Abstract
Cast Ni-Si-B alloys have the potential for high-temperature applications because of their high resistance to wear, impact, corrosion, and oxidation at elevated temperatures due to an appropriate balance of hard phases and austenite that ensures a good compromise between toughness and hardness. In this work, NiSi3B2 specimens, fabricated by the lost-wax casting process, were investigated. Given the complex multiphase cast microstructure, a differential scanning calorimeter (DSC-TGA) analysis was employed to characterize the reactions that occur during solidification and the resulting phases were characterized using scanning electron microscopy (SEM), with energy-dispersive microanalysis (EDS) and backscattered electron (BSE) image and X-ray diffraction (XRD). Due to the presence of hard phases, machining of the Ni-Si-B components can pose additional difficulties. Therefore, the conditions of the solution heat treatment, which might lead to the homogenization of the microstructure, consequently improving its machinability, were also investigated. The results of the heat-treated samples indicated that the dissolution of the eutectic constituent is accompanied by a significant decrease in the hardness (approximately 17%). It is important to emphasize that the solution heat treatments carried out reduced the hardness without affecting the percentage of borides, which will allow improving the machinability without adversely affecting the alloy performance in service.
1. Introduction
Nickel superalloys stand out for their high corrosion, abrasion, and mechanical resistance at high temperatures, competing in the market with other materials, namely austenitic stainless steels. These alloys are attractive for many applications: aerospace, chemical, oil and gas, nuclear, automotive, as well as tool and mold industries, since they with-stand severe wear conditions, corrosive environments, and thermal fatigue [1,2]. The alloys specifically for molds for the glass industry must also exhibit inert behavior, ensuring that there are no reactions at the interface between the liquid glass and the mold surface. Besides, these alloys must show superior toughness and thermal fatigue resistance to withstand a large number of thermal cycles at high temperatures. Another important property is the thermal conductivity, as it determines the efficiency of heat transfer, allowing the glass to cool quickly and reducing the production cycle time [3,4,5].
For the above reasons, Ni-Si-B alloys have the potential for use in the glass industry due to their high resistance to wear, impact, corrosion, and oxidation at high temperatures. Typically, Ni-Si-B alloys are processed by casting into shaped components or used as hard coatings [6,7]. The major disadvantage of producing these alloys in the form of hard coatings is the formation of metastable phases during rapid cooling, increasing the susceptibility to cracking [8,9,10,11]. For cast alloys [12], there is also a great microstructural complexity due to variations in the chemical composition of the melt and in the rate of cooling imposed by the wall thickness of the cast components. A microstructure composed of nickel-rich austenite and hard phases, such as borides (Ni2B, Ni3B) and eutectic constituents of silicides (Ni2Si, Ni5Si2, Ni3Si2) and austenite, can be produced. However, limited information on the kinetics of the formation of those phases under casting conditions is currently available [13,14]. Furthermore, the hardness of the material depends on the alloying elements and, therefore, it is of great importance to select a chemical composition that allows a balance of hard phases and austenite, ensuring a good compromise between toughness and hardness [15,16,17]. On the other hand, machining of Ni-Si-B components can pose additional difficulties if the hardness is too high [18]. Thus, the purpose of this study is to characterize the reactions that occur during casting and the resulting phases. The conditions of the solution heat treatment, which might lead to the transformation of the microstructure, reducing the hardness and, consequently, improving the machinability of the alloy, are also discussed. This softening would be beneficial when the machining of the assembling components is necessary; however, it may adversely affect the performance in service of those components [19,20,21,22].
2. Materials and Methods
Cylindrical specimens of 20 mm in diameter and 75 mm in length were fabricated by the lost-wax casting process. The metallic charge was melted in an induction furnace with a capacity of 65 kg and liquid argon was used to protect the surface of the melt, minimizing the oxidation of the molten metal. After adjusting the temperature at 1280 °C, the metal was cast directly into the investment mold pre-heated at 980 °C. The chemical analysis of the molten metal was performed by optical emission spectrometry (MAXx LMM05, Spectro, Kleve, Germany) and inductively coupled plasma-atomic emission spectroscopy. The nominal chemical composition of the prepared NiSi3B2 alloy is provided in Table 1.

Table 1.
Nominal chemical composition of the NiSi3B2 alloy (wt.%).
