Effect of Intercritical Annealing on the Microstructure and Mechanical Properties of 0.1C-13Cr-3Ni Martensitic Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results
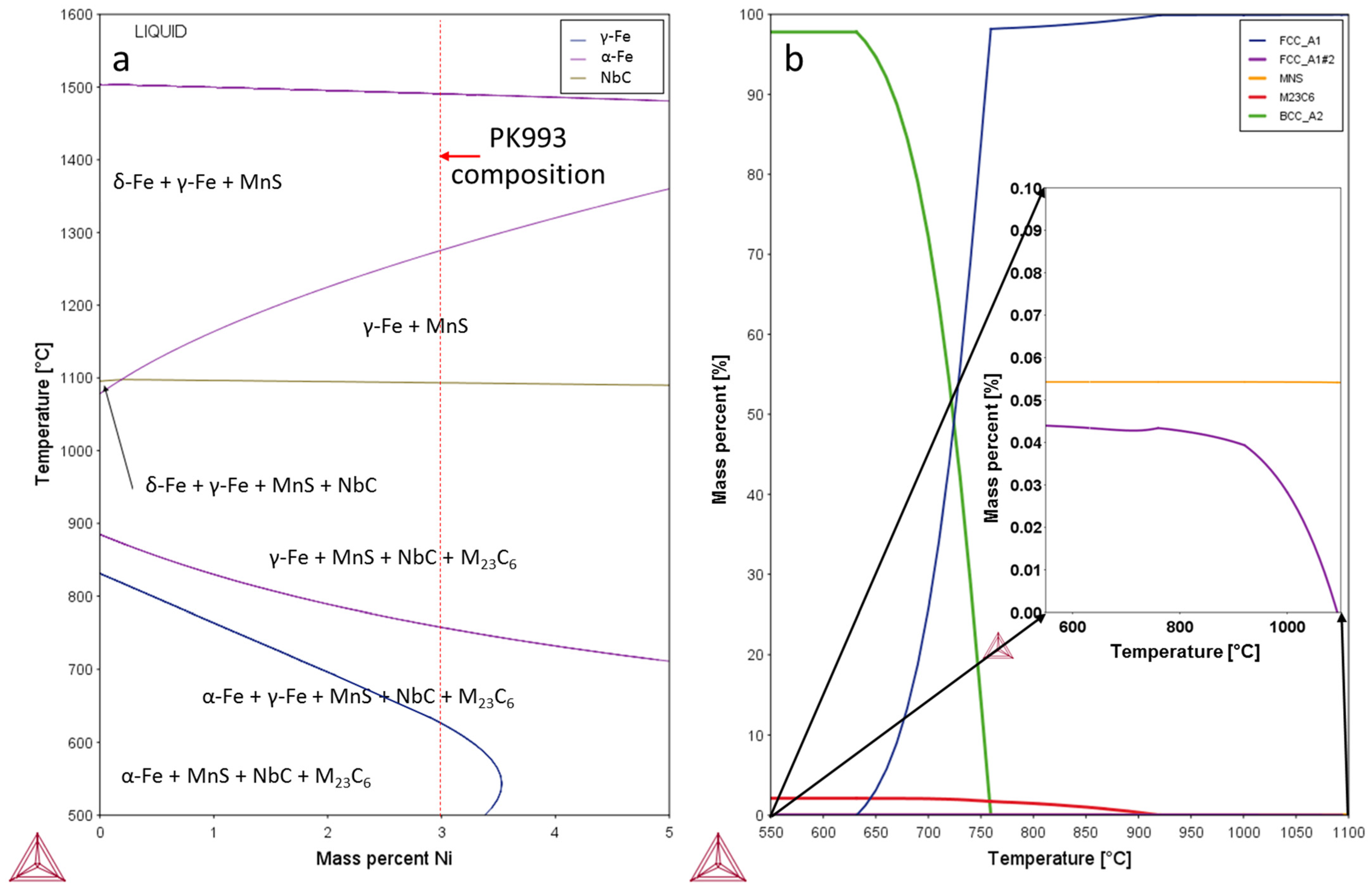
3.1. Thermodynamic Modelling
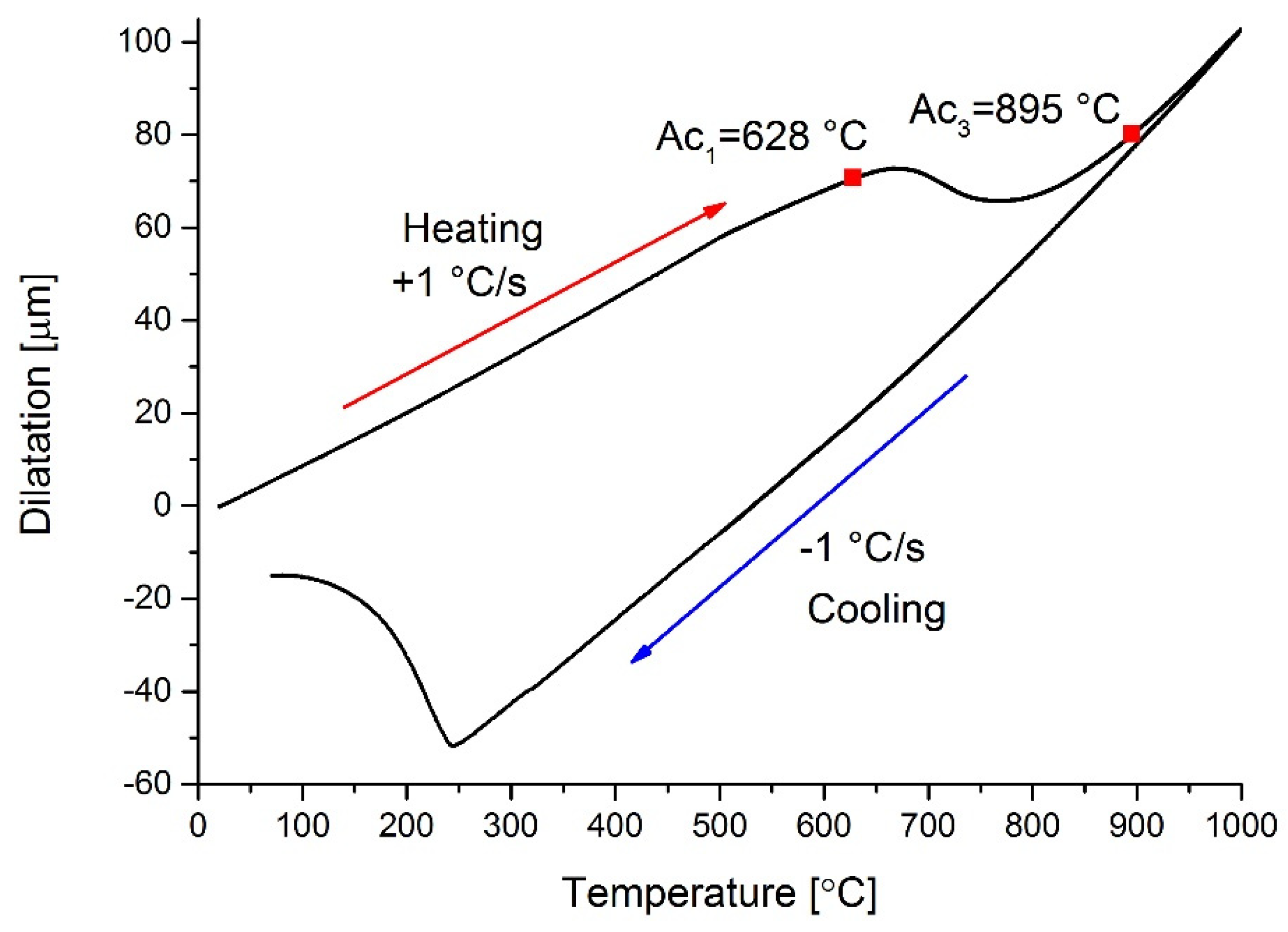
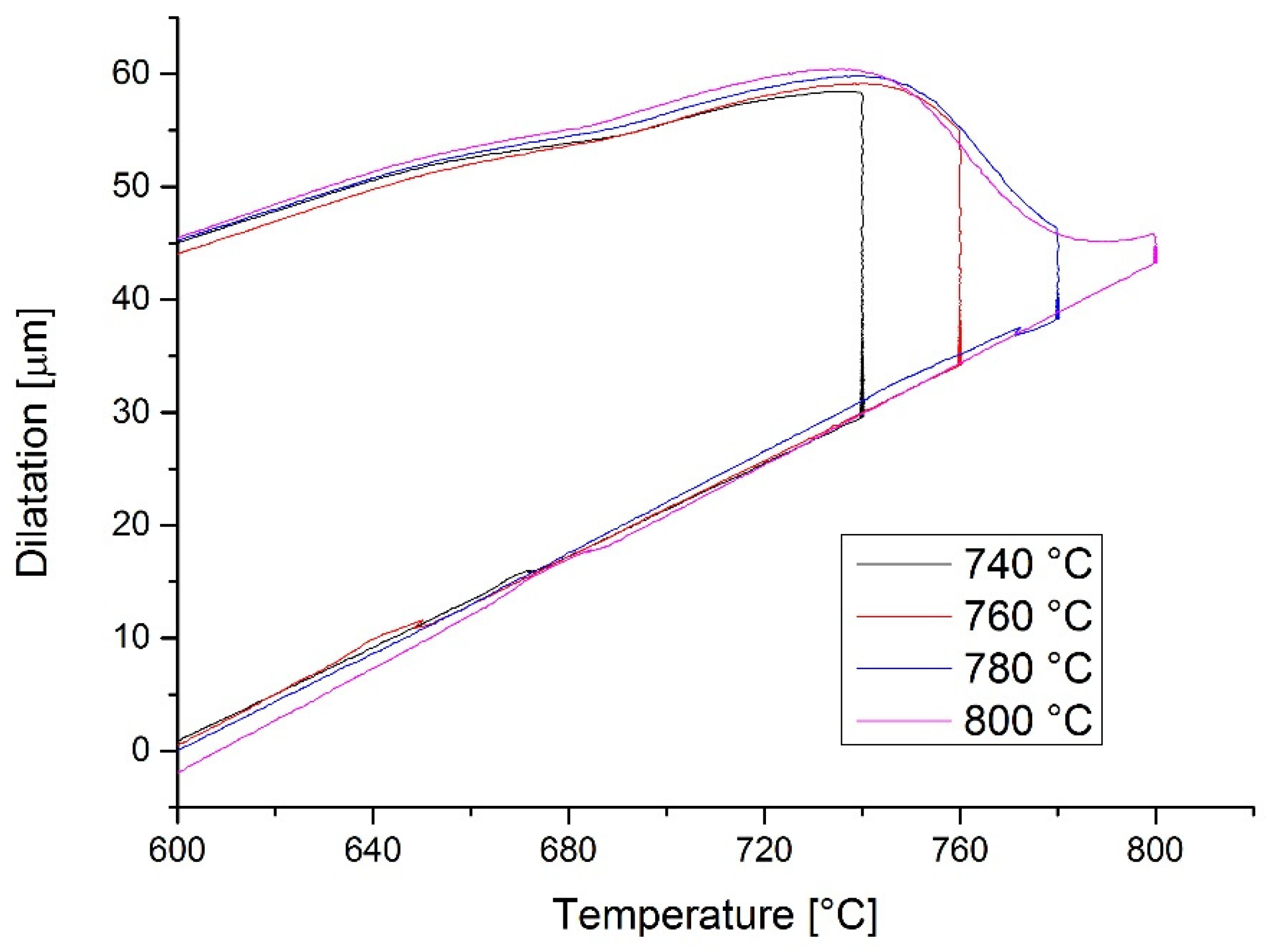
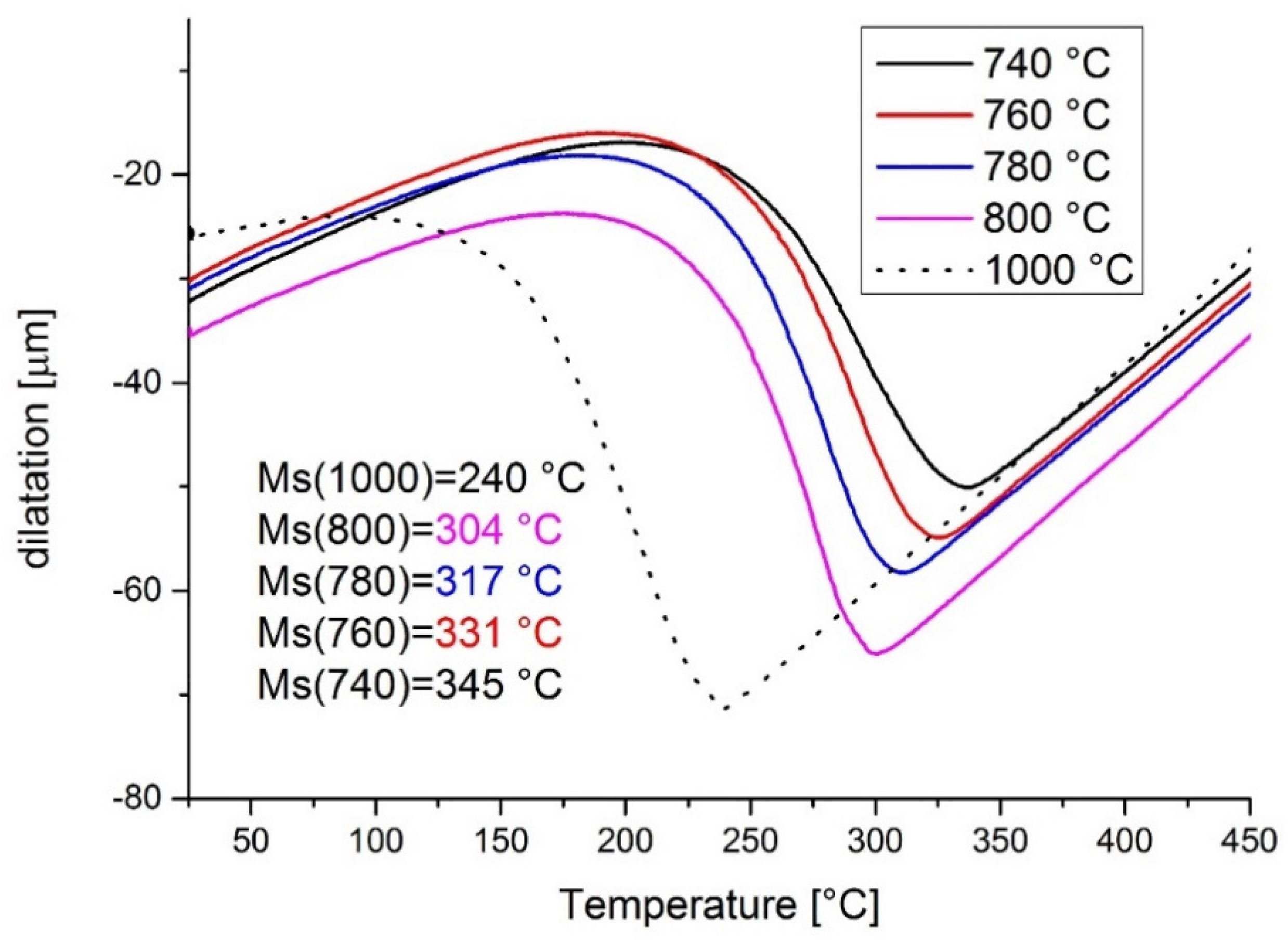
3.2. Dilatometry
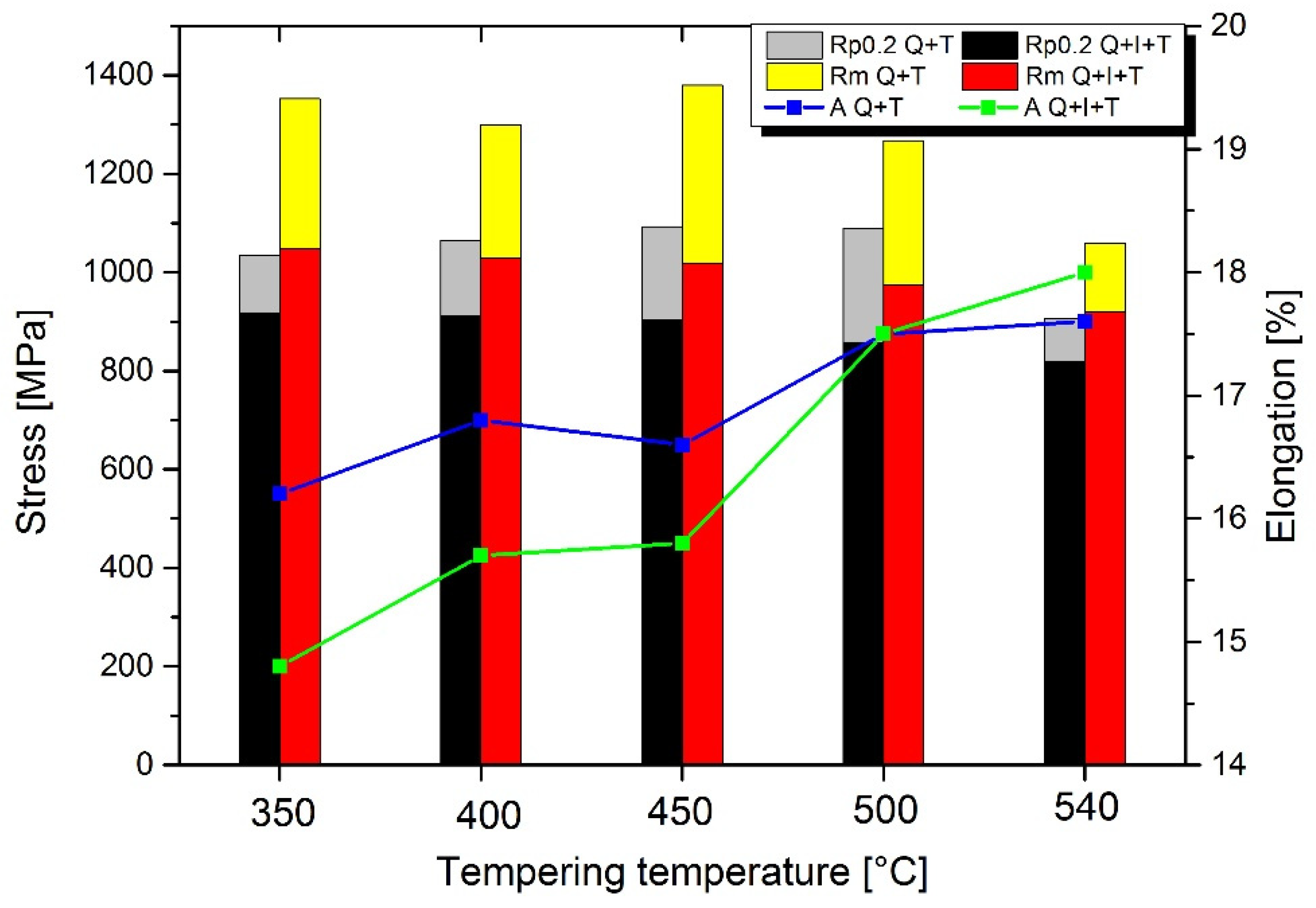
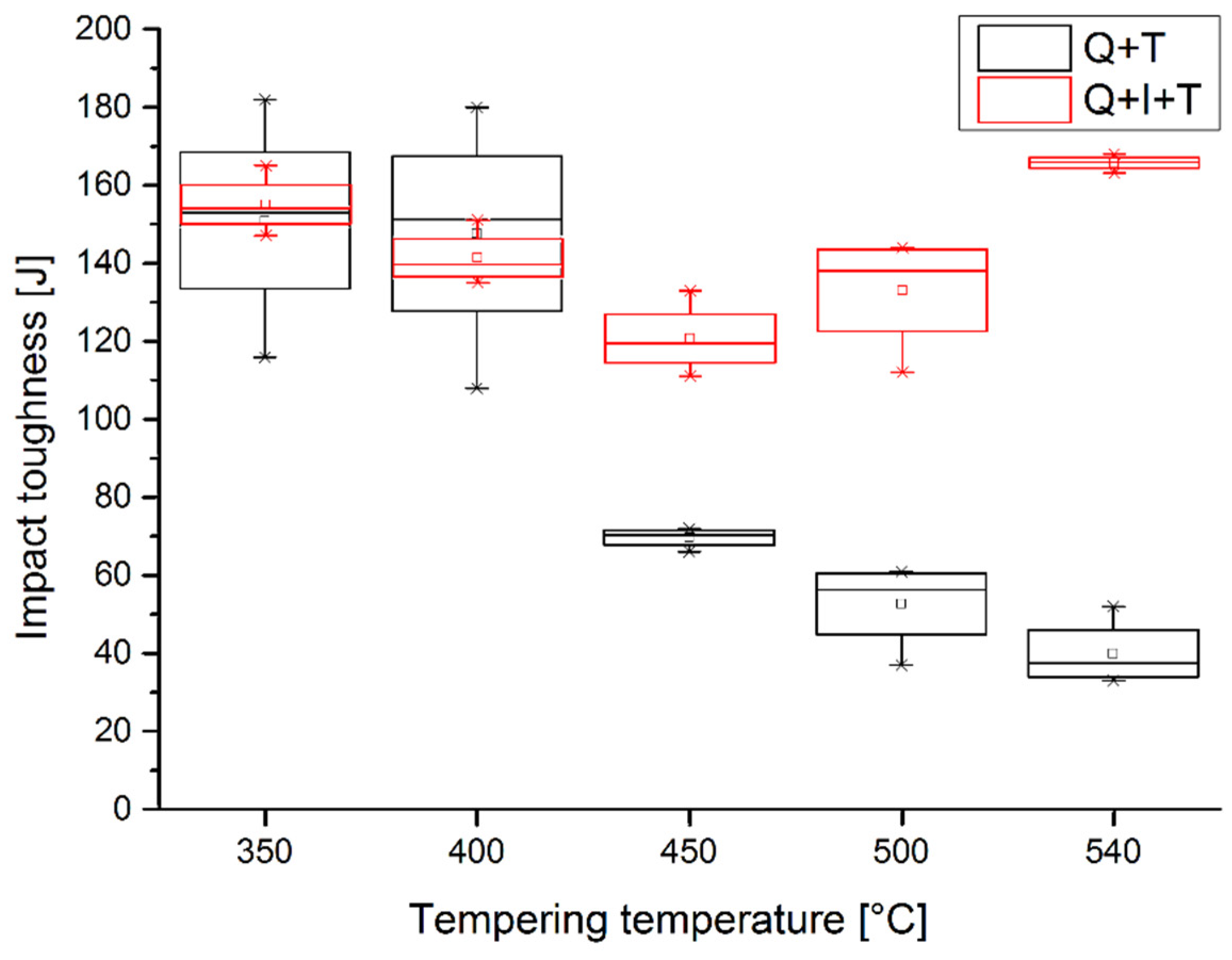
3.3. Mechanical Properties
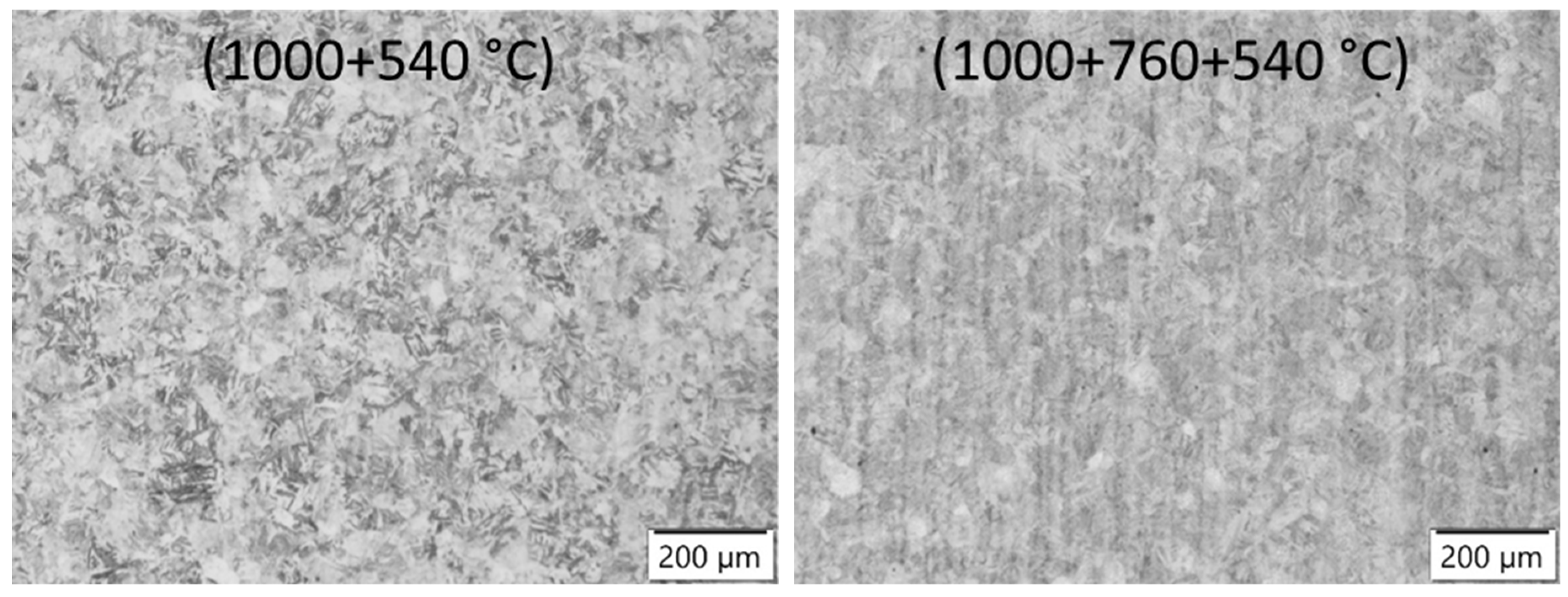
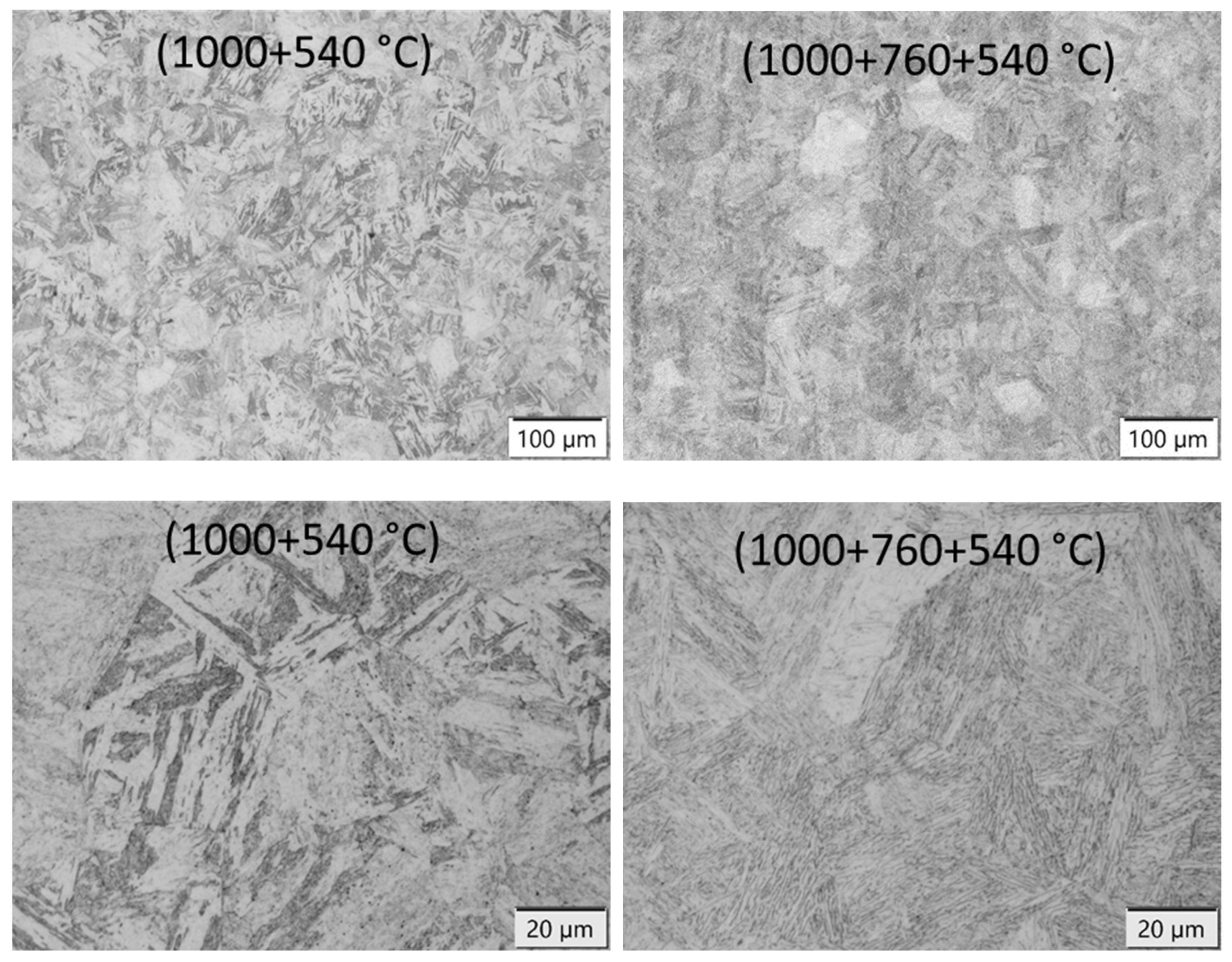
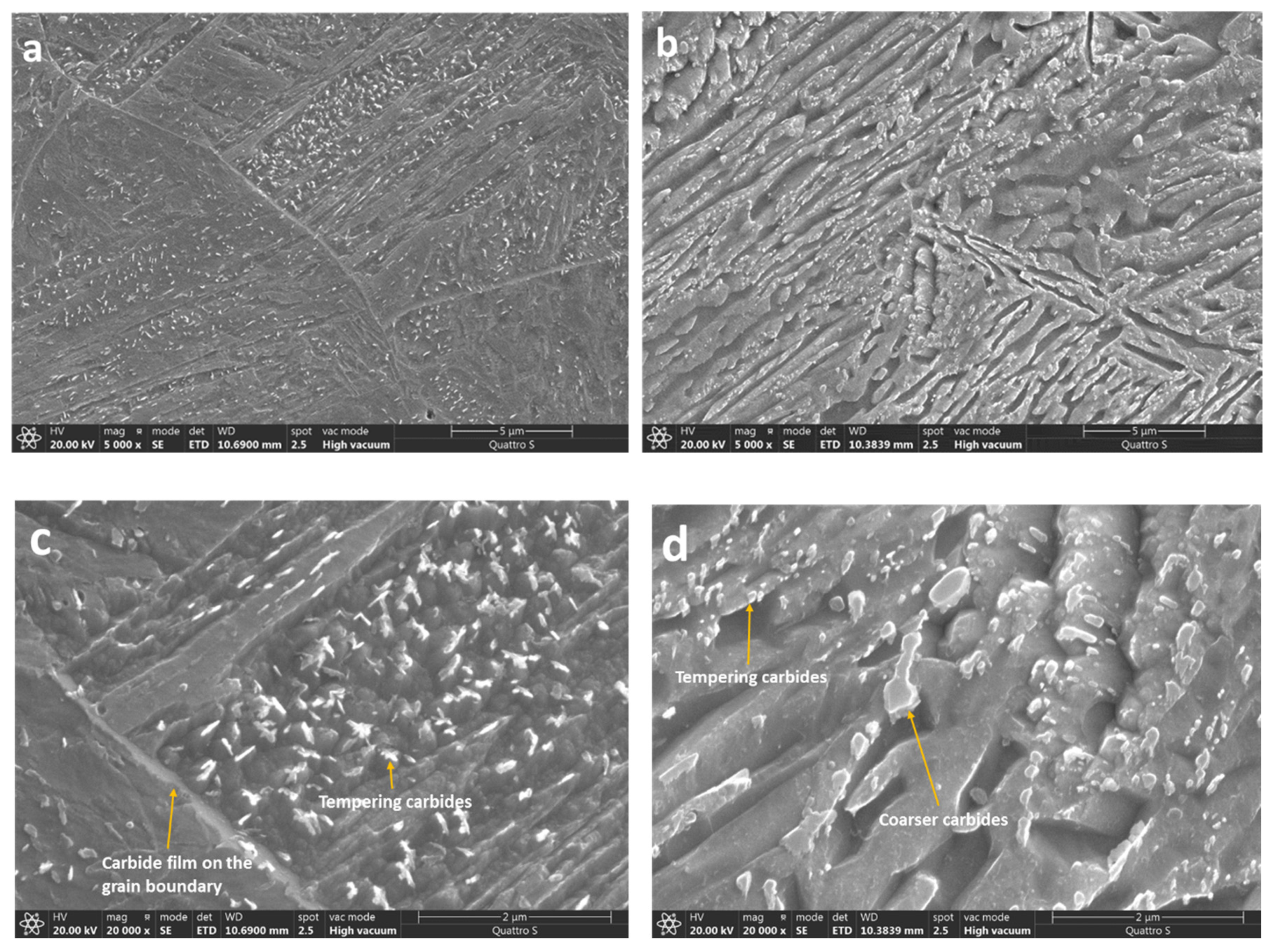
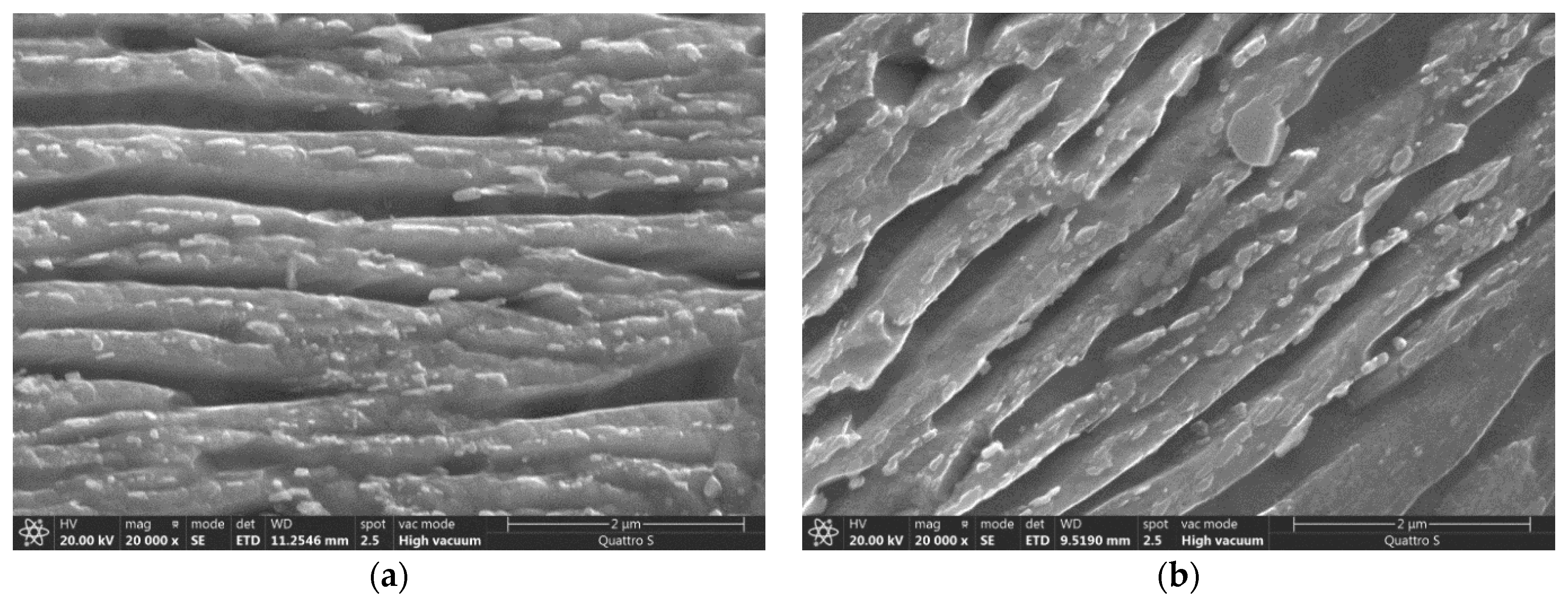
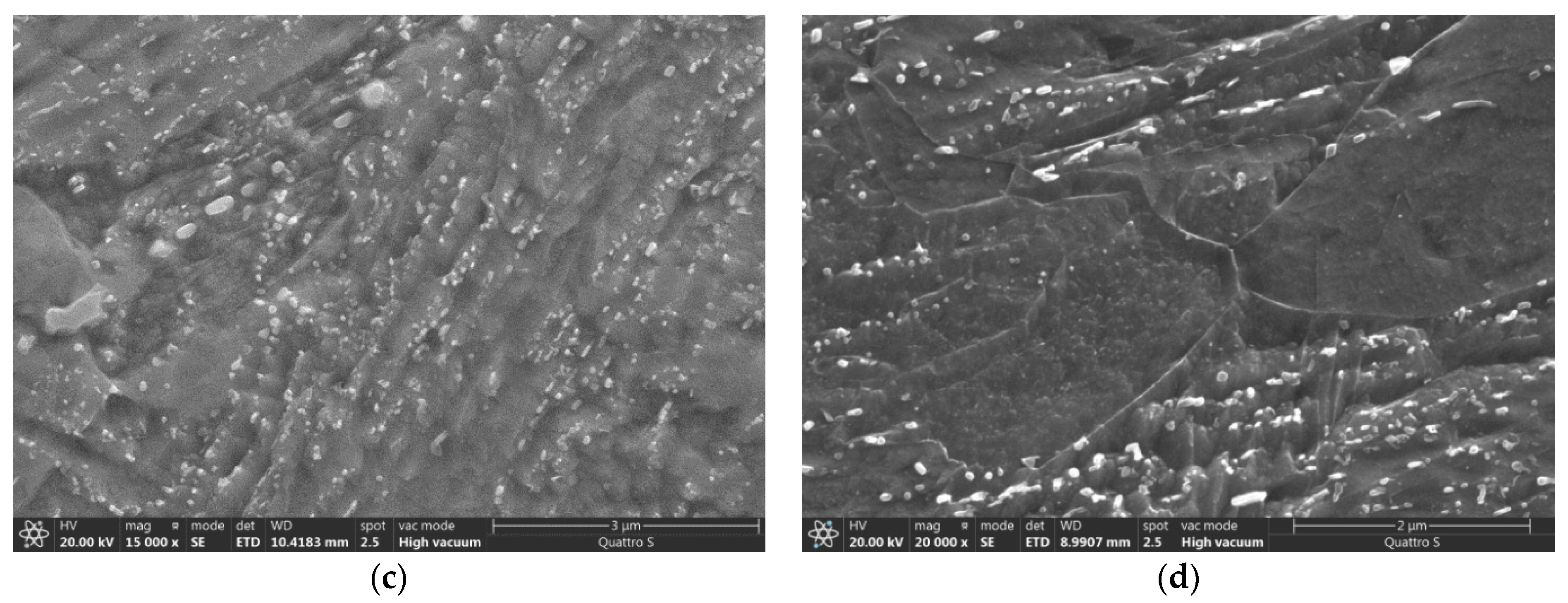
3.4. Microstructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dalmau, A.; Richard, C.; Igual-Muñoz, A. Degradation mechanisms in martensitic stainless steels: Wear, corrosion and tribocorrosion appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Talha, M.; Behera, C.K.; Sinha, O.P. A review on nickel-free nitrogen containing austenitic stainless steels for biomedical applications. Mater. Sci. Eng. C 2013, 33, 3563–3575. [Google Scholar] [CrossRef]
- Baddoo, N.R. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Gupta, R.K.; Birbilis, N. The influence of nanocrystalline structure and processing route on corrosion of stainless steel: A review. Corros. Sci. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Alam, M.K.; Mehdi, M.; Urbanic, R.J.; Edrisy, A. Mechanical behavior of additive manufactured AISI 420 martensitic stainless steel. Mater. Sci. Eng. A 2020, 773, 138815. [Google Scholar] [CrossRef]
