Experimental Apparent Stern–Geary Coefficients for AZ31B Mg Alloy in Physiological Body Fluids for Accurate Corrosion Rate Determination
Abstract
1. Introduction
2. Materials and Methods
3. Results
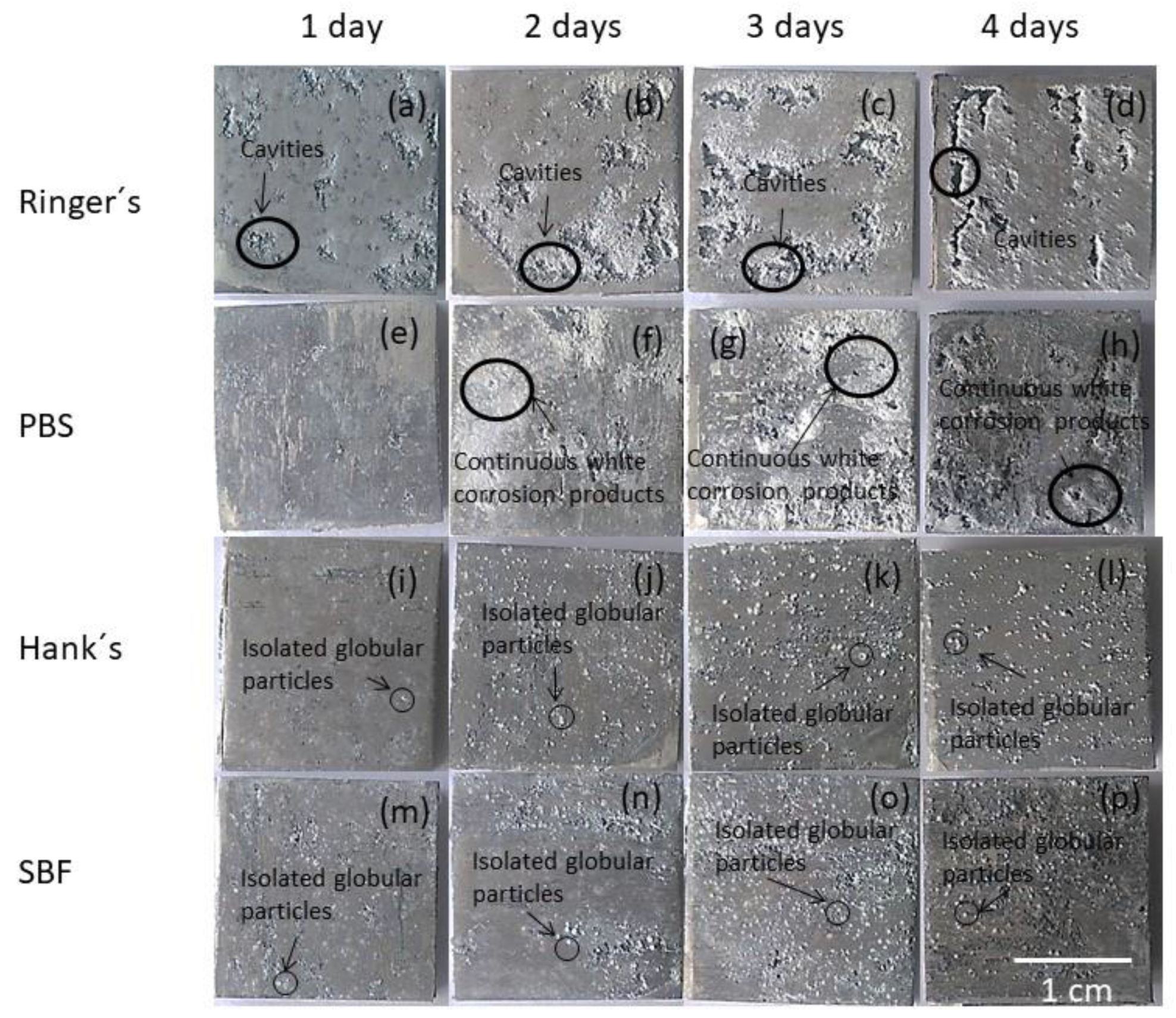
3.1. Macrostructural Surface Analysis
3.2. Weight Loss Measurements
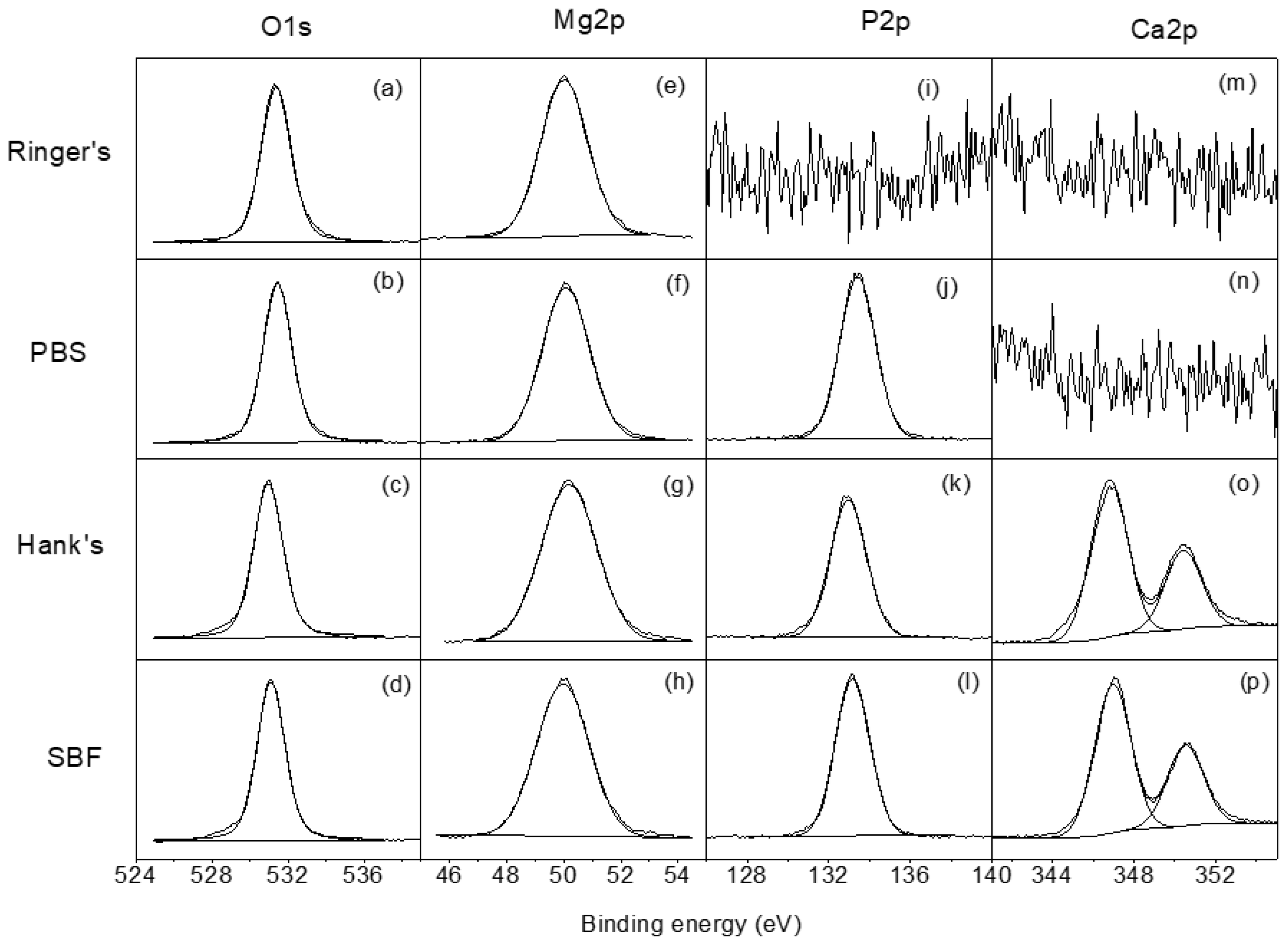
3.3. Differences in the Surface Chemistry of the Corrosion Layers Formed on the AZ31B Alloys after Immersion in Ringer’s, PBS, Hank’s, and SBF Solutions
3.4. Electrochemical Tests
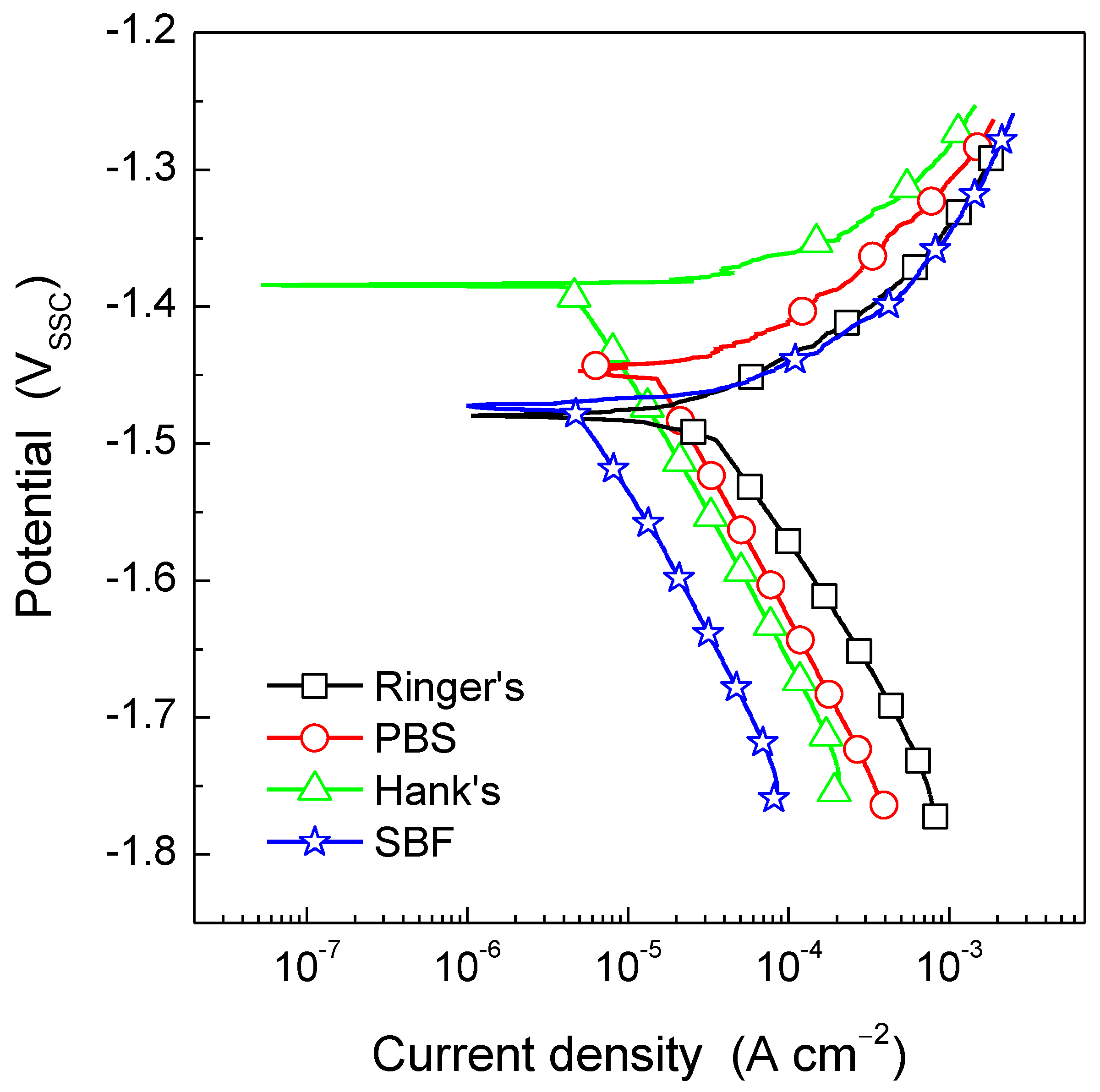
3.4.1. Open Circuit Potential Variation and Potentiodynamic Polarization Testing
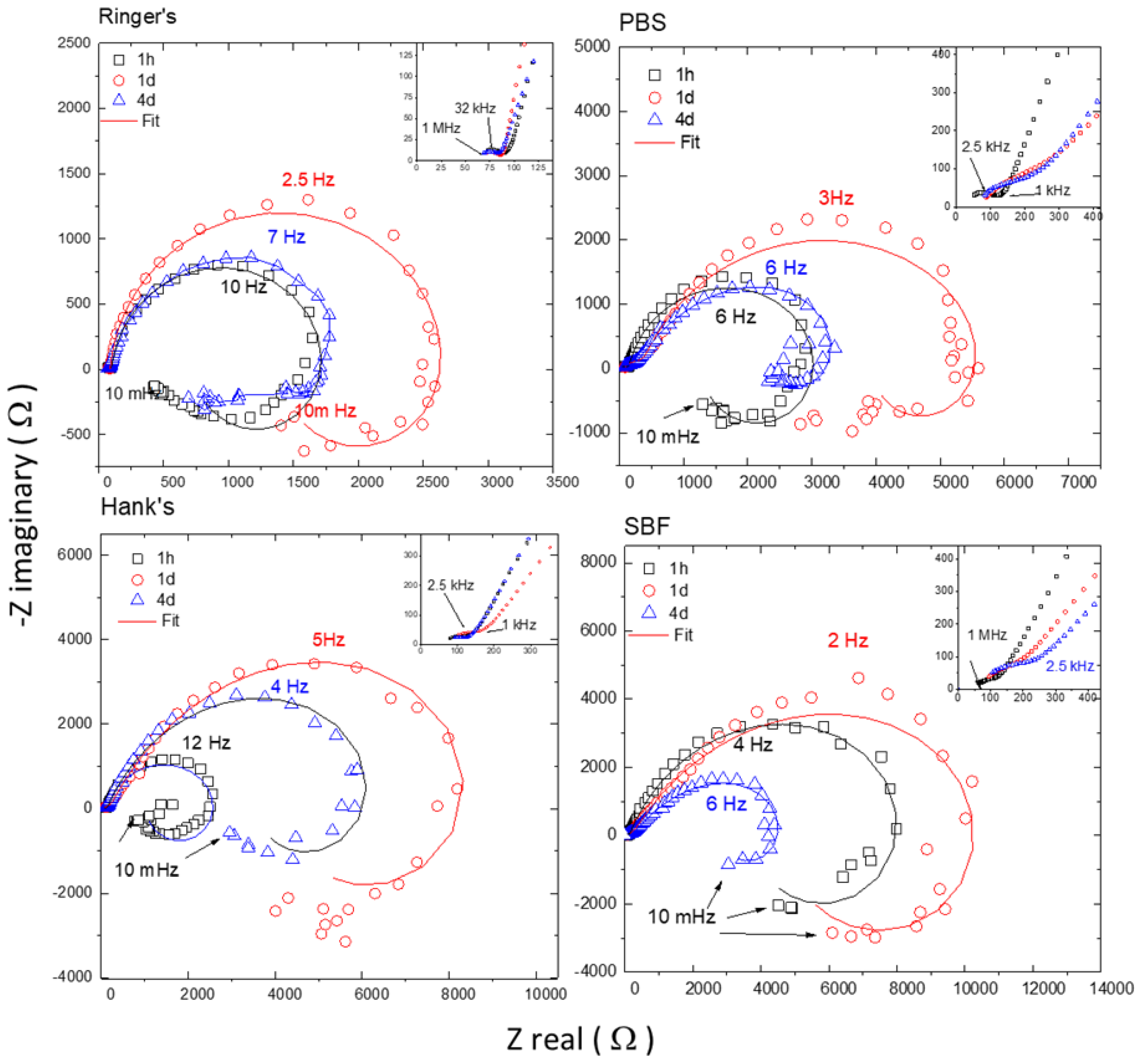
3.4.2. Electrochemical Impedance Spectroscopy (EIS) Testing
4. Discussion
4.1. Relationship between the Surface Chemistry of the Corrosion Layers Formed on Magnesium Alloys and Their Corrosion Resistance in Saline Solutions
4.2. Determination of Apparent Stern–Geary Coefficient (B′) for the Estimation of Corrosion Rate
5. Conclusions
- EIS measurements have been demonstrated to be useful for the quantitative determination of the corrosion rate of AZ31B alloy in SBF’s solutions.
