Effects of Bismuth on the Microstructure, Properties, and Interfacial Reaction Layers of Sn-9Zn-xBi Solders
Abstract
:1. Introduction
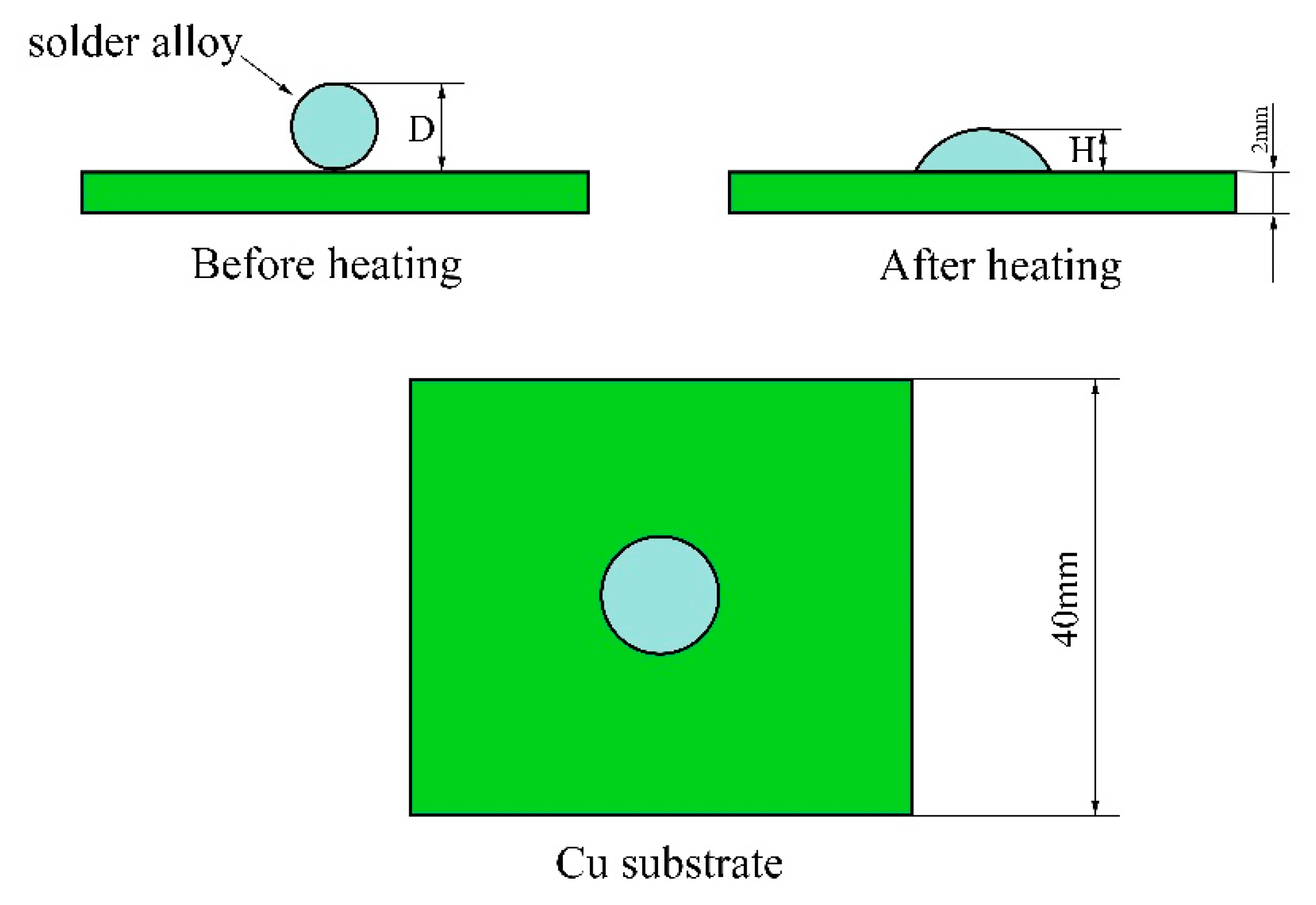
2. Materials and Methods