Thermal analysis was carried out using a differential scanning calorimeter (DSC-TGA Netzsch STA 449 F3 Jupiter, Selb, Germany). The samples were heated and cooled at a rate of 10 °C/min to determine the melting temperature and the phase transformation temperatures of the NiSi3B2 alloy.
The DSC results helped to set the temperature of the solution heat treatments, which were performed to study the process of dissolution of the hard phases. The solution heat treatments were carried out in a muffle furnace at 930 °C and 980 °C under different holding times, 0.5 and 3 h, followed by water quenching.
The microstructure of the alloy in the as-cast and heat-treated condition was characterized by scanning electron microscopy (SEM), using an FEI QUANTA 400 FEG (FEI Company, Hillsboro, OR, USA) with energy-dispersive microanalysis (EDS) and backscattered electron (BSE) image. X-ray diffraction (XRD, Cu Kα radiation, Bruker D8 Discover, Billerica, Massachusetts, EUA), with a scanning range of 20° to 100° (2θ), was also used for phases identification. Metallographic samples were prepared using standard techniques of grinding and polishing. The samples were etched with a solution of 7 g FeCl3, 5 mL HCl, and 100 mL H2O to reveal the phases present that were quantified by image analysis with optical microscopy (OM) using a Leica DM 4000M with a DFC 420 camera (Leica Microsystems, Wetzlar, Germany). The number and magnification of the fields were selected according to the microstructural features, and 20 random fields with a magnification of 100× were acquired.
The bulk hardness of the specimens was evaluated using Vickers hardness tests (universal hardness tester DuraVision 20, EMCO-TEST Prüfmaschinen GmbH, Kuchl, Austria) under a load of 294.2 N and conducting 10 tests in each specimen. Moreover, the microindentation hardness of the phases present in the microstructure was also determined using a Duramin-1 (Duramin-1, Struers Inc., Cleveland, OH, USA) hardness tester and making ten indentations in each constituent with a test load in the range of 25 g to 100 g.
3. Results and Discussion
3.1. Characterization of the Solidification Phases of the NiSi3B2 Alloy
The measurement of the melting point and phase transformation temperatures during solidification by thermal analysis (DSC) was significantly useful to interpret the as-cast microstructure. Figure 1 shows the DSC results after melting and cooling of the NiSi3B2 alloy. The melting occurs between 1074.1 °C (the onset point) and 1091.0 °C (the peak temperature). Furthermore, the cooling curve indicates two solidification peaks. The higher temperature peak at 955.0 °C corresponds to the nickel borides (Ni3B) formation and the peak temperature at 919.8 °C is with respect to the eutectic formation (Ni-γ-Ni3Si). Based on the equilibrium ternary diagrams of Ni-Si-B [23], it is expected that the first constituent to form is the nickel boride (Ni3B) and the remaining liquid is consumed by the eutectic formation at a lower temperature. This solidification process is consistent with the as-cast microstructure depicted in Figure 2.
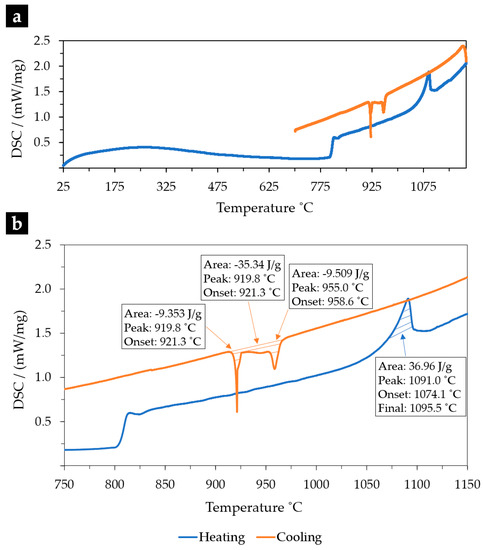
Figure 1.
(a) DSC heating and cooling curves with rates of 10 °C/min of the NiSi3B2 alloy; (b) zoomed in, showing the main phase transformations.