- Koležnik, M.; Burja, J.; Šetina Batič, B.; Nagode, A.; Medved, J. De-oxidation of Pk942 steel with Ti and Zr. Mater. Tehnol. 2017, 51, 1031–1036. [Google Scholar] [CrossRef]
- Geng, H.; Wu, X.; Wang, H.; Min, Y. Effects of copper content on the machinability and corrosion resistance of martensitic stainless steel. J. Mater. Sci. 2008, 43, 83–87. [Google Scholar] [CrossRef]
- Al Dawood, M.; El Mahallawi, I.S.; Abd El Azim, M.E.; El Koussy, M.R. Thermal aging of 16Cr-5Ni-1Mo stainless steel Part 2—Mechanical property characterisation. Mater. Sci. Technol. 2004, 20, 370–374. [Google Scholar] [CrossRef]
- Garcia de Andres, C.; Alvarez, L.F.; Lopez, V.; Jimenez, J.A. Effects of carbide-forming elements on the response to thermal treatment of the X45Cr13 martensitic stainless steel. Ann. Oper. Res. 2000, 97, 131–141. [Google Scholar] [CrossRef]
- Al Dawood, M.; El Mahallawi, I.S.; Abd El Azim, M.E.; El Koussy, M.R. Thermal aging of 16Cr-5Ni-1Mo stainless steel Part 1—Microstructural analysis. Mater. Sci. Technol. 2004, 20, 363–369. [Google Scholar] [CrossRef]
- Marshall, A.W.; Farrar, J.C.M. Welding of Ferritic and martensitic 11–14% Cr STEELS—Part 1. Weld. Res. Abroad 2001, 45, 32–55. [Google Scholar]
- Marcuci, J.R.J.; Camilo, C.C.; Di Lorenzo, P.L.; Domingos de Almeida Rollo, J.M. Mathematical modeling of dilatometric behavior of 420A and 440C martensitic stainless steels used in surgical tools. Rev. Bras. Eng. Biomed. 2013, 29, 25–31. [Google Scholar] [CrossRef][Green Version]
