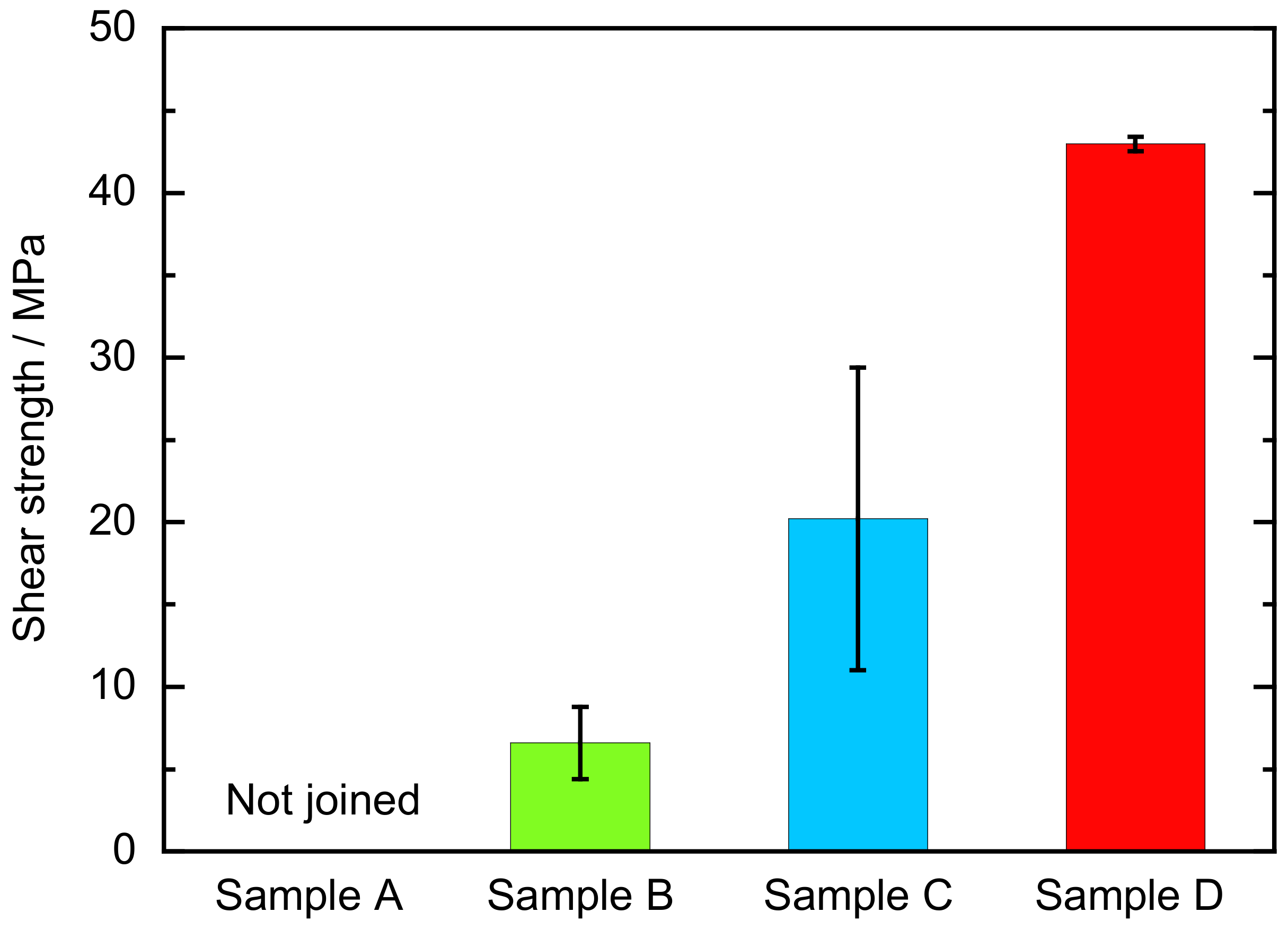
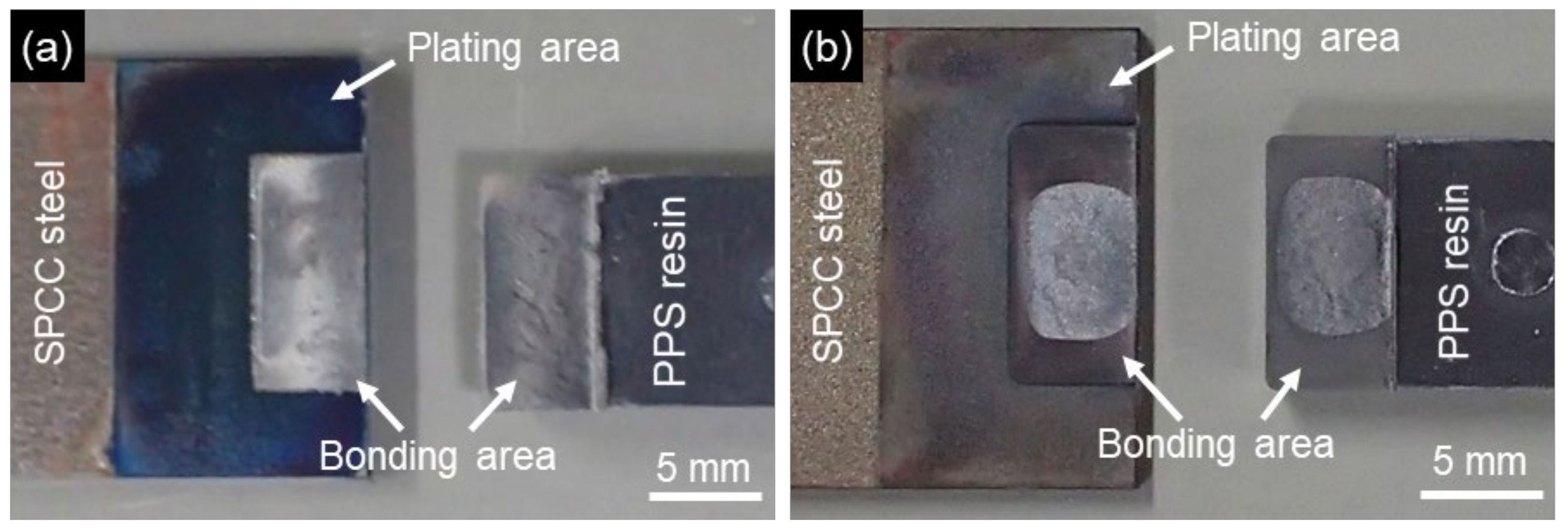
2.3. Evaluation of Bonding Strength
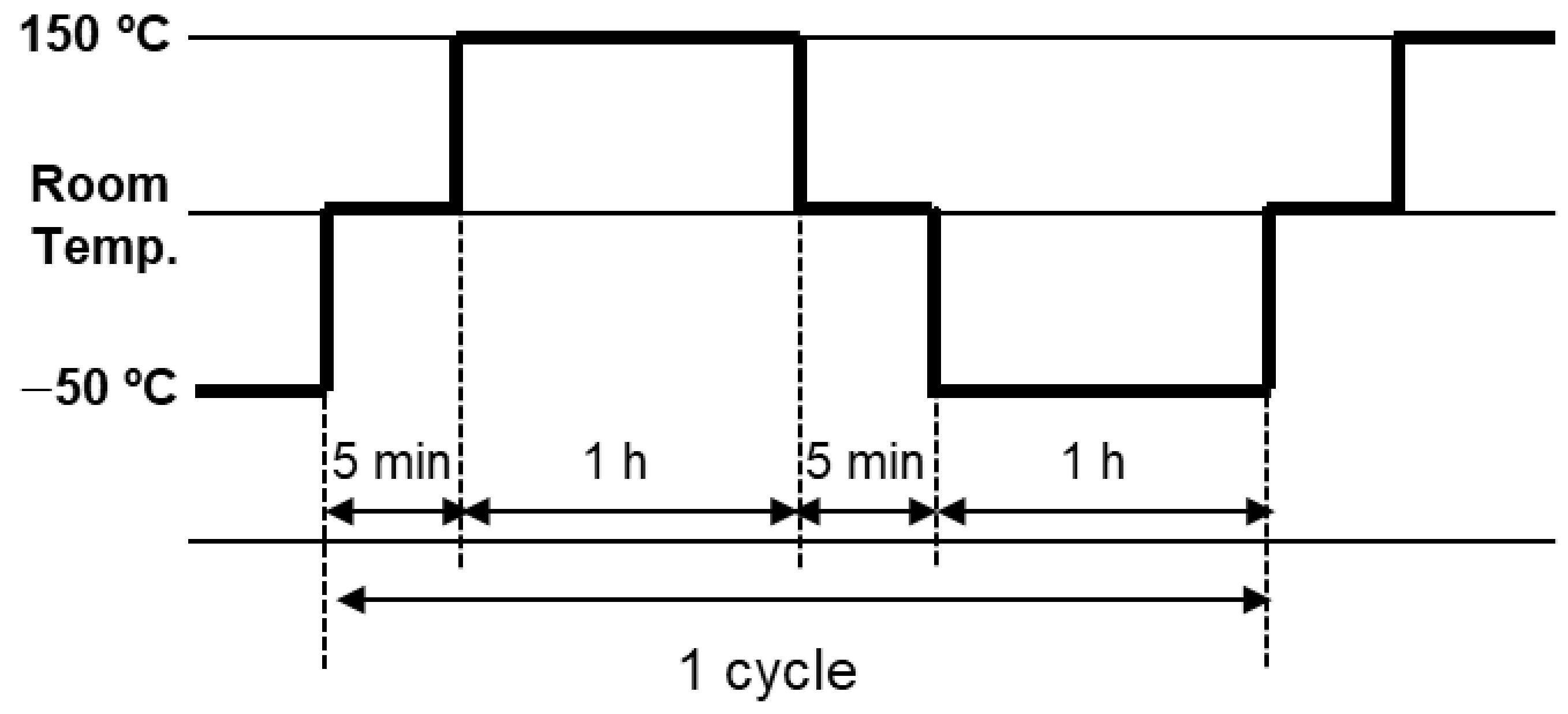
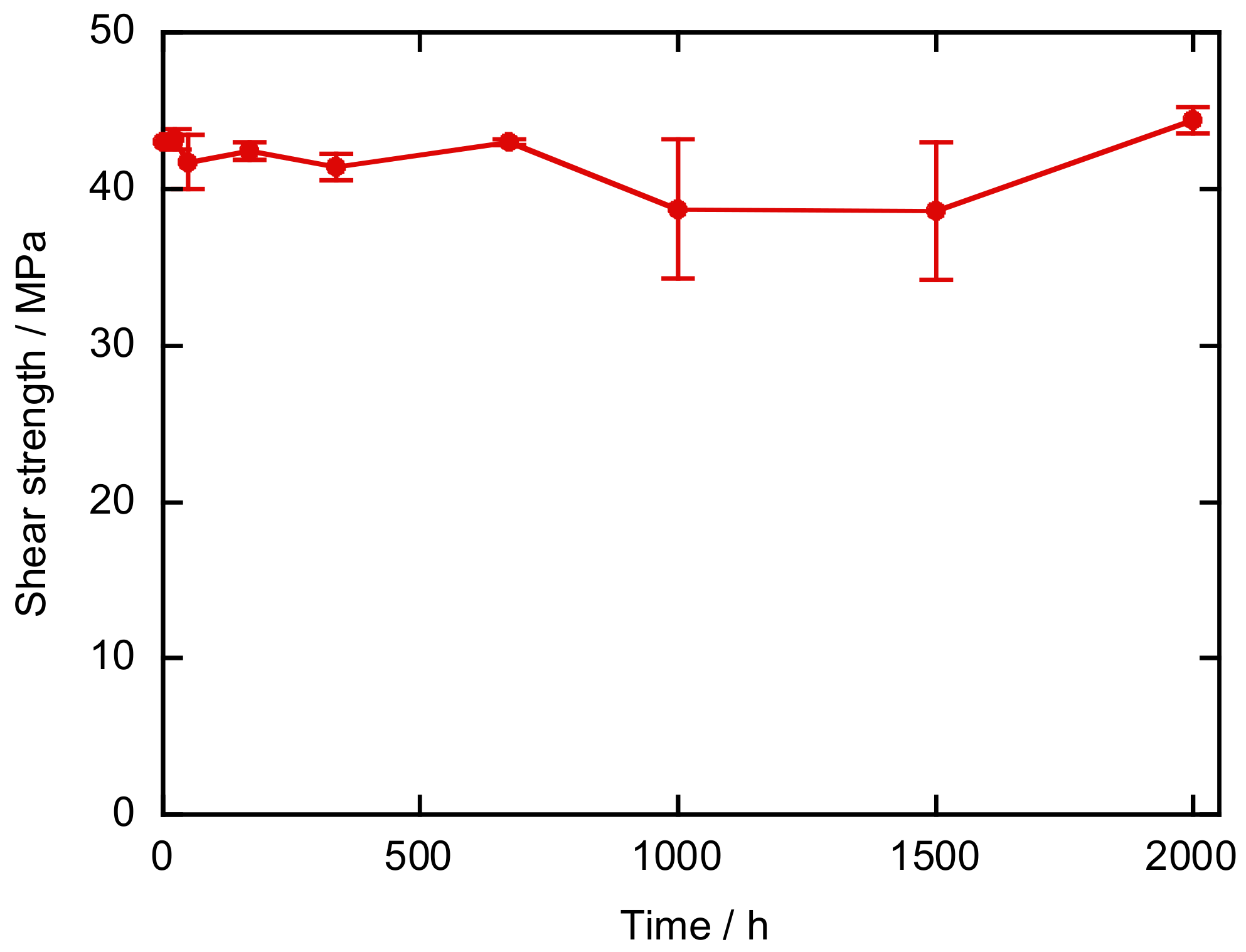
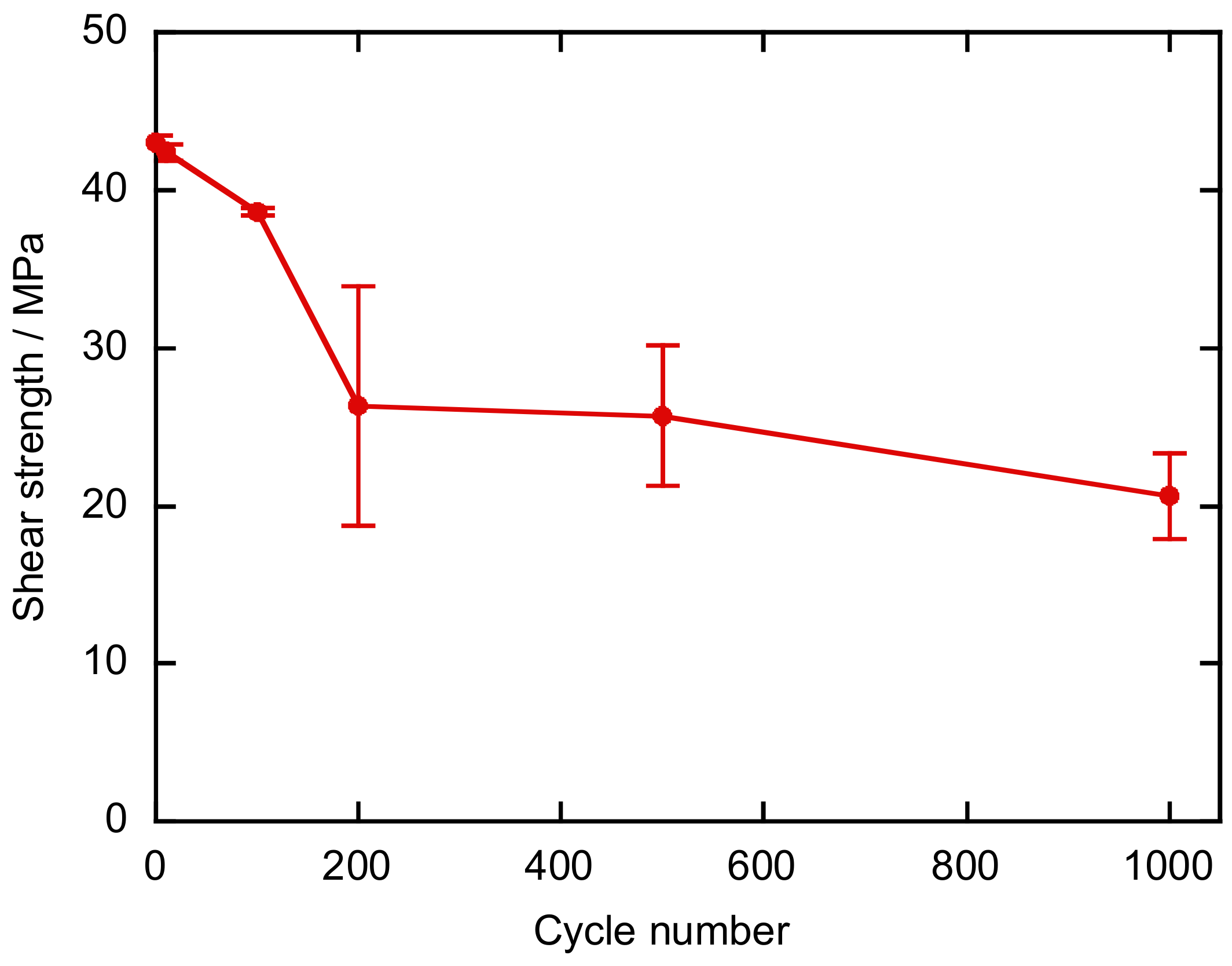
2.4. Durability Tests
2.4.1. High-Temperature and High-Humidity Test
2.4.2. Thermal Shock Test
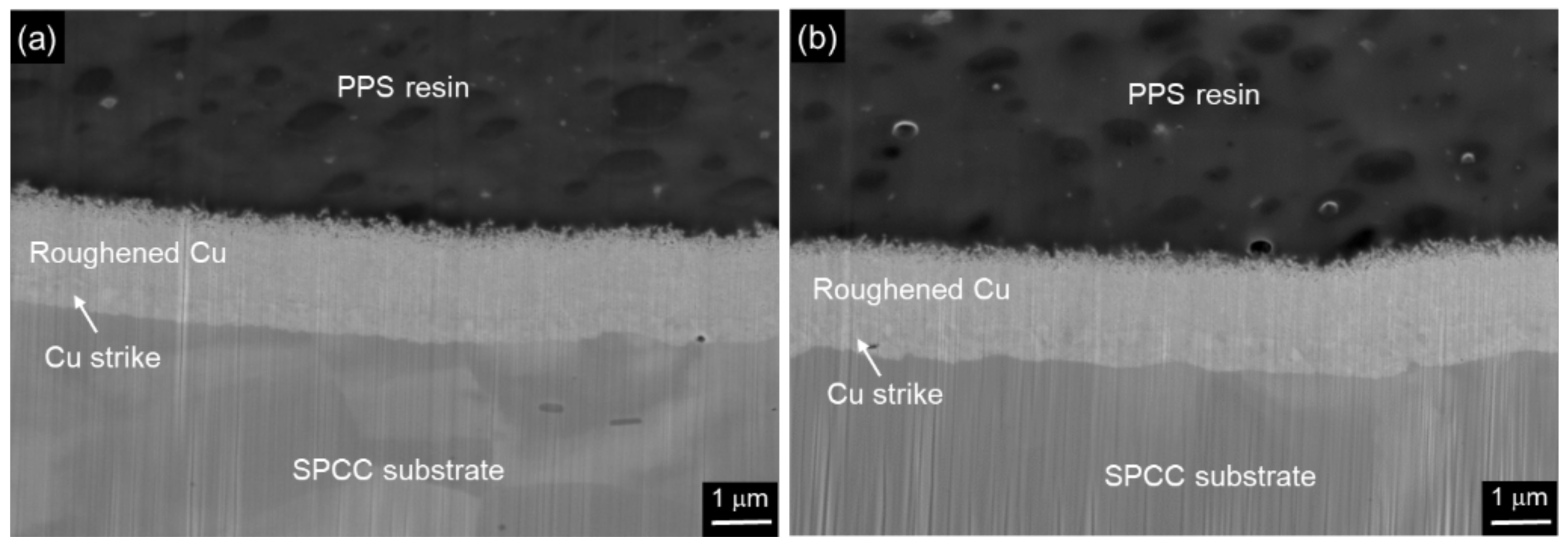
2.5. Structural Analysis
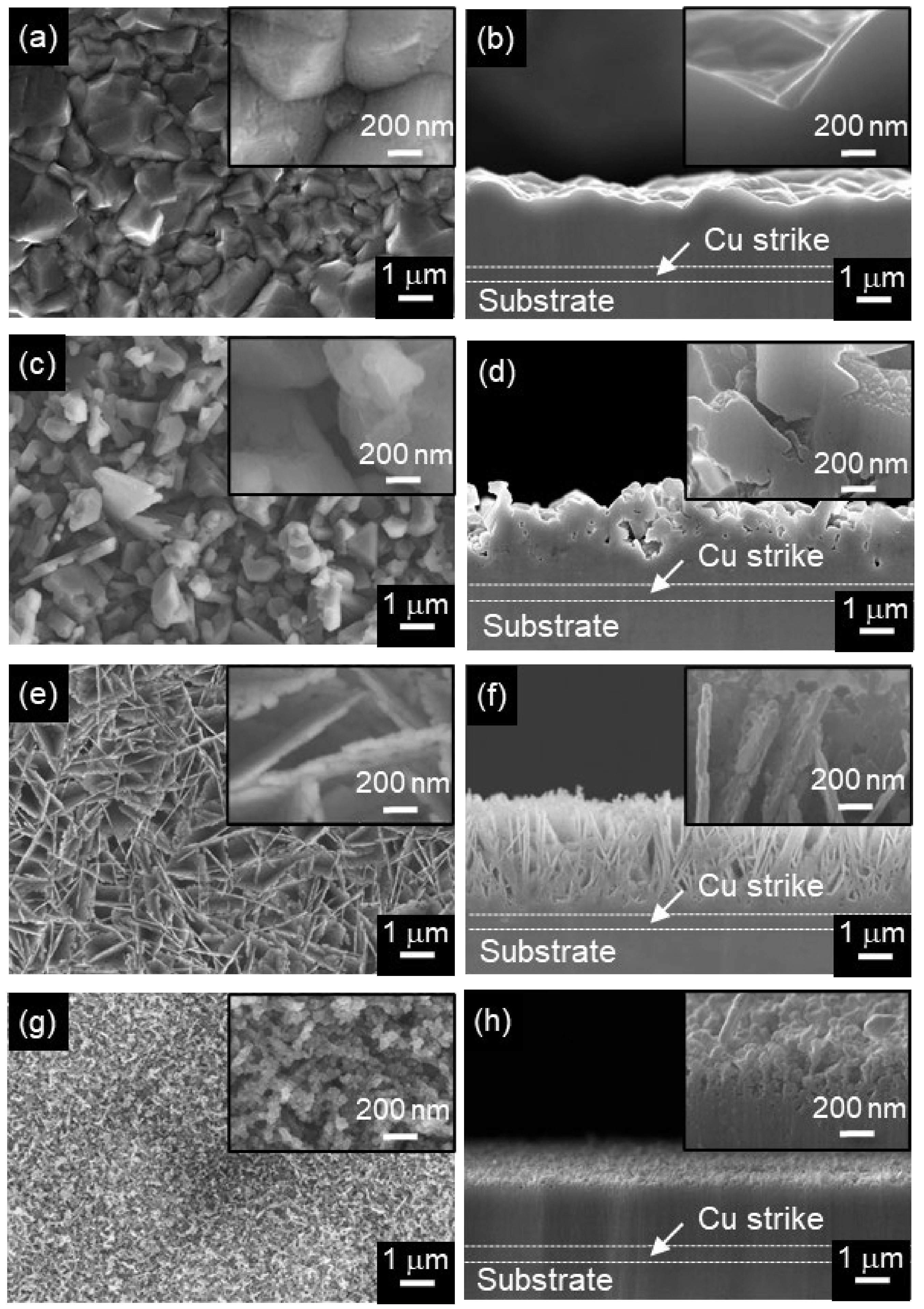
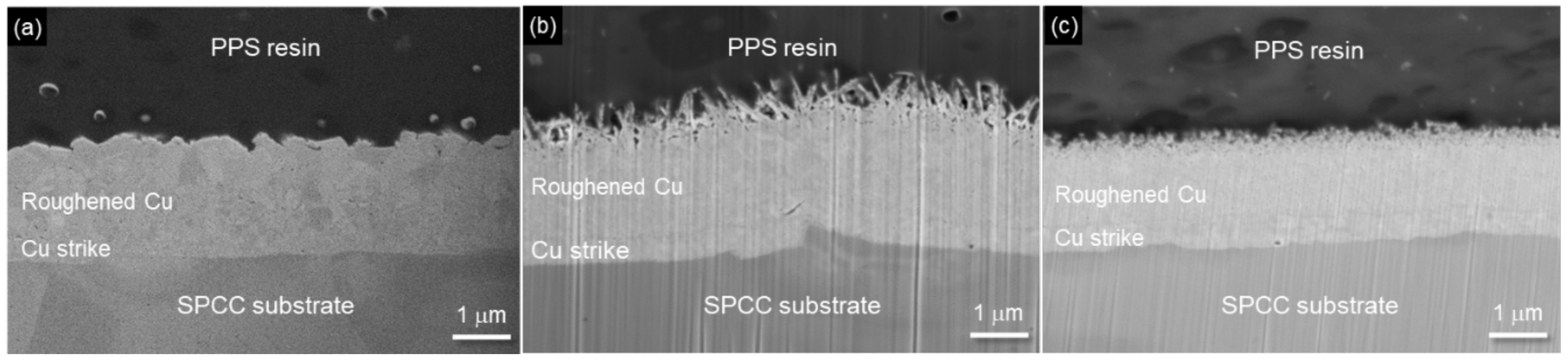
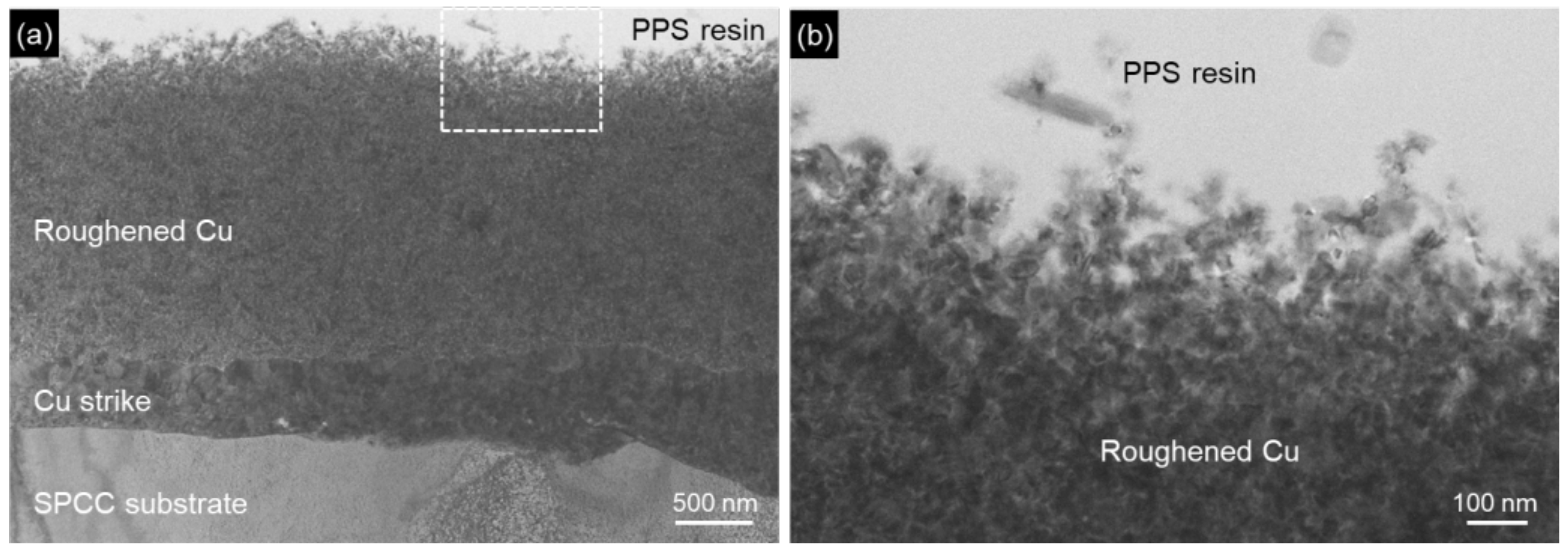
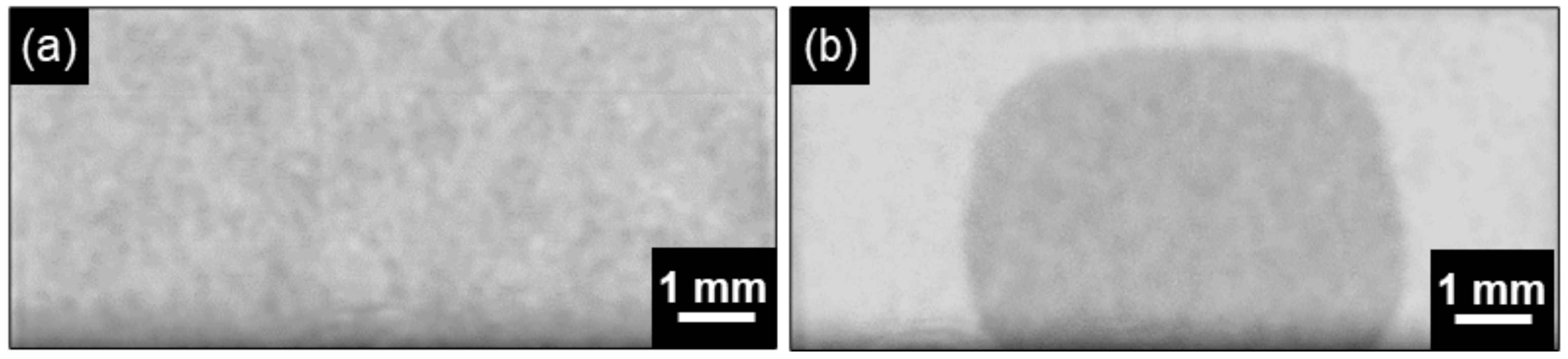
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goede, M.; Stehlin, M.; Rafflenbeul, L.; Kopp, G.; Beeh, E. Super light car—Lightweight construction thanks to a multi-material design and function integration. Eur. Trans. Res. Rev. 2009, 1, 5–10. [Google Scholar] [CrossRef]
- Amancio-Filho, S.T.; do Santos, J.F. Joining of polymers and polymer-metal hybrid structures: Recent developments and trend. Polym. Eng. Sci. 2009, 49, 1461–1476. [Google Scholar] [CrossRef]