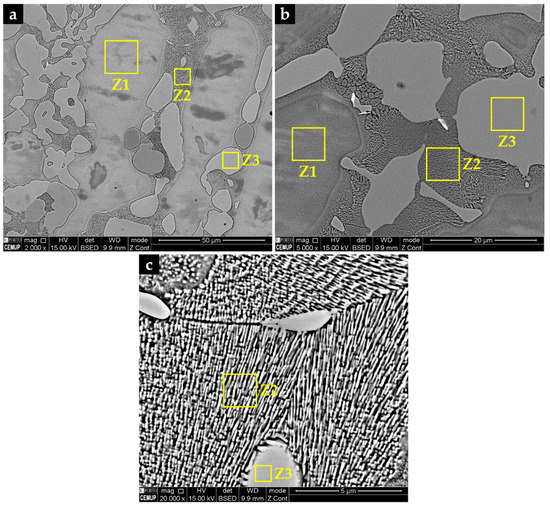
Figure 2.
(a) SEM-BSE images of the as-cast microstructure of NiSi3B2 alloy showing the nickel-austenite (Ni-γ) as Z1, eutectic constituent (Ni-γ-Ni3Si) as Z2, and nickel borides (Ni3B) as Z3; (b) the same as (a) at a higher magnification; (c) the fine eutectic constituent Z2 is more clearly shown at a higher magnification.
Figure 2 presents the microstructure of the specimens after solidification and cooling of the molten alloy from 1280 °C. The slow cooling inside of the pre-heated investment mold produced a microstructure composed of dispersed coarse particles of borides (Ni3B), nickel-rich austenite (Ni-γ), and a large volume fraction of eutectic constituent (Ni-γ-Ni3Si), as evidenced by the SEM-EDS and XRD analyses presented below. The fine eutectic constituent presents a rod-like morphology with very small rod distances. The shapes of the rods are circular and elongated in cross-section, as can be seen in Figure 2c.
Table 2 shows the results of the semi-quantitative analysis of the alloy by SEM-EDS. Due to the difficulty in analyzing the boron amount because of its small atomic weight, the results of the semi-quantitative analysis of nickel borides are not present in Table 2. However, combining the EDS analysis with the XRD pattern depicted in Figure 3, Z3 likely corresponds to nickel boride (Ni3B) and Z1 corresponds to nickel-austenite (Ni-γ) that takes chromium, carbon, iron, and silicon into solid-solution in a content of about 4 wt.%. Considering the limitation of analyzing the individual phases present within the eutectic constituent, Z2 yields the average composition of the eutectic constituent (Ni-γ-Ni3Si) containing a higher content of silicon (approximately 9 wt.%.).

Table 2.
SEM-EDS results of the as-cast NiSi3B2 alloy.
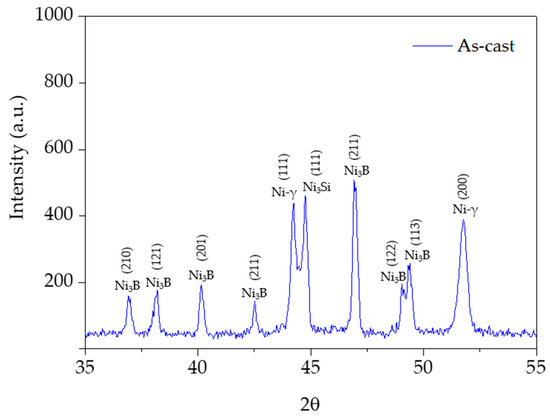
Figure 3.
XRD pattern of the as-cast NiSi3B2 specimen, exhibiting intense peaks at the range of 2θ = 35°–55°, corresponding to the Ni3B, Ni3Si and nickel-austenite (Ni-γ) phases.
3.2. Influence of the Solution Heat Treatment Parameters on the Microstructure of the NiSi3B2 Alloy
The solution heat treatment time and temperature determine the level of homogenization of the microstructure. Figure 4 shows the microstructural changes that occurred at 930 °C and 980 °C for 0.5 and 3 h. The increase in the holding time leads to the dissolution of the eutectic constituent (Ni-γ-Ni3Si), and it seems that the kinetics of the dissolution is faster at 980 °C. The holding time of 3 h is sufficient to promote the dissolution of the eutectic constituent (Z2) and to obtain a microstructure almost composed of nickel-austenite (Z1) and nickel borides (Z3), as seen in Figure 4e. Important also is the high content of nickel borides present after heat treatments: 42 ± 3% after 3 h at 930 °C and 980 °C. These results are similar to that of the as-cast sample (42 ± 2%), indicating that the solution heat treatments did not promote the dissolution of the nickel borides for the temperatures tested.