3. Results and Discussion
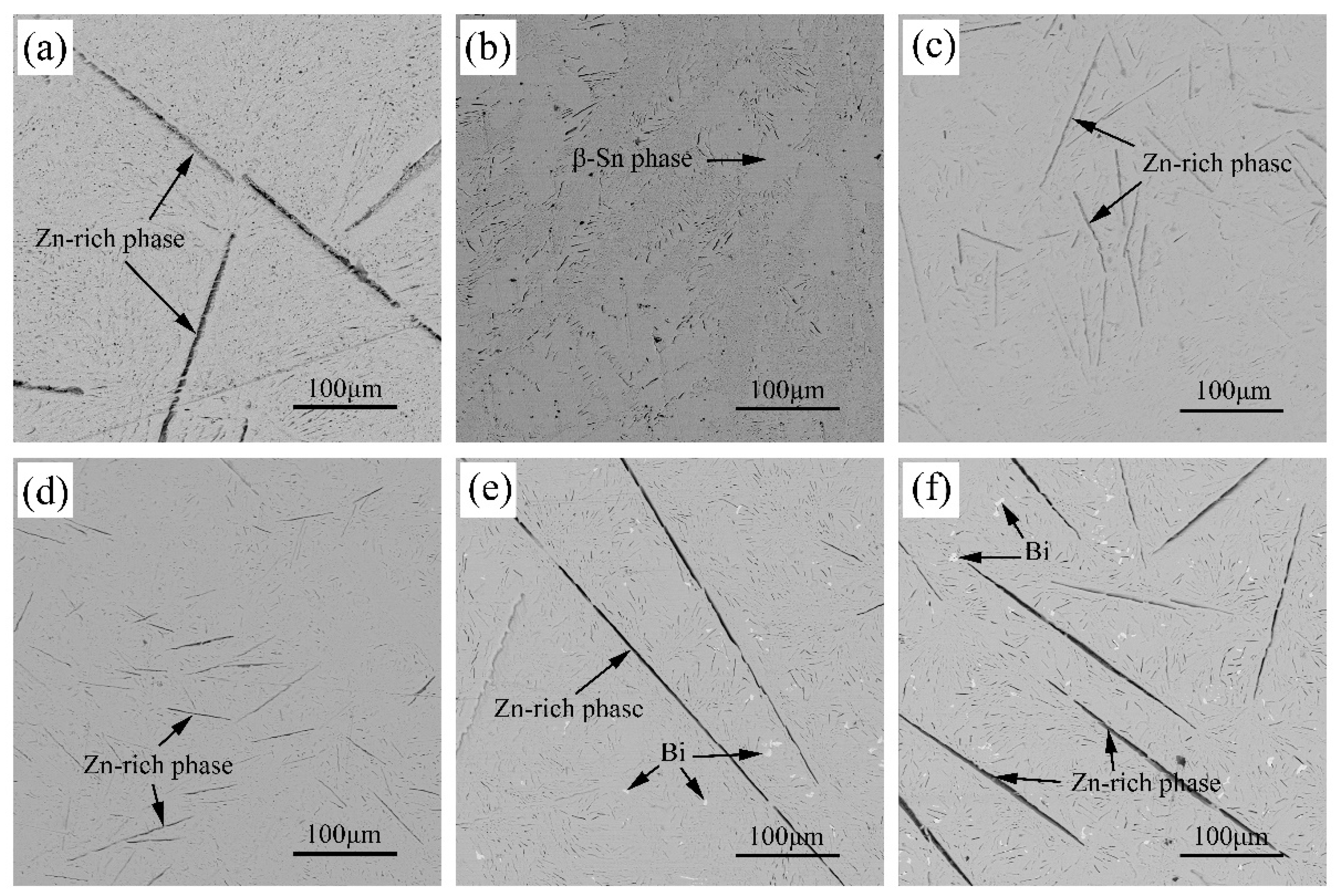
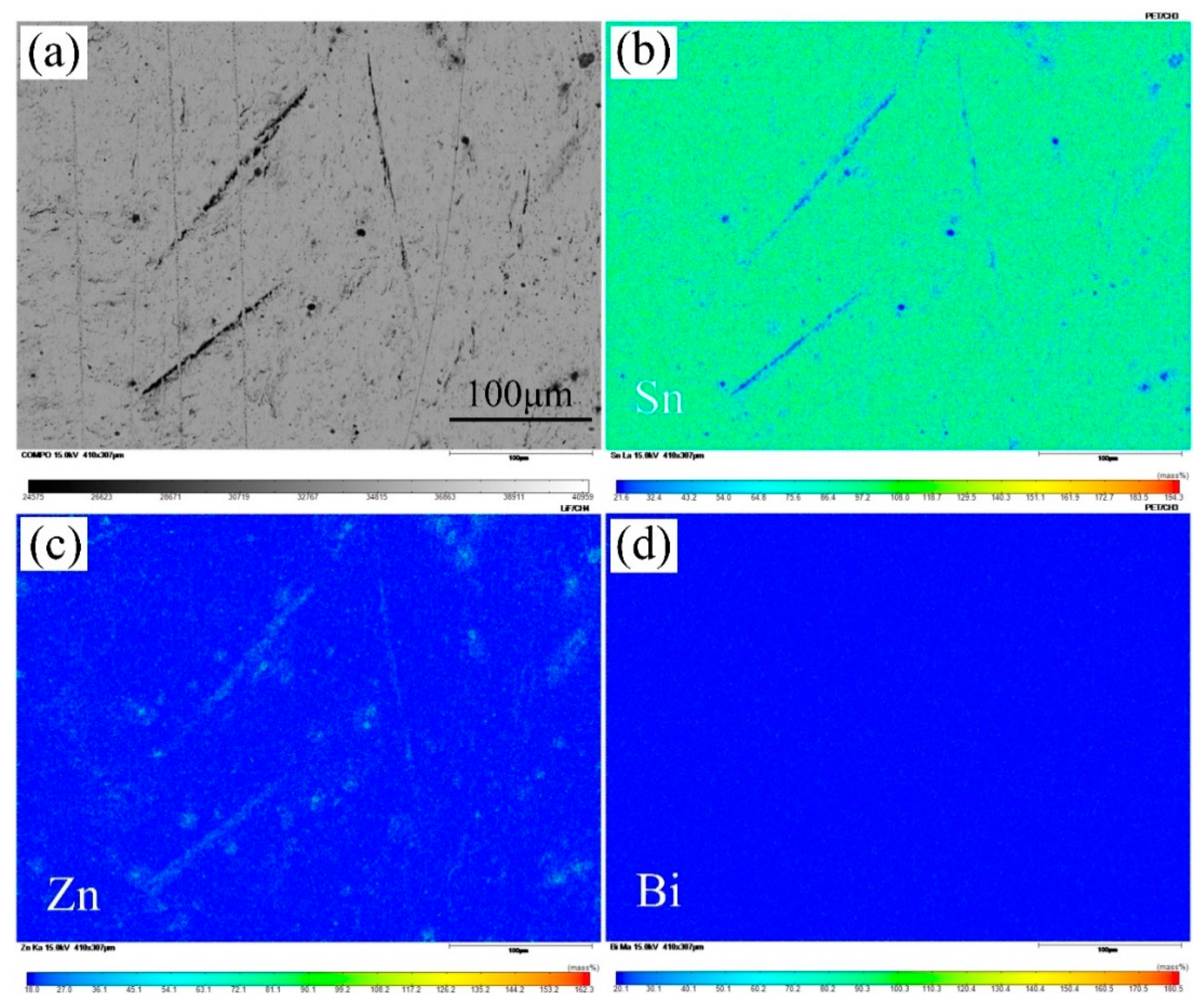
3.1. Microstructure
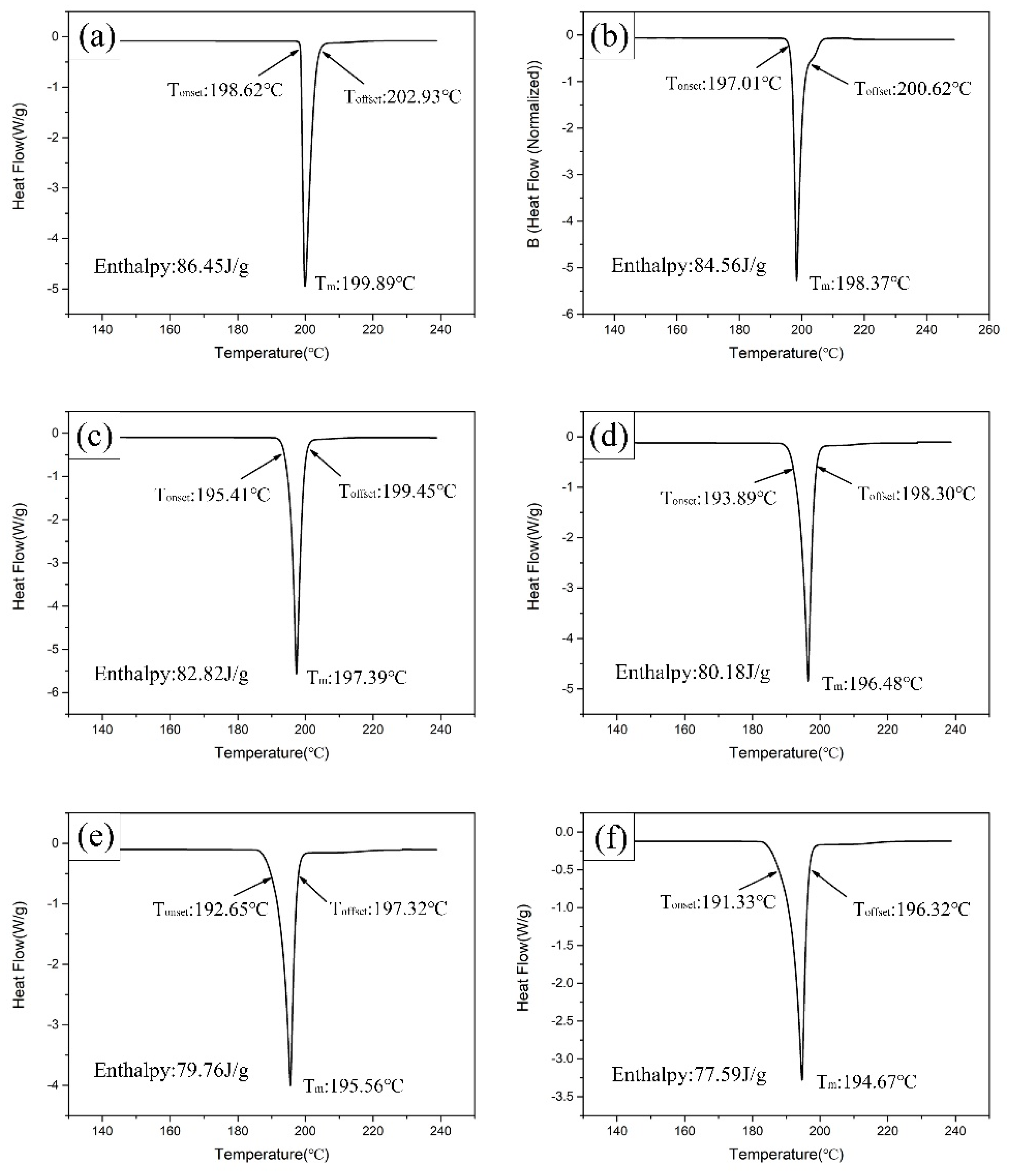
3.2. Melting Properties