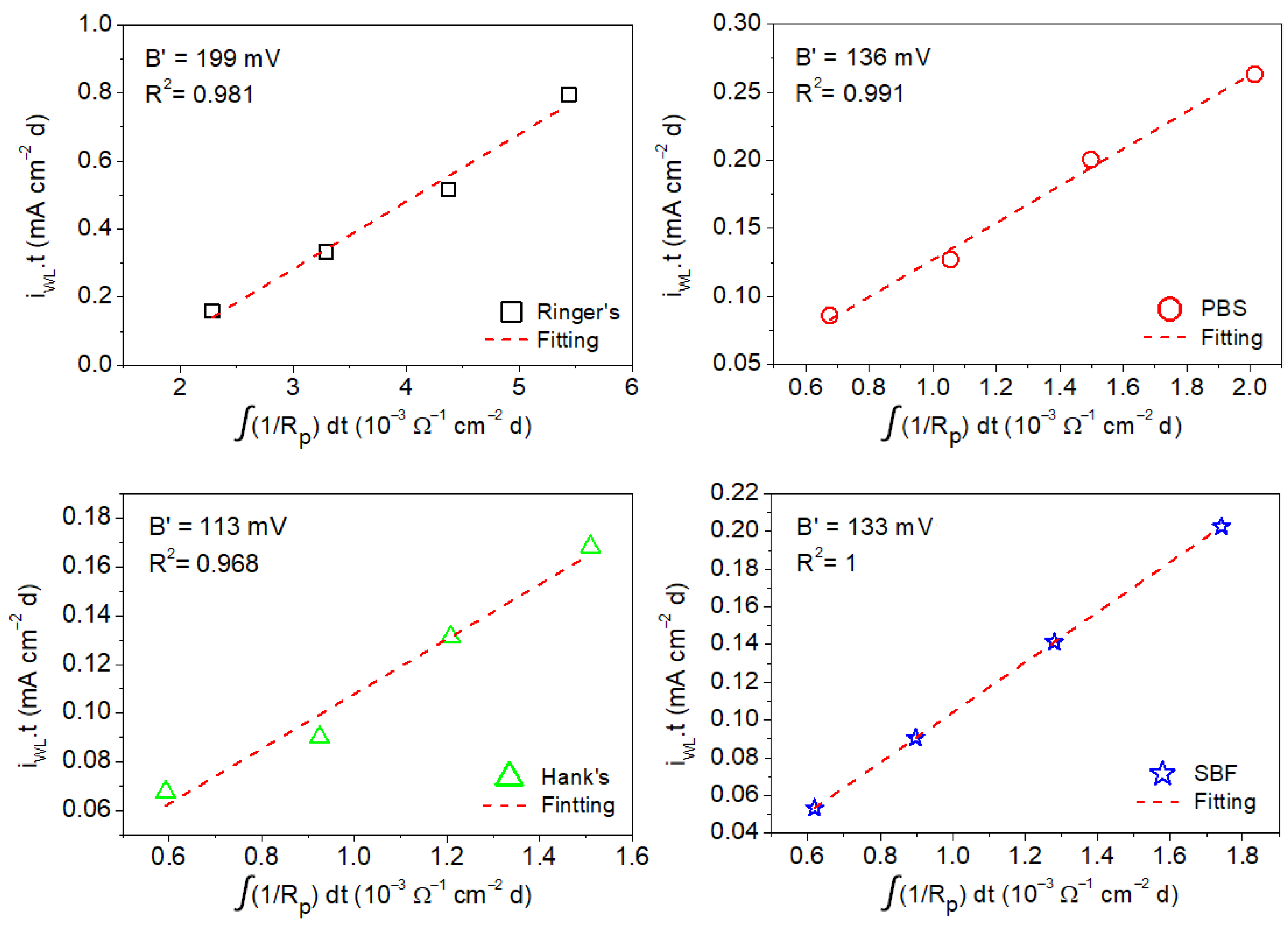
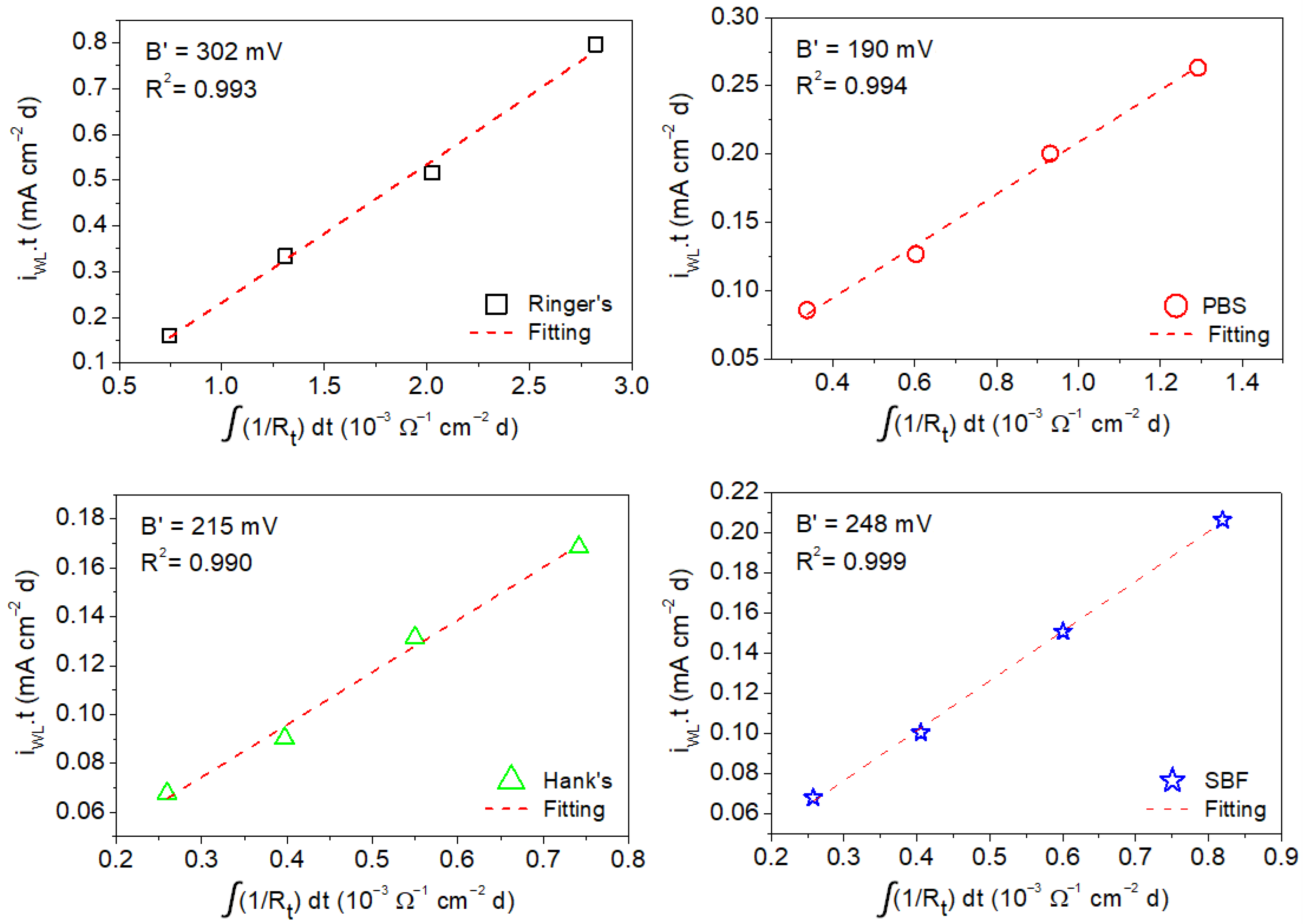
- The apparent Stern–Geary coefficient (B′) was estimated using data obtained via gravimetric experiments and the integration of the reciprocal values of the charge transfer resistance (Rt) and polarization resistance (Rp) obtained from the EIS diagrams. This apparent Stern–Geary coefficient is essential to obtain accurate corrosion rates using EIS measurements.
- Apparent Stern–Geary coefficients determined in Ringer’s, PBS, Hank’s, and SBF solutions at 37 °C after different immersion times as a function of the time-integrated reciprocal of the following: (i) Rp yielded the following B′ values: 199 ± 16 mV (Ringer’s), 136 ± 7 mV (PBS), 113 ± 12 mV (Hank’s), and 133 ± 4 mV (SBF); (ii) Rt yielded the following B′ values: 302 ± 14 mV (Ringer’s), 190 ± 9 mV (PBS), 215 ± 12 mV (Hank’s), and 248 ± 5 mV (SBF).
- All B′ values were significantly higher than the B values obtained from the polarization curves in this study. This is not new. Corrosion rates obtained by numerous researchers from weight loss measurements found values up to 15 times higher than those obtained by electrochemical techniques.