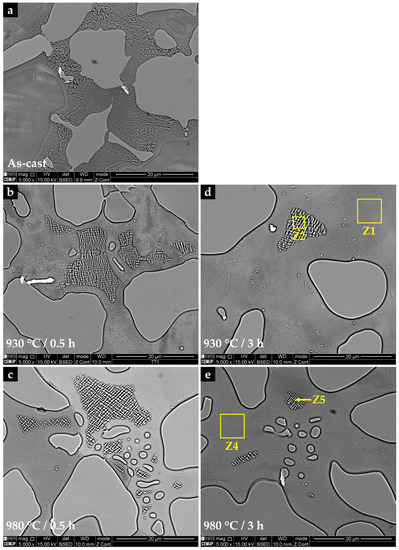
Figure 4.
Effect of the solution heat treatment on the microstructure of NiSi3B2 alloy: (a) the as-cast condition; (b) 930 °C/0.5 h; (c) 980 °C/0.5 h; (d) 930 °C/3 h; and (e) 980 °C/3 h.
The SEM-EDS results of the heat-treated specimens demonstrate the enrichment of austenite in silicon during the solution heat treatment. Table 3 shows the results of the specimen heat-treated at 930 °C and 980 °C for 3 h where this enrichment of about 2 wt.% can be verified compared to the as-cast sample (Table 2).

Table 3.
SEM-EDS results of the NiSi3B2 alloy solutionized at 930 °C and 980 °C for 3 h.
From the XRD spectra (the XRD pattern 980 °C/3 h is similar to that of the 930 °C/3 h, and so it was not included in Figure 5), a higher intensity of the Ni-γ (111) peak is observed and the Ni3Si peak is not detected (Figure 5). These results indicate that the reduction of the eutectic constituent (Ni-γ-Ni3Si) to values below the XRD detection limit and the consequent enrichment of austenite in silicon are certainly due to atomic diffusion. The stability of the borides is evident and, besides, new peaks in the spectra are not visible, which agrees with the SEM images.
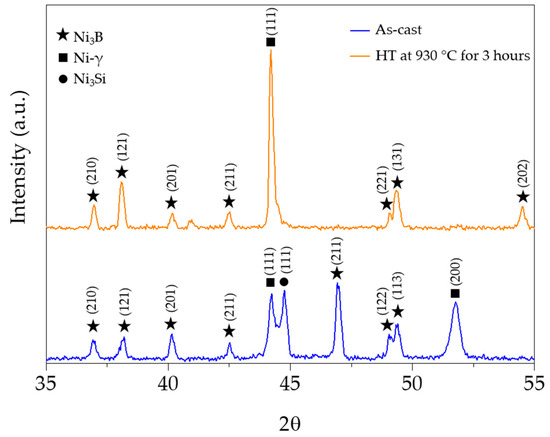
Figure 5.
XRD patterns of the as-cast and heat-treated NiSi3B2 specimens.
The enrichment of austenite in silicon during solution heat treatment enhances its hardness, as shown in Figure 6 (from 171 HV to 183 HV and 207 HV after 0.5 h at 930 °C and 980 °C, respectively). With the increase in holding time, the hardness of the austenite maintains stable for 930 °C and 980 °C. On the other hand, the dissolution of the eutectic regions results in a considerable softening of those regions during the dissolution process (from 593 HV to 522 HV and 483 HV after 0.5 h at 930 °C and 980 °C, respectively). The hardness average of the borides in the as-cast condition (1124 HV) also does not change significantly with the solution heat treatment. This variation is attributed to the smaller test load used that created smaller indentations that are much more sensitive to the measurement variation. Therefore, as shown in Figure 7, the microstructural transformations result in a significant decrease (17%) in the hardness of specimens from 396 HV to 329 HV and 328 HV after 3 h at 930 °C and 980 °C, respectively.