- Prieto, G.; Tuckart, W.R.; Ipiña, J.E.P. Influence of a cryogenic treatment on the fracture toughness of an AISI 420 martensitic stainless steel. Mater. Tehnol. 2017, 51, 591–596. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Li, D.; Li, Y. Investigation of the evolution of retained austenite in Fe-13%Cr-4%Ni martensitic stainless steel during intercritical tempering. Mater. Des. 2015, 84, 385–394. [Google Scholar] [CrossRef]
- Ma, X.P.; Wang, L.J.; Qin, B.; Liu, C.M.; Subramanian, S.V. Effect of N on microstructure and mechanical properties of 16Cr5Ni1Mo martensitic stainless steel. Mater. Des. 2012, 34, 74–81. [Google Scholar] [CrossRef]
- Niessen, F.; Grumsen, F.B.; Hald, J.; Somers, M.A.J. Formation and stabilization of reverted austenite in supermartensitic stainless steel. Metall. Res. Technol. 2018, 115, 402. [Google Scholar] [CrossRef]
- Lumney, T.; Morton, I. Nanostructure Control of Materials. In Nanoengineering of Metallic Materials; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Mirzadeh, H. Effects of Grain Size on Mechanical Properties and Work-Hardening Behavior of AISI 304 Austenitic Stainless Steel. Steel Res. Int. 2019, 90, 1900153. [Google Scholar] [CrossRef]
- Bilmes, P.D.; Solari, M.; Llorente, C.L. Characteristics and effects of austenite resulting from tempering of 13Cr-NiMo martensitic steel weld metals. Mater. Charact. 2001, 46, 285–296. [Google Scholar] [CrossRef]