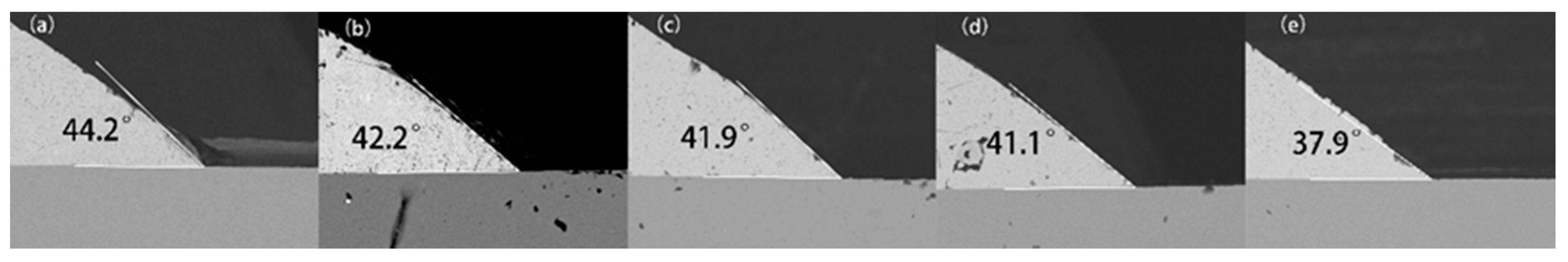
3.3. Wettability
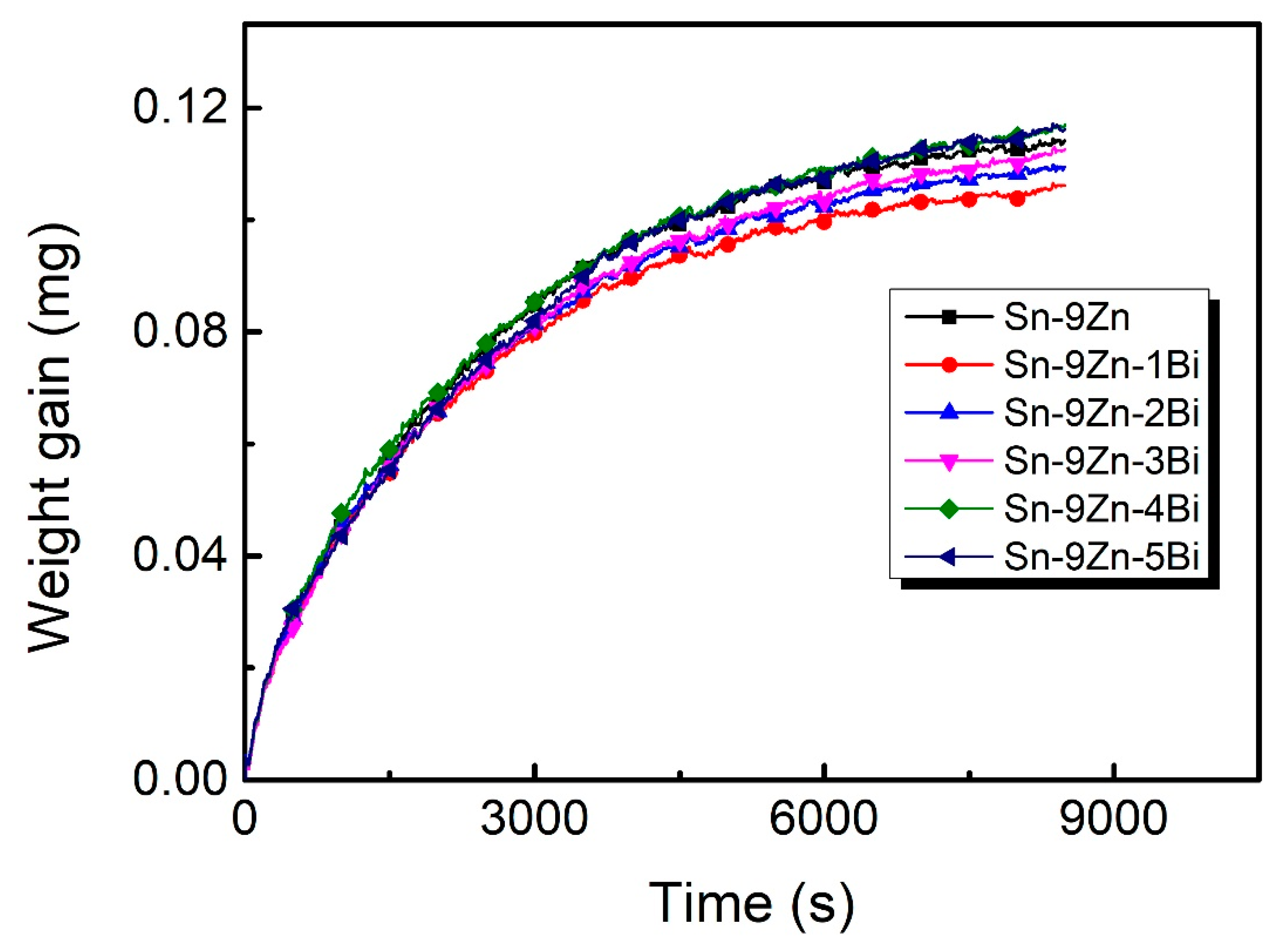
3.4. Oxidation Resistance
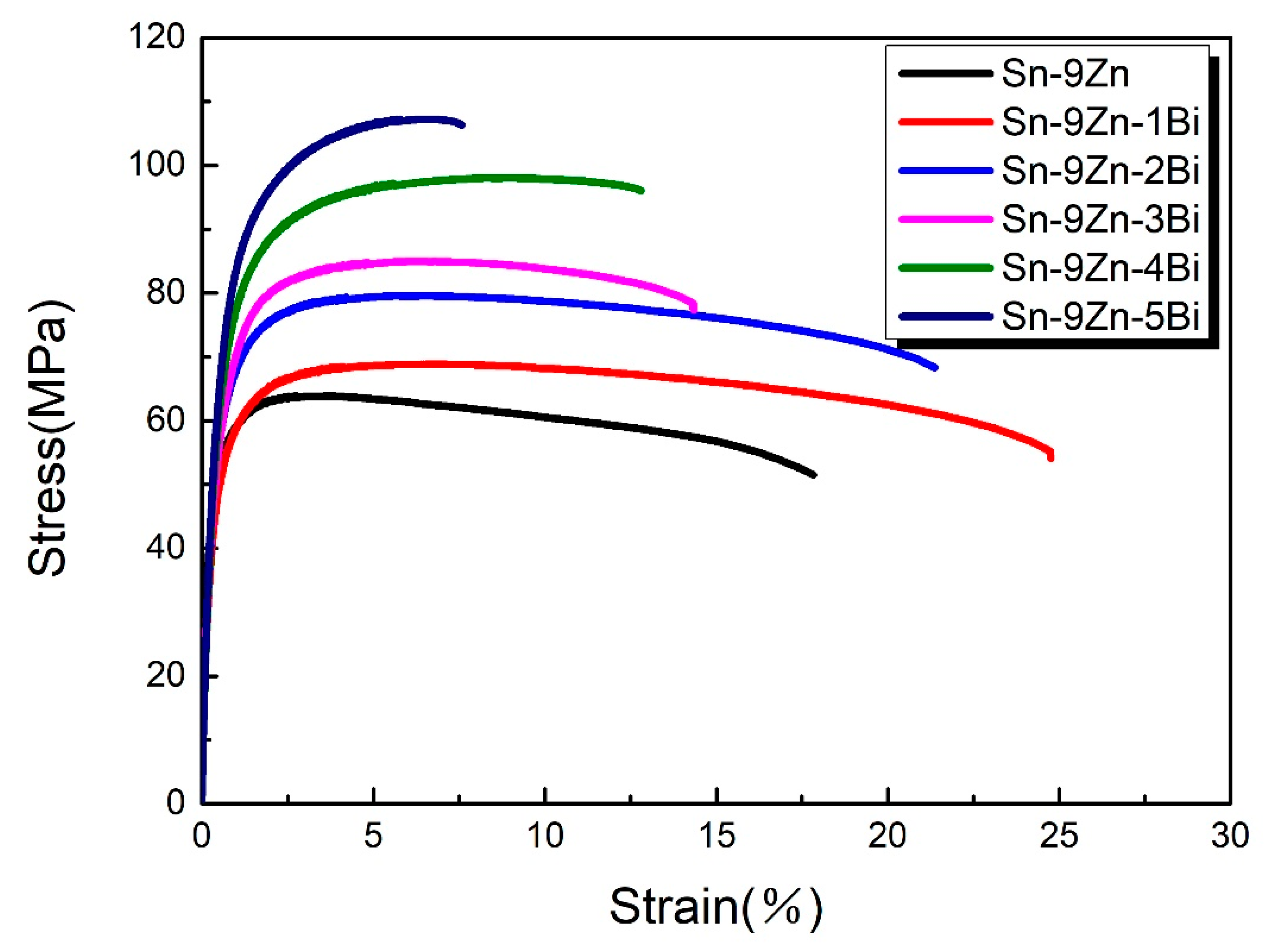
3.5. Mechanical Properties