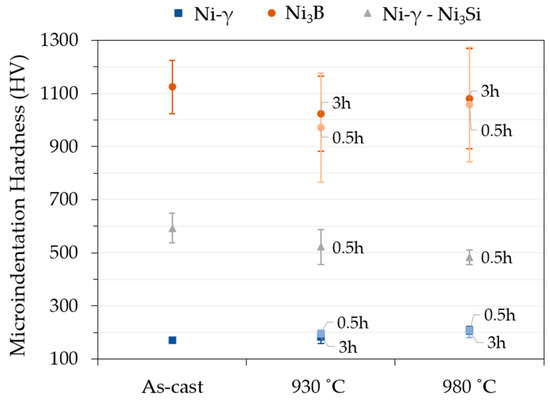
Figure 6.
Microindentation hardness (HV) of the phases present in the as-cast and heat-treated microstructures in the range of 25 g to 100 g.
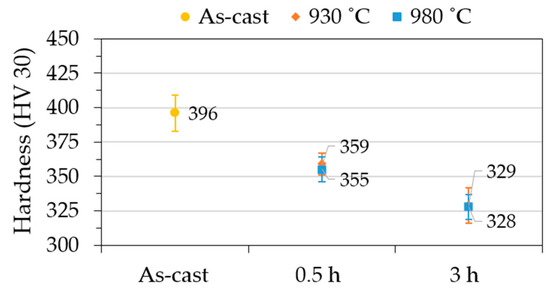
Figure 7.
Effect of the solution heat treatment on the hardness of the NiSi3B2 alloy.
4. Conclusions
The results obtained in this study indicate the following conclusions:
- The microstructure of the as-cast NiSi3B2 alloy consists of nickel-rich austenite (Ni-γ) with coarse particles of borides (Ni3B) and a large volume fraction of eutectic constituent (Ni-γ-Ni3Si).
- The microstructure can be modified by solution heat treatment (at 930 °C or 980 °C), resulting in the dissolution of the eutectic constituent, with faster dissolution kinetics at 980 °C.
- The dissolution of the eutectic phases is accompanied by solid solution hardening of the austenite.
- The volume fraction of the nickel borides of 42 ± 3% is similar to that of the as-cast condition (42 ± 2%), indicating that the solution heat treatment does not promote the dissolution of borides at the temperatures tested.
- The solutionized microstructure is significantly more homogeneous and the bulk hardness decreases considerably, approximately 17% (from 396 HV to 329 HV and 328 HV after 3 h at 930 °C and 980 °C, respectively).
Author Contributions
Conceptualization, G.M.G.; formal analysis, L.M.M.R. and M.F.V.; funding acquisition, L.M.M.R.; investigation, G.M.G., A.B.M. and P.L.; supervision, L.M.M.R. and M.F.V.; validation, L.M.M.R. and M.F.V.; writing—original draft, G.M.G.; writing—review and editing, L.M.M.R. and M.F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FEDER funds through the program P2020|COMPETE (project POCI-01-0247-FEDER-039836).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to CEMUP (Centro de Materiais da Universidade do Porto) for expert assistance with SEM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, J.R. ASM Specialty Handbook-Nickel, Cobalt, and Their Alloys; ASM International: Geauga County, OH, USA, 2000. [Google Scholar]
- Jalilian, F.; Jahazi, M.; Drew, R.A.L. Microstructural evolution during transient liquid phase bonding of Inconel 617 using Ni–Si–B filler metal. Mater. Sci. Eng. A 2006, 423, 269–281. [Google Scholar] [CrossRef]
- Manns, P.; Döll, W.; Kleer, G. Glass in contact with mould materials for container production. Glas. Ber. Glass Sci. Technol. 1995, 68, 389–399. [Google Scholar]
- He, L.Z.; Zheng, Q.; Sun, X.F.; Guan, H.R.; Hu, Z.Q.; Tieu, A.K.; Lu, C.; Zhu, H.T. Effect of carbides on the creep properties of a Ni-base superalloy M963. Mater. Sci. Eng. A 2005, 397, 297–304. [Google Scholar] [CrossRef]
- Shulga, A.V. Boron and carbon behavior in the cast Ni-base superalloy EP962. J. Alloys Compd. 2007, 436, 155–160. [Google Scholar] [CrossRef]
- Cape, A.T.; Calif, M. Nickel-Silicon-Boron Alloys. U.S. Patent 2743177A, 24 April 1956. [Google Scholar]
- Kornienko, E.; Nikulina, A.; Belousova, N.; Lazurenko, D.; Ivashutenko, A.; Kuz’min, V. Structural features of Ni-Cr-Si-B materials obtained by different technologies. IOP Conf. Ser: Mater. Sci. Eng. 2016, 156, 012020. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Hashim, F.A.; Al-Mohaisen, A.M.N. Microstructure and phase analysis of brazing bonds for stainless steel (AISI 316L and 431) to carbon steel (A516 G70) using a Ni–Si–B filler metal alloy. Mater. Sci. Eng. 2020, 881. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.; Liu, Y.; Gao, Y.; Di, C. Wear and heat shock resistance of Ni-WC coating on mould copper plate fabricated by laser. J. Mater. Res. Technol. 2020, 9, 8283–8288. [Google Scholar] [CrossRef]