- Analysis of the results showed that if B values obtained from polarization curves are used instead of B′ for icorr determination using Rp in the Stern–Geary equation, the real corrosion rate (as determined by weight loss) is underestimated by between one and two orders of magnitude. Therefore, it is suggested that the B constant used in the Stern–Geary equation cannot be obtained by the direct determination of the Tafel slopes.
- The increase of the Rt values in the impedance spectra observed for Hank’s and SBF solutions compared to those obtained for PBS and Ringer’s solutions tends to reflect the precipitation of hydroxyapatite-like compounds observed by XPS analysis on the surface of the corrosion layer formed during exposure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Xue, F.; Ma, R.; Etim, I.-I.N.; Hao, X.; Dong, J.; Ke, W. Electrochemical noise analysis on the pit corrosion susceptibility of biodegradable AZ31 magnesium alloy in four types of simulated body solutions. J. Mater. Sci. Technol. 2018, 34, 1876–1884. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, E. Biocorrosion behavior of magnesium alloy in different simulated fluids for biomedical application. Mater. Sci. Eng. C 2009, 29, 1691–1696. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Dong, J.; Ke, W. Effect of corrosion product films on the in vitro degradation behavior of Mg-3% Al-1% Zn (in wt %) alloy in hank’s solution. J. Mater. Sci. Technol. 2018, 34, 1756–1764. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.Q.; Chen, C.F.; Gu, Y.H. Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros. Sci. 2015, 91, 7–28. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Zainal Abidin, N.I. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Staiger, M.P.; Feyerabend, F.; Willumeit, R.; Sfeir, C.S.; Zheng, Y.F.; Virtanen, S.; Müeller, W.D.; Atrens, A.; Peuster, M.; Kumta, P.N.; et al. Summary of the panel discussions at the 2nd Symposium on Biodegradable Metals, Maratea, Italy. Mater. Sci. Eng. B 2011, 176, 1596–1599. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Stormer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Curioni, M.; Liu, Z. Correlation between electrochemical impedance measurements and corrosion rates of Mg-1Ca alloy in simulated body fluid. Electrochim. Acta 2018, 264, 101–108. [Google Scholar] [CrossRef]
- Mraied, H.; Wang, W.; Cai, W. Influence of Chemical Heterogeneity and Microstructure on the Corrosion Resistance of Biodegradable WE43 Magnesium Alloys. J. Mater. Chem. B 2019, 7, 6399–6411. [Google Scholar] [CrossRef]
- Khalifeh, S.; Burleigh, T.D. Super-hydrophobic stearic acid layer formed on anodized high purified magnesium for improving corrosion resistance of bioabsorbable implants. J. Magnes. Alloys 2018, 6, 327–336. [Google Scholar] [CrossRef]
- Han, L.; Li, X.; Bai, J.; Xue, F.; Zheng, Y.; Chu, C. Effects of flow velocity and different corrosion media on the in vitro bio-corrosion behaviors of AZ31 magnesium alloy. Mater. Chem. Phys. 2018, 217, 300–307. [Google Scholar] [CrossRef]
- Veleva, L.; Fernandez-Olaya, M.G.; Feliu, S. Initial stages of AZ31B magnesium alloy degradation in Ringer’s solution: Interpretation of EIS, mass loss, hydrogen evolution data and scanning electron microscopy observations. Metals 2018, 8, 933. [Google Scholar] [CrossRef]