- Karlsen, M.; Hjelen, J.; Grong, Ø.; Rørvik, G.; Chiron, R.; Schubert, U.; Nilsen, E. SEM/EBSD based in situ studies of deformation induced phase transformations in supermartensitic stainless steels. Mater. Sci. Technol. 2008, 24, 64–72. [Google Scholar] [CrossRef]
- LeBrun, T.; Nakamoto, T.; Horikawa, K.; Kobayashi, H. Effect of retained austenite on subsequent thermal processing and resultant mechanical properties of selective laser melted 17-4 PH stainless steel. Mater. Des. 2015, 81, 44–53. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W.; Wilson, E.A.; Grey, R.W. Improving Toughness of PH13-8 Stainless Steel through Intercritical Annealing. ISIJ Int. 2003, 43, 1622–1629. [Google Scholar] [CrossRef]
- Iwabuchi, Y.; Kobayashi, I.; Ohmori, M.; Kikuchi, M. Precipitation and decomposition behaviors of reverted austenite in 13Cr-4Ni stainless steel. Nippon Kinzoku Gakkaishi/J. Jpn. Inst. Met. 1994, 58, 411–417. [Google Scholar] [CrossRef]
- Liu, Y.R.; Ye, D.; Yong, Q.L.; Su, J.; Zhao, K.Y.; Jiang, W. Effect of heat treatment on microstructure and property of Cr13 super martensitic stainless steel. J. Iron Steel Res. Int. 2011, 18, 60–66. [Google Scholar] [CrossRef]
- Nakada, N.; Tsuchiyama, T.; Takaki, S.; Miyano, N. Temperature dependence of austenite nucleation behavior from lath martensite. ISIJ Int. 2011, 51, 299–304. [Google Scholar] [CrossRef]
- Da Silva, G.F.; Tavares, S.S.M.; Pardal, J.M.; Silva, M.R.; De Abreu, H.F.G. Influence of heat treatments on toughness and sensitization of a Ti-alloyed supermartensitic stainless steel. J. Mater. Sci. 2011, 46, 7737–7744. [Google Scholar] [CrossRef]