- Tanigawa, D.; Abe, N.; Tsukamoto, M.; Hayashi, Y.; Yamazaki, H.; Tatsumi, Y.; Yoneyama, M. The effect of particle size on the heat affected zone during laser cladding of Ni–Cr–Si–B alloy on C45 carbon steel. Opt. Lasers Eng. 2018, 101, 23–27. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, W.; Ge, Y.; Zhou, J.; Cui, Z. Microstructure and mechanical properties of Ni-Cr-Si-B-Fe composite coating fabricated through laser additive manufacturing. J. Alloys Compd. 2018, 747, 401–407. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Cygan, R. Analysis of temperature distribution in shell mould during thin-wall superalloy casting and its effect on the resultant microstructure. Arch. Civ. Mech. Eng. 2018, 18, 1441–1450. [Google Scholar] [CrossRef]
- Ajao, J.; Hamar-Thibault, S. Influence of additions on the solidification behaviour of Ni-B alloys—crystallography of Ni-Ni3B eutectic. J. Mater. Sci. 1988, 23, 1112–1125. [Google Scholar] [CrossRef]
- Fraga-Chávez, K.L.; Castro-Román, M.J.; Herrera-Trejo, M.; Ramírez-Vidaurri, L.E.; Aguilera-Luna, I. Phase Selection during Solidification of Ni-10.95 at. % and B-3.23 at. % Si Alloy. Metals 2017, 6, 187. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Zhang, D. Phase selection of undercooled solidification of Ni–4.5 wt% B alloy. J. Mater. Res. 2013, 28, 3347–3354. [Google Scholar] [CrossRef]
- Zhu, C.L.; Wang, Q.; Wang, Y.M.; Qiang, J.B.; Dong, C. Ni-based B–Fe–Ni–Si–Ta bulk metallic glasses designed using cluster line, minor alloying, and element substitutions. Intermetallics 2010, 18, 791–795. [Google Scholar] [CrossRef]
- Siredey-Schwaller, N.; Hamel-Akré, J.; Peltier, L.; Hazotte, A.; Bocher, P. Solidification sequence of Ni-Si-Cr 3wt% B brazing alloys. Weld. World 2017, 61, 1253–1265. [Google Scholar] [CrossRef]
- Thellaputta, G.R.; Chandra, P.S.; Rao, C.S.P. Machinability of Nickel Based Superalloys: A Review. Mater. Today Proc. 2017, 4, 3712–3721. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Effect of Heat Treatment on the Characteristics of Electroless Ni-B, Ni-B-W and Ni-B-Mo Coatings. Mater. Today Proc. 2018, 5, 3306–3315. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Hassan, H.B.; Attyia, A.M. Influence of deposition temperature and heat treatment on the performance of electroless Ni–B films. Surf. Coat. Technol. 2010, 205, 2348–2354. [Google Scholar] [CrossRef]
- Diabb, J.; Juárez-Hernandez, A.; Colas, R.; Castillo, A.G.; García-Sanchez, E.; Hernandez-Rodriguez, M.A.L. Boron influence on wear resistance in nickel-based alloys. Wear 2009, 267, 550–555. [Google Scholar] [CrossRef]
- de Sousa, J.M.S.; Ratusznei, F.; Pereira, M.; de Medeiros Castro, R.; Curi, E.I.M. Abrasion resistance of Ni-Cr-B-Si coating deposited by laser cladding process. Tribol. Int. 2020, 143, 106002. [Google Scholar] [CrossRef]
- Moreau, E.D.; Corbin, S.F. Application of Diffusion Path Analysis to Understand the Mechanisms of Transient Liquid-Phase Bonding in the Ni-Si-B System. Metall. Mater. Trans. A 2019. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).