- Fajardo, S.; Glover, C.F.; Williams, G.; Frankel, G.S. The Source of Anodic Hydrogen Evolution on Ultra High Purity Magnesium. Electrochim. Acta 2016, 212, 510–521. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.-L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef]
- Bland, L.G.; King, A.D.; Birbilis, N.; Scully, J.R. Assessing the corrosion of commercially pure magnesium and commercial AZ31B by electrochemical impedance, mass-loss, hydrogen collection and inductively coupled plasma optical emission spectrometry solution analysis. Corrosion 2015, 71, 128–145. [Google Scholar] [CrossRef]
- Hsieh, M.K.; Dzombak, D.A.; Vidie, R.D. Bridging gravimetric and electrochemical approaches to determine the corrosion rate of metals and metal alloys in cooling systems: Bench scale evaluation method. Ind. Eng. Chem. Res. 2010, 49, 9117–9123. [Google Scholar] [CrossRef]
- Feliu, S.; Maffiotte, C.; Samaniego, A.; Galvan, J.C.; Barranco, V. Effect of the chemistry and structure of the native oxide surface film on the corrosion properties of commercial AZ31 and AZ61 alloys. Appl. Surf. Sci. 2011, 257, 8558–8568. [Google Scholar] [CrossRef]
- Delgado, M.C.; Garcia-Galvan, F.R.; Barranco, V.; Feliu, S., Jr. A measuring approach to assess the corrosion rate of magnesium alloys using electrochemical impedance measurements. In Magnesium Alloys; Aliofkhazraei, M., Ed.; Intech: Rijeka, Croatia, 2017; pp. 129–159. [Google Scholar]
- Liu, Y. Corrosion Behaviour of Biodegradable Mg-1Ca Alloy in Simulated Body Fluid. Ph.D. Thesis, The University of Manchester, Manchester, UK, March 2018. [Google Scholar]
- Liu, C.L.; Wang, Y.J.; Zeng, R.C.; Zhang, X.M.; Huang, W.J.; Chu, P.K. In Vitro corrosion degradation behaviour of Mg–Ca alloy in the presence of albumin. Corros. Sci. 2010, 52, 3341–3347. [Google Scholar] [CrossRef]
- ISO 16428, Implants for Surgery—Test Solutions and Environmental Conditions for Static and Dynamic Corrosion Tests on Implantable Materials and Medical Devices. 2005. Available online: https://www.iso.org/standard/30280.html (accessed on 9 November 2018).
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- ZView Software, Version 3.1c; Scribner Associates Inc.: Southern Pines, NC, USA, 2007.