- Bojack, A.; Zhao, L.; Morris, P.F.; Sietsma, J. In-situ determination of austenite and martensite formation in 13Cr6Ni2Mo supermartensitic stainless steel. Mater. Charact. 2012, 71, 77–86. [Google Scholar] [CrossRef]
- Leem, D.S.; Lee, Y.D.; Jun, J.H.; Choi, C.S. Amount of retained austenite at room temperature after reverse transformation of martensite to austenite in an Fe-13%Cr-7%Ni-3%Si martensitic stainless steel. Scr. Mater. 2001, 45, 767–772. [Google Scholar] [CrossRef]
- Martinek, P.; Hradil, D.; Malina, J. Selection of Materials for Components of Linearstepper Motors for Nuclear Power Plants. Int. J. Mech. Prod. Eng. 2019, 7, 38–43. [Google Scholar]
- Godbole, K.; Das, C.R.; Joardar, J.; Albert, S.K.; Ramji, M.; Panigrahi, B.B. Toughening of AISI 410 Stainless Steel Through Quenching and Partitioning and Effect of Prolonged Aging on Microstructure and Mechanical Properties. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 3377–3383. [Google Scholar] [CrossRef]
- Escobar, J.D.; Oliveira, J.P.; Salvador, C.A.F.; Tschiptschin, A.P.; Mei, P.R.; Ramirez, A.J. Double-step inter-critical tempering of a supermartensitic stainless steel: Evolution of hardness, microstructure and elemental partitioning. Mater. Charact. 2019, 158, 109994. [Google Scholar] [CrossRef]
- Abbasi-Khazaei, B.; Mollaahmadi, A. Rapid Tempering of Martensitic Stainless Steel AISI420: Microstructure, Mechanical and Corrosion Properties. J. Mater. Eng. Perform. 2017, 26, 1626–1633. [Google Scholar] [CrossRef]