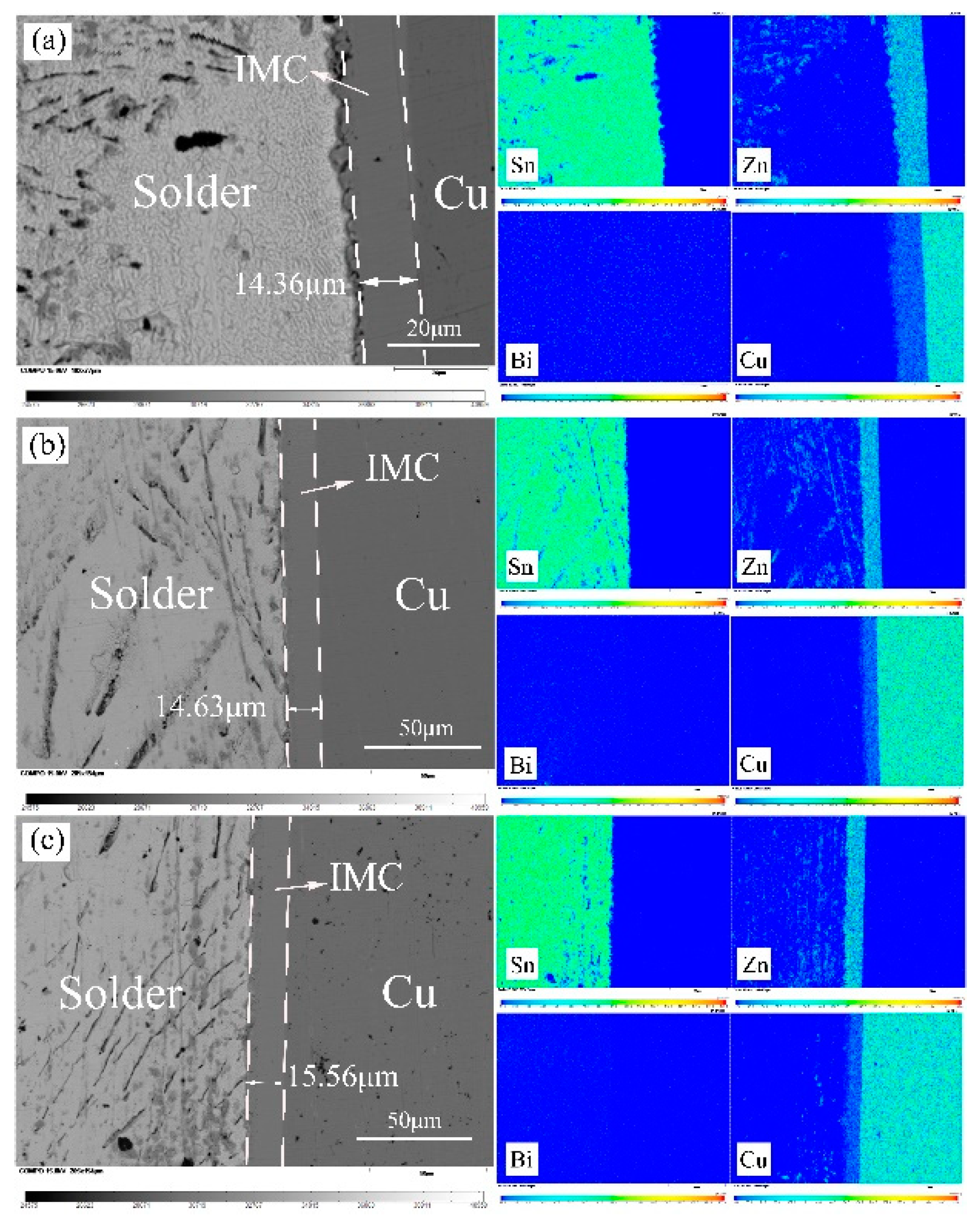
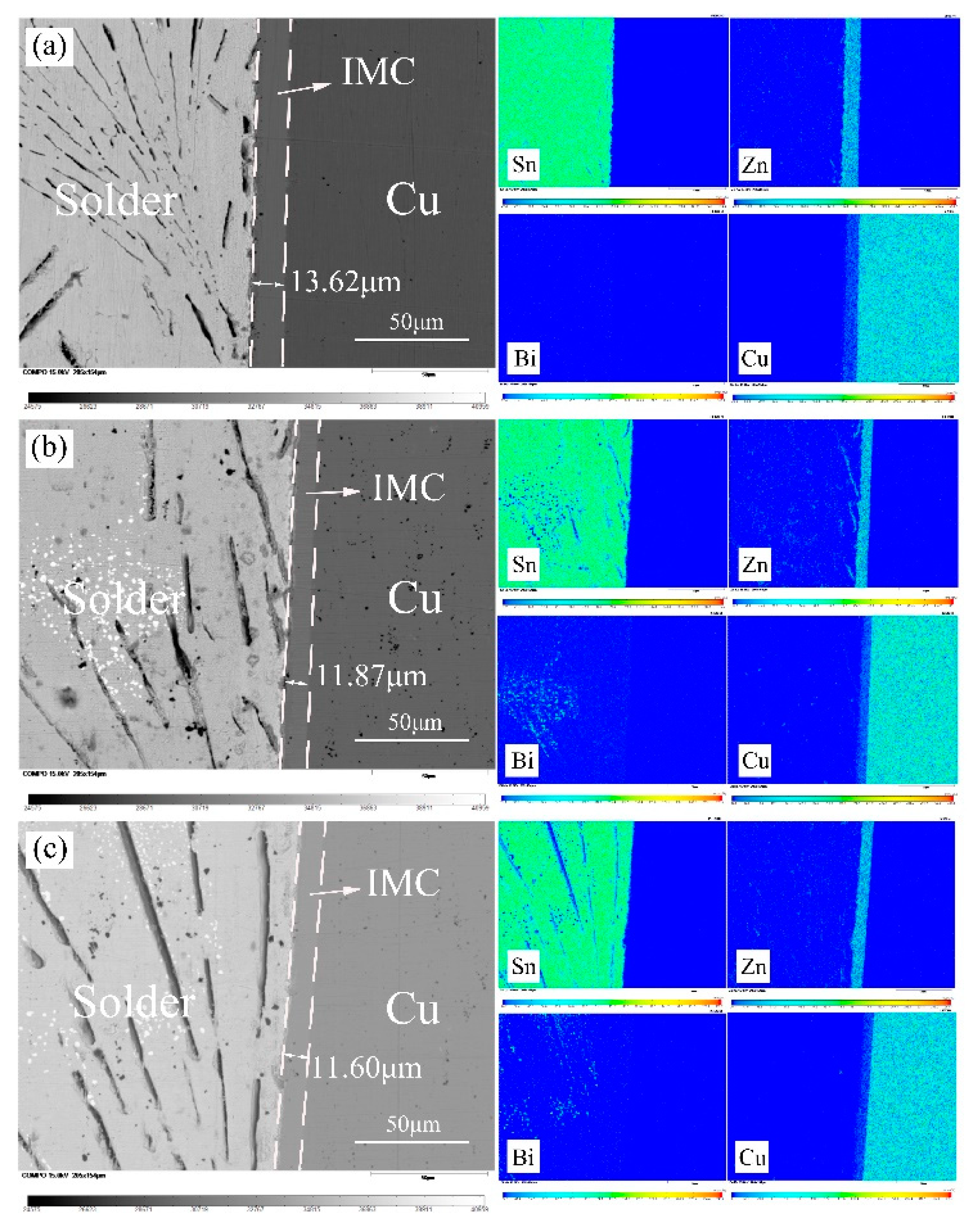
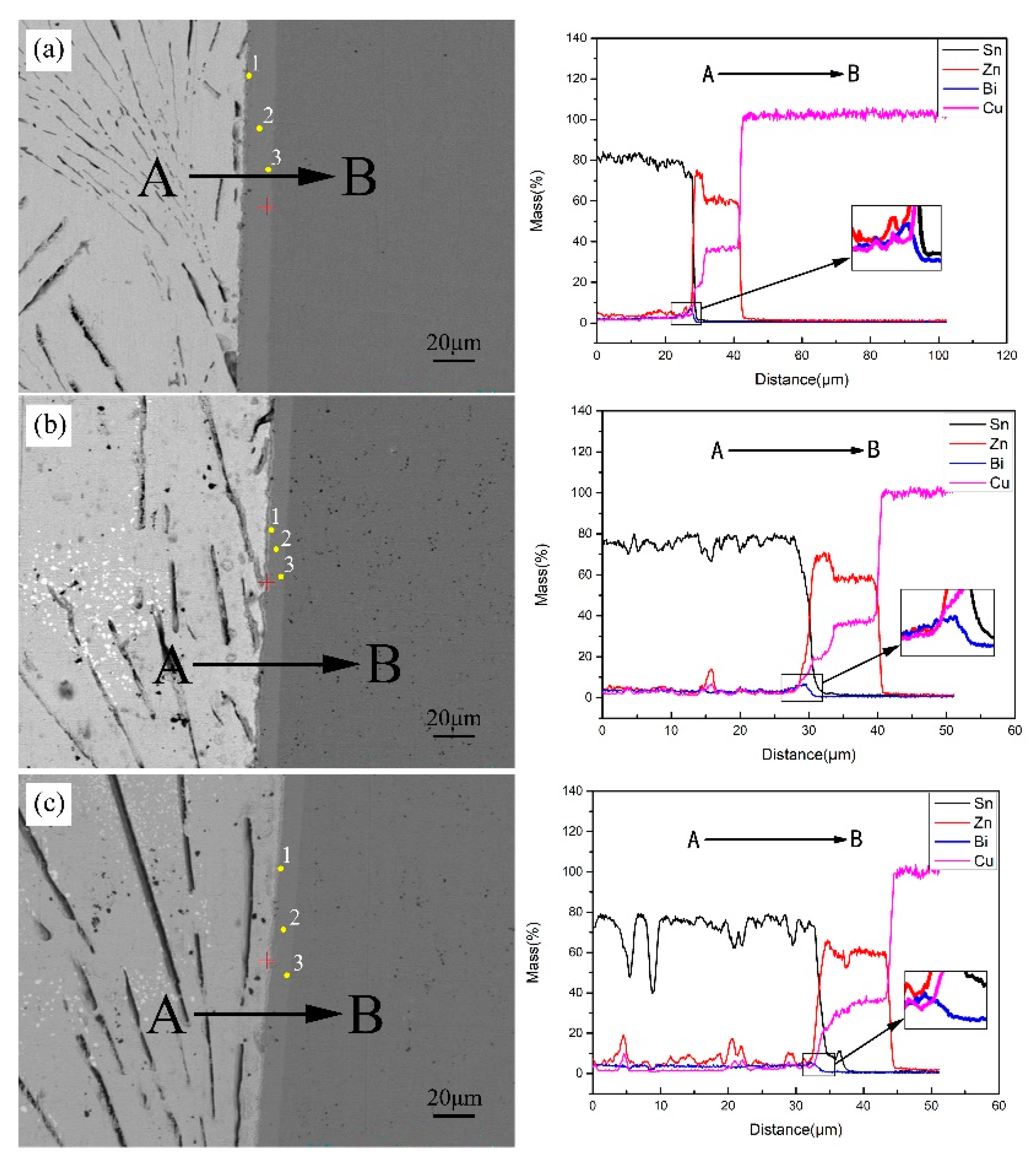
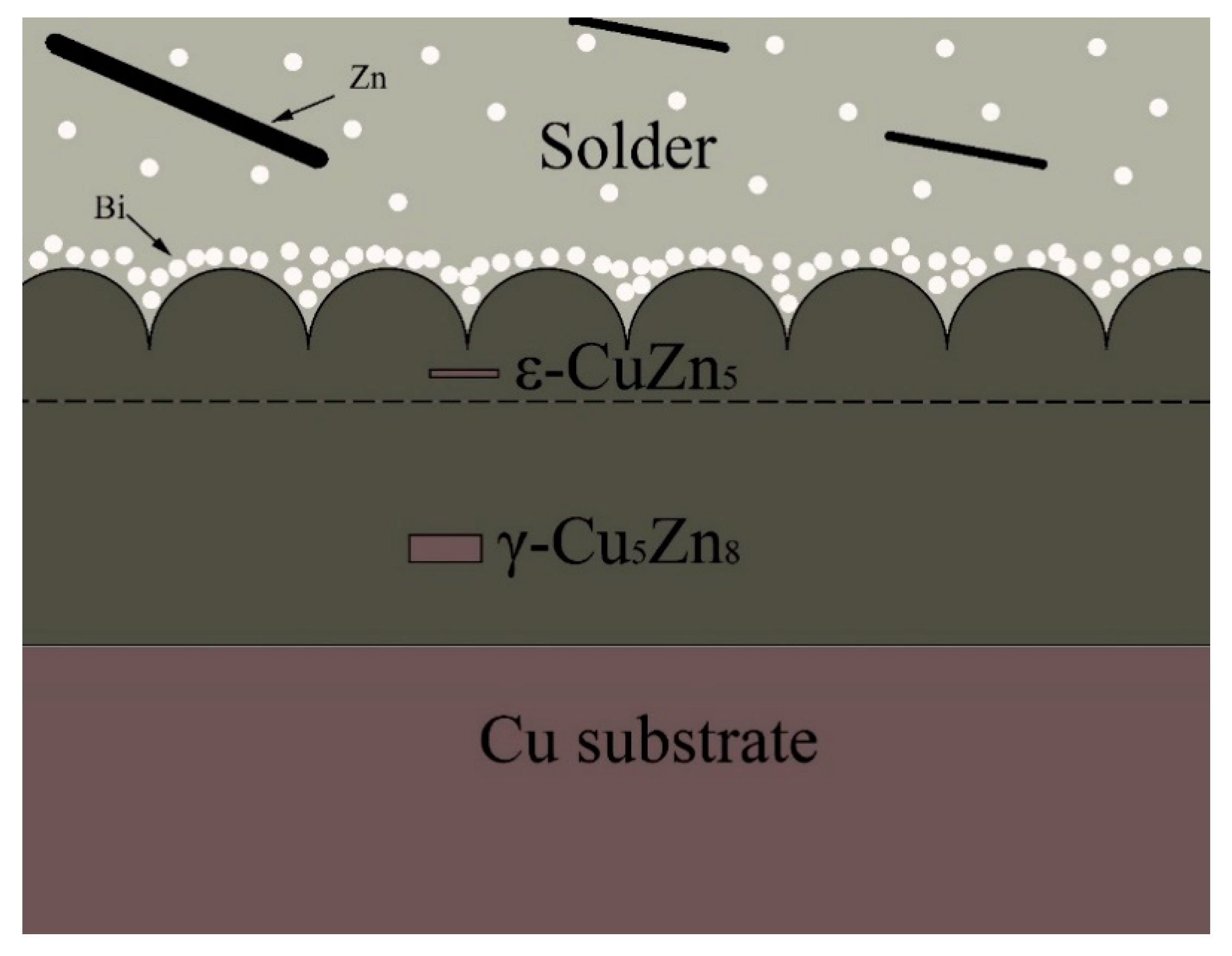
3.6. The Interfacial Reaction in Sn-9Zn-xBi/Cu Joints