- Wang, H.; Kawamoto, Y.; Nishimoto, K. A laser system for titanium and polyethylene terephthalate plastic controlled by multiple signal sources. IEEE T. Ind. Electron. 2019, 66, 1255–1263. [Google Scholar] [CrossRef]
- Liu, F.C.; Liao, J.; Nakata, K. Joining of metal to plastic using friction lap welding. Mater. Des. 2014, 54, 236–244. [Google Scholar] [CrossRef]
- Seto, M.; Asami, Y.; Itakura, M.; Tanaka, H.; Yamabe, M. Influence of molding conditions on joining strength of injection molded parts joined with metal and resin, Yamabe. J. Jpn. Soc. Polym. Process 2015, 27, 68–74. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Taki, K.; Nakamura, S.; Takayama, T.; Nemoto, A.; Ito, H. Direct joining of a laser-ablated metal surface and polymers by precise injection molding. Microsyst. Technol. 2016, 22, 31–38. [Google Scholar] [CrossRef]
- Fabrin, P.A.; Hoikkanen, M.E.; Vuorinen, J.E. Adhesion of thermoplastic elastomer on surface treated aluminum by injection molding. Polym. Eng. Sci. 2007, 47, 1187–1191. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Boll, J.V.; Holm, A.H.; Hojsholt, R.; Balling, P. Ultra-high-strength micro-mechanical interlocking by injection molding into laser-structured surfaces. Int. J. Adhes. Adhes. 2010, 30, 485–488. [Google Scholar] [CrossRef]
- Roesner, A.; Scheik, S.; Olowinsky, A.; Gillner, A.; Reisgen, U.; Schleser, M. Laser assisted joining of plastic metal hybrids. Phys. Procedia 2011, 12, 370–377. [Google Scholar] [CrossRef]
- Amend, P.; Pfindel, S.; Schmidt, M. Thermal joining of thermoplastic metal hybrids by means of mono- and polychromatic radiation. Phys. Procedia 2013, 41, 98–105. [Google Scholar] [CrossRef]
- Kurakake, Y.; Farazila, Y.; Miyashita, Y.; Otsuka, Y.; Mutoh, Y. Effect of molten pool shape on tensile shear strength of dissimilar materials laser spot joint between plastic and metal. JLMN-J. Laser Micro/Nanoeng. 2013, 8, 161–164. [Google Scholar] [CrossRef]