- Žužek, B.; Vodopivec, F.; Jenko, M.; Podgornik, B. Effect of creep strain on creep rate in the temperature range 550–640 °C. Mater. Tehnol. 2014, 48, 545–548. [Google Scholar]
- Kaňa, V.; Krutiš, V.; Bořil, P.; Záděra, A.; Rimko, M. Influence of Heat Treatment and Nickel Content on the Properties of the GX4CrNi13-4 Steel. Arch. Metall. Mater. 2021, 66, 37–41. [Google Scholar] [CrossRef]
- Burja, J.; Šuler, B.; Nagode, A. Effect of ageing temperature on reverse austenite content in AISI 630 stainless steel. Materwiss. Werksttech. 2019, 50, 405–411. [Google Scholar] [CrossRef]
- Chakraborty, G.; Das, C.R.; Albert, S.K.; Bhaduri, A.K.; Paul, V.T.; Panneerselvam, G.; Dasgupta, A. Study on tempering behaviour of AISI 410 stainless steel. Mater. Charact. 2015, 100, 81–87. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, Y. Influence of carbides on the high-temperature tempered martensite embrittlement of martensitic heat-resistant steels. Mater. Sci. Eng. A 2016, 670, 256–263. [Google Scholar] [CrossRef]


| C | Si | Mn | P | S | Cr | Ni | Mo | V | Cu |
| 0.12 | 0.24 | 0.4 | 0.012 | 0.002 | 12.9 | 2.93 | 0.14 | 0.03 | 0.09 |
| W | Al | B | Ti | Nb | Co | H (ppm) | As | Sb | Sn |
| 0.04 | 0.009 | 0.00066 | 0.001 | 0.004 | 0.02 | 4.5 | 0.006 | 0.004 | 0.007 |
| No. | Tγ 1 (°C) | TI 2 (°C) | TA 3 (°C) | Sample Type |
|---|---|---|---|---|
| 1 | 1000 | - | 350 | 3xtensile, 3xCharpy |
| 2 | 1000 | - | 400 | 3xtensile, 3xCharpy |
| 3 | 1000 | - | 450 | 3xtensile, 3xCharpy |