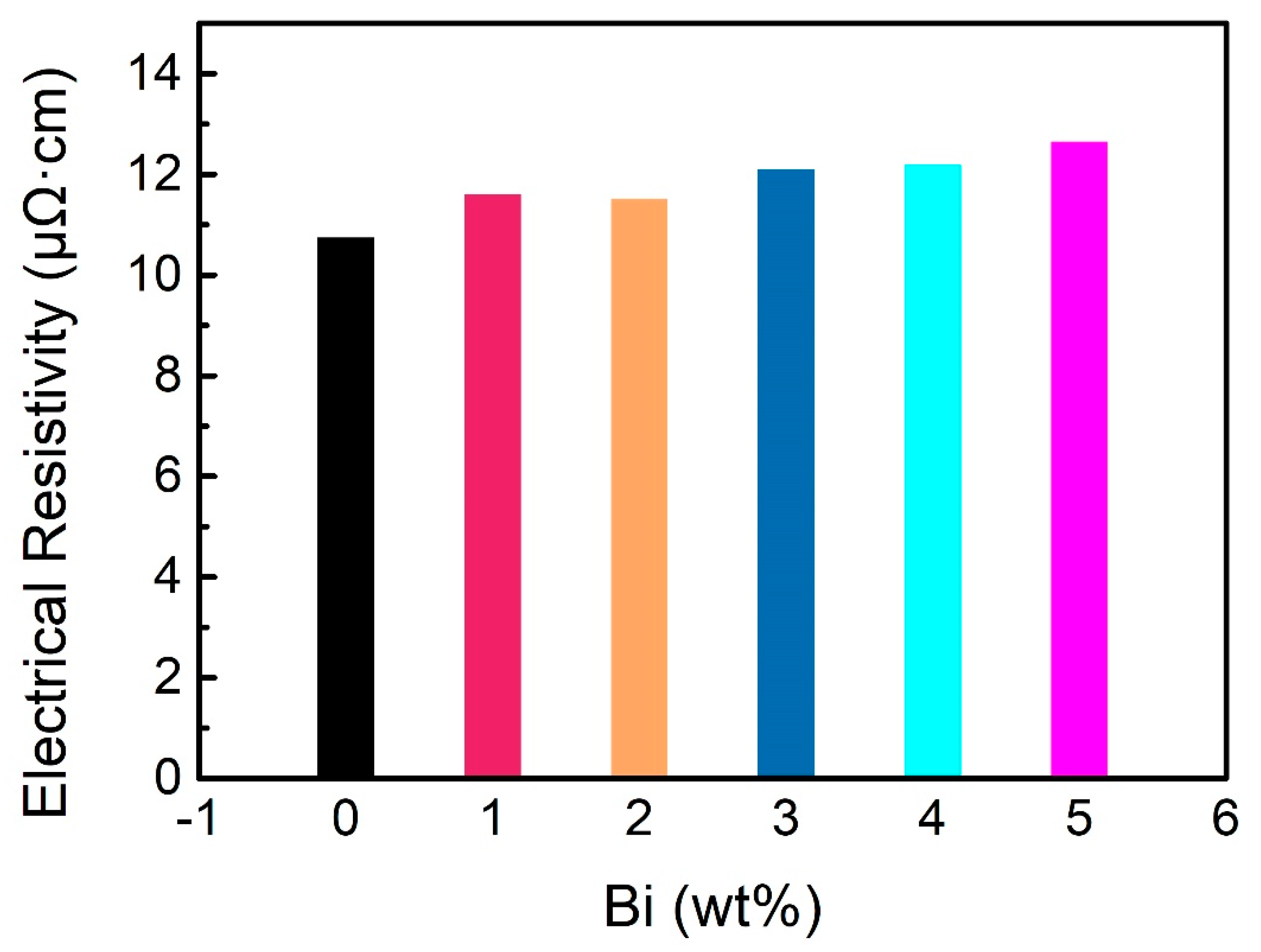
3.7. Electrical Conductivity
4. Conclusions
- (a)
- The microstructure of Sn-9Zn-xBi solder alloy consists of a light gray β-Sn matrix, a coarse black acicular Zn-rich phase, and a Sn-Zn eutectic mixture. With the increase in Bi content, the Zn-rich phase disappears at first and then becomes bigger, then white granular Bi precipitates from the Sn matrix.