- Kim, W.S.; Yun, I.H.; Lee, J.J.; Jung, H.T. Evaluation of mechanical interlock effect on adhesion strength of polymer-metal interfaces using micro-patterned surface topography. Int. J. Adhes. Adhes. 2010, 30, 408–417. [Google Scholar] [CrossRef]
- Kimura, F.; Kadoya, S.; Kajihara, Y. Effects of molding conditions on injection molded direct joining using a metal with nano-structured surface. Precis. Eng. 2016, 45, 203–208. [Google Scholar] [CrossRef]
- Ramani, K.; Moriarty, B. Thermoplastic bonding to metals via injection molding for macro-composite manufacture. Polym. Eng. Sci. 1998, 35, 870–877. [Google Scholar] [CrossRef]
- Harris, A.F.; Beevers, A. The effects of grid-blasting on surface properties for adhesion. Int. J. Adhes. Adhes. 1999, 19, 445–452. [Google Scholar] [CrossRef]
- Lucchetta, G.; Marinello, F.; Bariani, P.F. Aluminum sheet surface roughness correlation with adhesion in polymer metal hybrid overmolding. CIRP Ann. Manuf. Technol. 2011, 60, 559–562. [Google Scholar] [CrossRef]
- Arai, S.; Sugawara, R.; Shimizu, M.; Inoue, J.; Horita, M.; Nagaoka, T.; Itabashi, M. Excellent bonding strength between steel and thermoplastic resin using roughed electrodeposited Ni/CNT composite layer without adhesives. Mater. Lett. 2020, 263, 127241. [Google Scholar] [CrossRef]
- Arai, S.; Sugawara, R.; Shimizu, M.; Inoue, J.; Horita, M.; Nagaoka, T.; Itabashi, M. Superior durability of dissimilar material joint between steel and thermoplastic resin with roughened electrodeposited nickel interlayer. Adv. Eng. Mater. 2020, 22, 2000739. [Google Scholar] [CrossRef]
- Arai, S.; Kitamura, T. Simple method for fabrication of three-dimensional (3D) copper nanostructured architecture by electrodeposition. ECS Electrochem. Lett. 2014, 3, D7–D9. [Google Scholar] [CrossRef][Green Version]