| 4 | 1000 | - | 500 | 3xtensile, 3xCharpy |
| 5 | 1000 | - | 540 | 3xtensile, 3xCharpy |
| 6 | 1000 | 760 | 350 | 3xtensile, 3xCharpy |
| 7 | 1000 | 760 | 400 | 3xtensile, 3xCharpy |
| 8 | 1000 | 760 | 450 | 3xtensile, 3xCharpy |
| 9 | 1000 | 760 | 500 | 3xtensile, 3xCharpy |
| 10 | 1000 | 760 | 540 | 3xtensile, 3xCharpy |
| 11 | 1000 | 740 | - | dilatometer |
| 12 | 1000 | 760 | - | dilatometer |
| 13 | 1000 | 780 | - | dilatometer |
| 14 | 1000 | 800 | - | dilatometer |
| 15 | 1000 | - | - | dilatometer |
| T/°C | γ | Fe | C | Si | Mn | Cr | Ni | Mo | V | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 1000 | 100 | 83.23 | 0.12 | 0.24 | 0.4 | 12.9 | 2.93 | 0.14 | 0.030 | 0.009 |
| 800 | 98.45 | 84.18 | 0.036 | 0.24 | 0.40 | 12.07 | 2.97 | 0.07 | 0.020 | 0.009 |
| 780 | 98.32 | 84.28 | 0.029 | 0.24 | 0.40 | 11.98 | 2.98 | 0.06 | 0.019 | 0.009 |
| 760 | 98.21 | 84.36 | 0.022 | 0.24 | 0.40 | 11.91 | 2.98 | 0.05 | 0.018 | 0.009 |
| 740 | 71.01 | 84.19 | 0.018 | 0.24 | 0.48 | 11.51 | 3.50 | 0.04 | 0.014 | 0.007 |
| Tempering (°C) | Q + T | Q + I + T | ||||
|---|---|---|---|---|---|---|
| HB | HRC | HV | HB | HRC | HV | |
| 350 | 410 ± 10 | 41.1 ± 1.1 | 418 ± 12 | 336 ± 9 | 33.1 ± 1.3 | 351 ± 12 |
| 400 | 410 ± 8 | 42.0 ± 0.5 | 420 ± 8 | 333 ± 8 | 32.8 ± 0.9 | 344 ± 10 |
| 450 | 422 ± 8 | 42.7 ± 0.7 | 433 ± 10 | 335 ± 5 | 33.7 ± 0.2 | 346 ± 5 |
| 500 | 418 ± 10 | 41.8 ± 1 | 421 ± 10 | 306 ± 3 | 30.8 ± 0.5 | 318 ± 4 |
| 540 | 324 ± 3 | 33.4 ± 0.3 | 333 ± 5 | 280 ± 2 | 25.7 ± 0.1 | 296 ± 3 |