- (b)
- The melting temperature, solidus temperature, and liquidus temperature decreased with the increasing Bi addition, but the melting range increased.
- (c)
- The spreading rate increased and the wetting angle decreased with the increase in Bi content from 1 to 5 wt.%. The addition of Bi obviously improved the wettability of the Sn-9Zn-xBi solder alloy, which can be attributed to the Bi element reducing the surface tension of the molten solder alloy.
- (d)
- Adding a small amount of Bi can improve the oxidation resistance of the Sn-9Zn solder alloy. However, if the content of Bi is more than 3 wt.%, the oxidation resistance of the Sn-9Zn-xBi alloy will decrease. This is because the Zn-rich phase becomes bigger with an increasing Bi addition, which is harmful to the oxidation resistance.
- (e)
- Bi atoms would precipitate out from the matrix and produce a lot of brittle particles, which leads to an increase in strength and a significant decrease in plasticity.
- (f)
- Two reaction layers were formed at the Sn-Zn/Cu interface. They are ε-CuZn5, close to the solder, and γ-Cu5Zn8, closed to the Cu substrate, respectively. The formation of the IMC layer is mainly controlled by a diffusion mechanism.
- (g)
- The formation of solid solution and Bi particles in the solder alloy, leading to an increase in the scattering of electrons, is mainly responsible for the increase in the resistivity of the alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Xue, S.-B.; Gao, L.-L.; Sheng, Z.; Ye, H.; Xiao, Z.-X.; Zeng, G.; Chen, Y.; Yu, S.-L. Development of Sn–Zn lead-free solders bearing alloying elements. J. Mater. Sci. Mater. Electron. 2010, 21, 1–15. [Google Scholar] [CrossRef]
- Kroupa, A.; Watson, A.; Mucklejohn, S.; Ipser, H.; Dinsdale, A.; Andersson, D. Chapter 5—Lead-free soldering: Environmentally friendly electronics. In Green and Sustainable Manufacturing of Advanced Material; Singh, M., Ohji, T., Asthana, R., Eds.; Elsevier: Oxford, UK, 2016; pp. 101–134. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, C.-M.; Pecht, M. A review of lead-free solders for electronics applications. Microelectron. Reliab. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Howes, P.D.; Mannan, S.H. A review: On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Xu, J.; Xue, S.; Xue, P.; Long, W.-M.; Zhang, Q.-K. Study on microstructure and properties of Sn–0.3Ag–0.7Cu solder bearing Nd. J. Mater. Sci. Mater. Electron. 2016, 27, 8771–8777. [Google Scholar] [CrossRef]
- Bieler, T.R.; Lee, T.-K. Lead-free solder. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2010; pp. 1–12. [Google Scholar] [CrossRef]
- Liu, J.-C.; Zhang, G.; Ma, J.-S.; Suganuma, K. Ti addition to enhance corrosion resistance of Sn–Zn solder alloy by tailoring microstructure. J. Alloys Compd. 2015, 644, 113–118. [Google Scholar] [CrossRef]
- Islam, R.A.; Chan, Y.C.; Jillek, W.; Islam, S. Comparative study of wetting behavior and mechanical properties (microhardness) of Sn–Zn and Sn–Pb solders. Microelectron. J. 2006, 37, 705–713. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.; Xue, F. Properties of low melting point Sn–Zn–Bi solders. J. Alloys Compd. 2005, 397, 260–264. [Google Scholar] [CrossRef]
- Chen, W.X.; Xue, S.B.; Wang, H.; Wang, J.X.; Han, Z.J.; Gao, L.L. Effects of Ag on microstructures, wettabilities of Sn–9Zn–xAg solders as well as mechanical properties of soldered joints. J. Mater. Sci. Mater. Electron. 2010, 21, 461–467. [Google Scholar] [CrossRef]
- Xue, P.; Xue, S.-B.; Shen, Y.-F.; Zhu, H.; Gao, L.L. Study on properties of Sn-9Zn-Ga solder bearing Nd. J. Mater. Sci. Mater. Electron. 2012, 23, 1272–1278. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, D.; Fang, Y.-L.; Xue, F. Investigation on properties of Sn-8Zn-3Bi lead-free solder by Nd addition. J. Alloys Compd. 2009, 480, 903–907. [Google Scholar] [CrossRef]
- Mahdavifard, M.H.; Sabri, M.F.M.; Shnawah, D.A.; Said, S.M.; Badruddin, I.A.; Rozali, S. The effect of iron and bismuth addition on the microstructural, mechanical, and thermal properties of Sn-1Ag-0.5Cu solder alloy. Microelectron. Reliab. 2015, 55, 1886–1890. [Google Scholar] [CrossRef]
- Eid, E.A.; El-Khawas, E.H.; Abd-Elrahman, A.S. Impact of Sb additives on solidification performance, microstructure enhancement and tensile characteristics of Sn-6.5Zn-0.3Cu Pb-free solder alloy. J. Mater. Sci. Mater. Electron. 2019, 30, 6507–6518. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Liu, Y.; Min, Z. Developments of high strength Bi-containing Sn0.7Cu lead-free solder alloys prepared by directional solidification. J. Alloys Compd. 2015, 625, 241–250. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hashem, H.A.; Radwan, N.; El-Tantawy, F.; Dalloul, T.R.; Mansour, N.A.; Abd-Elmoniem, H.M.; Lotfy, E.H. Robust effects of Bi doping on microstructure development and mechanical properties of hypoeutectic Sn–6.5Zn solder alloy. J. Mater. Sci. Mater. Electron. 2016, 27, 2950–2962. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Wen, G.-H.; Sheng, G.-M. Influence of rapid solidification on Sn-8Zn-3Bi alloy characteristics and microstructural evolution of solder/Cu joints during elevated temperature aging. Trans. Nonferrous Met. Soc. China 2017, 27, 234–240. [Google Scholar] [CrossRef]