- Arai, S.; Mendsaikhan, M.; Nishimura, K. Fabrication of a uniformly tin-coated three-dimensional copper nanostructured architecture by electrodeposition. J. Electrochem. Soc. 2016, 163, D54–D56. [Google Scholar] [CrossRef]
- Shimizu, M.; Mendsaikhan, M.; Arai, S. Li-insertion/extraction properties of three-dimensional Sn electrode prepared by facile electrodeposition method. J. Appl. Electrochem. 2017, 47, 727–734. [Google Scholar] [CrossRef]
- Shimizu, M.; Yatsuzuka, R.; Horita, M.; Yamamoto, T.; Arai, S. Design of Roughened current collector by bottom-up approach using the electroplating technique: Charge-discharge performance of a Sn negative-electrode for Na-ion batteries. J. Phys. Chem. C 2017, 121, 27285–27294. [Google Scholar] [CrossRef]
- ISO 19095-3, Plastics-Evaluation of the Adhesion Interface Performance in Plastic-Metal Assemblies—Part 3: Test Methods; ISO: Geneva, Switzerland, 2015.
- Iwasaki, R.; Sato, C.; Yamabe, H. Characterization of the water diffusion on the interphase of the epoxy resin adhesive/metal and the visualization of the water accumulation on the interphase using fluorescence. J. Adhesion Soc. Jpn. 2007, 43, 81–88. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Munz, D.; Yang, Y.Y. Stress near the edge of bonded dissimilar materials described by two stress intensity factors. Int. J. Fract. 1993, 60, 169–177. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Munz, D. Stress singularities in a dissimilar materials joint with edge tractions under mechanical and thermal loadings. Int. J. Solid Struct. 1997, 34, 1199–1216. [Google Scholar] [CrossRef]
- Wada, J. Kikai Zairyo, 2nd ed.; Ohmsha Ltd.: Tokyo, Japan, 1991. [Google Scholar]
- List of Physical Properties of PPS. Available online: https://www.tosoh.co.jp/product/petrochemicals/polymer/assets/pps_49.pdf (accessed on 1 April 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, S.; Iwashita, R.; Shimizu, M.; Inoue, J.; Horita, M.; Nagaoka, T.; Itabashi, M. Fabrication of Roughened Electrodeposited Copper Coating on Steel for Dissimilar Joining of Steel and Thermoplastic Resin. Metals 2021, 11, 591. https://doi.org/10.3390/met11040591
Arai S, Iwashita R, Shimizu M, Inoue J, Horita M, Nagaoka T, Itabashi M. Fabrication of Roughened Electrodeposited Copper Coating on Steel for Dissimilar Joining of Steel and Thermoplastic Resin. Metals. 2021; 11(4):591. https://doi.org/10.3390/met11040591
Chicago/Turabian StyleArai, Susumu, Ryosuke Iwashita, Masahiro Shimizu, Junki Inoue, Masaomi Horita, Takashi Nagaoka, and Masami Itabashi. 2021. "Fabrication of Roughened Electrodeposited Copper Coating on Steel for Dissimilar Joining of Steel and Thermoplastic Resin" Metals 11, no. 4: 591. https://doi.org/10.3390/met11040591
APA StyleArai, S., Iwashita, R., Shimizu, M., Inoue, J., Horita, M., Nagaoka, T., & Itabashi, M. (2021). Fabrication of Roughened Electrodeposited Copper Coating on Steel for Dissimilar Joining of Steel and Thermoplastic Resin. Metals, 11(4), 591. https://doi.org/10.3390/met11040591