| Tempering (°C) | Q + T | Q + I + T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Average | Standard Deviation | 1 | 2 | 3 | Average | St. Dev | |
| 350 | 116 | 182 | 155 | 151 | 33.2 | 153 | 147 | 165 | 155 | 9.2 |
| 400 | 155 | 180 | 108 | 147.7 | 36.6 | 151 | 138 | 135 | 141.3 | 8.5 |
| 450 | 66 | 72 | 71 | 69.7 | 3.2 | 133 | 118 | 111 | 120.7 | 11.2 |
| 500 | 60 | 37 | 61 | 52.7 | 13.6 | 143 | 144 | 112 | 133 | 18.2 |
| 540 | 33 | 35 | 52 | 40 | 10.4 | 163 | 166 | 168 | 165.7 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burja, J.; Šuler, B.; Češnjaj, M.; Nagode, A. Effect of Intercritical Annealing on the Microstructure and Mechanical Properties of 0.1C-13Cr-3Ni Martensitic Stainless Steel. Metals 2021, 11, 392. https://doi.org/10.3390/met11030392
Burja J, Šuler B, Češnjaj M, Nagode A. Effect of Intercritical Annealing on the Microstructure and Mechanical Properties of 0.1C-13Cr-3Ni Martensitic Stainless Steel. Metals. 2021; 11(3):392. https://doi.org/10.3390/met11030392
Chicago/Turabian StyleBurja, Jaka, Blaž Šuler, Marko Češnjaj, and Aleš Nagode. 2021. "Effect of Intercritical Annealing on the Microstructure and Mechanical Properties of 0.1C-13Cr-3Ni Martensitic Stainless Steel" Metals 11, no. 3: 392. https://doi.org/10.3390/met11030392
APA StyleBurja, J., Šuler, B., Češnjaj, M., & Nagode, A. (2021). Effect of Intercritical Annealing on the Microstructure and Mechanical Properties of 0.1C-13Cr-3Ni Martensitic Stainless Steel. Metals, 11(3), 392. https://doi.org/10.3390/met11030392