- Moon, K.-W.; Boettinger, W.J.; Kattner, U.R.; Handwerker, C.A.; Lee, D.-J. The effect of Pb contamination on the solidification behavior of Sn-Bi solders. J. Electron. Mater. 2001, 30, 45–52. [Google Scholar] [CrossRef]
- Erer, A.M.; Oguz, S.; Türen, Y. Influence of bismuth (Bi) addition on wetting characteristics of Sn-3Ag-0.5Cu solder alloy on Cu substrate. Eng. Sci. Technol. Int. J. 2018, 21, 1159–1163. [Google Scholar] [CrossRef]
- Jiang, J.; Lee, J.-E.; Kim, K.-S.; Suganuma, K. Oxidation behavior of Sn–Zn solders under high-temperature and high-humidity conditions. J. Alloys Compd. 2008, 462, 244–251. [Google Scholar] [CrossRef]
- Yang, C.-F.; Chen, S.-W.; Wu, K.-H.; Chin, T.-S. Interfacial reactions of Sn-8wt.%Zn-3wt.%Bi solder with Cu, Ag, and Ni substrates. J. Electron. Mater. 2007, 36, 1524–1530. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Liu, L.; Wu, P. Cu5Zn8 growth reversion in Cu/Sn-8Zn-3Bi/Cu during discontinuous electromigration. J. Electron. Mater. 2012, 41, 2045–2050. [Google Scholar] [CrossRef]
- Suganuma, K.; Niihara, K.; Shoutoku, T.; Nakamura, Y. Wetting and interface microstructure between Sn–Zn binary alloys and Cu. J. Mater. Res. 1998, 13, 2859–2865. [Google Scholar] [CrossRef]
- Mayappan, R.; Ismail, A.B.; Ahmad, Z.A.; Ariga, T.; Hussain, L.B. The effect of crosshead speed on the joint strength between Sn-Zn-Bi lead-free solders and Cu substrate. J. Alloys Compd. 2007, 436, 112–117. [Google Scholar] [CrossRef]
- Kim, D.-G.; Jang, H.-S.; Jung, S.-B. Solid state intermetallic compound layer growth between Sn-8Zn-3Bi solder and bare copper substrate. J. Mater. Sci. Mater. Electron. 2005, 16, 523–528. [Google Scholar] [CrossRef]
- Jaffery, H.A.; Mohd Sabri, M.F.; Rozali, S.; Mahdavifard, M.H.; Shnawah, D.A. Effect of temperature and alloying elements (Fe and Bi) on the electrical resistivity of Sn-0.7Cu solder alloy. RSC Adv. 2016, 6, 58010–58019. [Google Scholar] [CrossRef]



| Alloy | Zn(wt.%) | Bi(wt.%) | Sn(wt.%) |
|---|---|---|---|
| Sn-9Zn | 9.03 | 0 | 90.97 |
| Sn-9Zn-1Bi | 9.14 | 1.01 | 89.85 |
| Sn-9Zn-2Bi | 8.88 | 2.02 | 89.10 |
| Sn-9Zn-3Bi | 8.76 | 3.01 | 88.23 |
| Sn-9Zn-4Bi | 8.91 | 3.98 | 87.11 |
| Sn-9Zn-5Bi | 8.85 | 4.87 | 86.28 |
| Alloy | Tonset (°C) | Toffset (°C) | Tm (°C) | Pasty Range (°C) | Enthalpy (J/g) |
|---|---|---|---|---|---|
| Sn-9Zn | 198.62 | 202.93 | 199.89 | 4.31 | 86.45 |
| Sn-9Zn-1Bi | 197.01 | 200.62 | 198.37 | 3.61 | 84.56 |
| Sn-9Zn-2Bi | 195.41 | 199.45 | 197.39 | 4.04 | 82.82 |
| Sn-9Zn-3Bi | 193.89 | 198.30 | 196.48 | 4.41 | 80.18 |
| Sn-9Zn-4Bi | 192.65 | 197.32 | 195.56 | 4.67 | 79.76 |
| Sn-9Zn-5Bi | 191.33 | 196.32 | 194.67 | 4.99 | 77.59 |
| Alloy | σ/MPa | δ/% |
|---|---|---|
| Sn-9Zn | 61 | 17.5 |
| Sn-9Zn-1Bi | 68 | 25.0 |
| Sn-9Zn-2Bi | 79 | 22.5 |
| Sn-9Zn-3Bi | 84 | 20.0 |
| Sn-9Zn-4Bi | 98 | 12.5 |
| Sn-9Zn-5Bi | 107 | 7.5 |
| Alloy | Location | Composition (Mol%) | |||
|---|---|---|---|---|---|
| Zn | Cu | Sn | Bi | ||
| Sn-9Zn | 1 | 64.39 | 32.5 | 2.11 | 0 |
| 2 | 64.07 | 35.56 | 0 | 0 | |
| 3 | 63.01 | 36.64 | 0.35 | 0 | |
| Sn-9Zn-1Bi | 1 | 80.55 | 17.28 | 2.14 | 0.03 |
| 2 | 64.98 | 34.64 | 0.36 | 0.02 | |
| 3 | 63.80 | 35.88 | 0.31 | 0.01 | |
| Sn-9Zn-2Bi | 1 | 78.50 | 17.22 | 4.24 | 0.04 |
| 2 | 65.01 | 34.34 | 0.65 | 0 | |
| 3 | 63.71 | 35.78 | 0.46 | 0.05 | |
| Sn-9Zn-3Bi | 1 | 80.93 | 17.94 | 1.12 | 0.01 |
| 2 | 64.43 | 35.07 | 0.48 | 0.01 | |
| 3 | 63.60 | 35.98 | 0.42 | 0 | |
| Sn-9Zn-4Bi | 1 | 79.03 | 18.43 | 2.50 | 0.05 |
| 2 | 63.49 | 35.48 | 1.03 | 0 | |
| 3 | 62.41 | 36.70 | 0.86 | 0.03 | |
| Sn-9Zn-5Bi | 1 | 77.22 | 18.57 | 4.17 | 0.03 |
| 2 | 65.86 | 33.60 | 0.54 | 0 | |
| 3 | 64.35 | 35.19 | 0.43 | 0.03 | |
| Alloys | Sn-9Zn | Sn-9Zn-1Bi | Sn-9Zn-2Bi | Sn-9Zn-3Bi | Sn-9Zn-4Bi | Sn-9Zn-5Bi |
|---|---|---|---|---|---|---|
| thickness (μm) | 14.03 | 14.88 | 15.90 | 13.37 | 11.85 | 11.61 |
| 14.76 | 15.14 | 15.39 | 13.62 | 11.60 | 11.59 | |
| 14.30 | 13.88 | 15.39 | 13.87 | 12.15 | 11.61 | |
| Average (μm) | 14.36 | 14.63 | 15.56 | 13.62 | 11.87 | 11.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.Z.; Li, C.J.; Yang, J.J.; Zhang, J.T.; Peng, J.B.; Zhou, G.J.; Pu, C.J.; Yi, J.H. Effects of Bismuth on the Microstructure, Properties, and Interfacial Reaction Layers of Sn-9Zn-xBi Solders. Metals 2021, 11, 538. https://doi.org/10.3390/met11040538
Peng YZ, Li CJ, Yang JJ, Zhang JT, Peng JB, Zhou GJ, Pu CJ, Yi JH. Effects of Bismuth on the Microstructure, Properties, and Interfacial Reaction Layers of Sn-9Zn-xBi Solders. Metals. 2021; 11(4):538. https://doi.org/10.3390/met11040538
Chicago/Turabian StylePeng, Yan Zhi, Cai Ju Li, Jiao Jiao Yang, Jia Tao Zhang, Ju Bo Peng, Guang Ji Zhou, Cun Ji Pu, and Jian Hong Yi. 2021. "Effects of Bismuth on the Microstructure, Properties, and Interfacial Reaction Layers of Sn-9Zn-xBi Solders" Metals 11, no. 4: 538. https://doi.org/10.3390/met11040538
APA StylePeng, Y. Z., Li, C. J., Yang, J. J., Zhang, J. T., Peng, J. B., Zhou, G. J., Pu, C. J., & Yi, J. H. (2021). Effects of Bismuth on the Microstructure, Properties, and Interfacial Reaction Layers of Sn-9Zn-xBi Solders. Metals, 11(4), 538. https://doi.org/10.3390/met11040538