- Jang, Y.; Collins, B.; Sankar, J.; Yun, Y. Effect of biologically relevant ions on the corrosion products formed on alloy AZ31B: An improved understanding of magnesium corrosion. Acta Biomater. 2013, 9, 8761–8770. [Google Scholar] [CrossRef] [PubMed]
- Mena-Morcillo, E.; Veleva, L. Degradation of AZ31 and AZ91 magnesium alloys in different physiological media: Effect of surface layer stability on electrochemical behavior. J. Magnes. Alloys 2020, 8, 667–675. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Tian, S.; Jing, Y.; Zhuang, J.; Guo, L.; Jia, D.; Zhou, Y. Hydrothermal fabrication of rGO/apatite layers on AZ31 magnesium alloy for enhanced bonding strength and corrosion resistance. Appl. Surf. Sci. 2019, 470, 430–438. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Li, X.-T.; Li, S.-Q.; Zhang, F.; Han, E.-H. In Vitro degradation of pure Mg in response to glucose. Sci. Rep. 2015, 5, 13026. [Google Scholar] [CrossRef]
- Pacha-Olivenza, M.A.; Galván, J.C.; Porro, J.A.; Lieblich, M.; Díaz, M.; Angulo, I.; Cordovilla, F.; García-Galván, F.R.; Fernández-Calderón, M.C.; González-Martín, M.L.; et al. Efficacy of laser shock processing of biodegradable Mg and Mg-1Zn alloy on their in vitro corrosion and bacterial response. Surf. Coat. Technol. 2020, 384, 125320. [Google Scholar] [CrossRef]
- Baril, G.; Pebere, N. The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions. Corros. Sci. 2001, 43, 471–484. [Google Scholar] [CrossRef]
- Curioni, M.; Salamone, L.; Scenini, F.; Santamaria, M. A mathematical description accounting for the superfluous hydrogen evolution and the inductive behaviour observed during electrochemical measurements on magnesium. Electrochim. Acta 2018, 274, 343–352. [Google Scholar] [CrossRef]
- Yuwono, J.A.; Taylor, C.D.; Frankel, G.S.; Birbilis, N.; Fajardo, S. Understanding the enhanced rates of hydrogen evolution on dissolving magnesium. Electrochem. Commun. 2019, 104, 106482. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.K.; Singh, R. Exploring environment friendly nickel electrodeposition on AZ91 magnesium alloy: Effect of prior surface treatments and temperature of the bath on corrosion behavior. Corros. Sci. 2019, 151, 1–19. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Narayanan, T.S.N.S. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution—Long term evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The apparent constant-phase-element behavior of a disk electrode with faradaic reactions: A global and local impedance analysis. J. Electrochem. Soc. 2007, 154, C99–C107. [Google Scholar] [CrossRef]
- Harrington, S.P.; Wang, F.; Devine, T.M. The structure and electronic properties of passive and prepassive films of iron in borate buffer. Electrochim. Acta 2010, 55, 4092–4102. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfa. Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébere, N.; Tribollet, B.; Vivier, V. An impedance investigation of the mechanism of pure magnesium corrosion in sodium sulfate solutions. J. Electrochem. Soc. 2007, 154, C108–C113. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Feliu, S. Influence of Microstructure and Composition on the Corrosion Behaviour of Mg/Al Alloys in Chloride Media. Electrochim. Acta 2008, 53, 7890–7902. [Google Scholar] [CrossRef]
- Santamaria, M.; di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef]
- Cui, Z.; Li, X.; Xiao, K.; Dong, C. Atmospheric corrosion of field-exposed AZ31 magnesium in a tropical marine environment. Corros. Sci. 2013, 76, 243–256. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, Y.; Lv, J.; Wang, X.; Li, J.; Wang, F. Transition of interface oxide layer from porous Mg(OH)2 to dense MgO induced by polyaniline and corrosion resistance of Mg alloy. Appl. Surf. Sci. 2015, 328, 247–254. [Google Scholar] [CrossRef]
- Feliu, S.; Veleva, L.; García-Galvan, F. Effect of Temperature on the Corrosion Behavior of Biodegradable AZ31B Magnesium Alloy in Ringer’s Physiological Solution. Metals 2019, 9, 591. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hiromoto, S. Effect of inorganic salts, amino acids and proteins on the degradation of pure magnesium in vitro. Mater. Sci. Eng. C 2009, 29, 1559–1568. [Google Scholar] [CrossRef]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, D.B.; Gopalakrishnan, P.; Ravi, K.R. Morphological studies on the development of chemical conversion coating on surface of Mg-4Zn alloy and its corrosion and bio mineralisation behaviour in simulated body fluid. J. Alloys Compd. 2020, 812, 152146. [Google Scholar] [CrossRef]
- Miao, H.; Huang, H.; Shi, Y.; Zhang, H.; Pei, J.; Yuan, G. Effects of solution treatment before extrusion on the microstructure, mechanical properties and corrosion of Mg-Zn-Gd alloy in vitro. Corros. Sci. 2017, 122, 90–99. [Google Scholar] [CrossRef]
- Pinto, R.; Ferreira, M.G.S.; Carmezim, M.J.; Montemor, M.F. Passive behavior of magnesium alloys (Mg–Zr) containing rare-earth elements in alkaline media. Electrochim. Acta 2010, 55, 2482–2489. [Google Scholar] [CrossRef]
- Zidoune, M.; Grosjean, M.H.; Roué, L.; Huot, J.; Schulz, R. Comparative study on the corrosion behavior of milled and unmilled magnesium by electrochemical impedance spectroscopy. Corros. Sci. 2004, 46, 3041–3055. [Google Scholar] [CrossRef]
- Hu, J.; Huang, D.; Zhang, G.; Song, G.L.; Guo, X. Research on the inhibition mechanism of tetraphenylporphyrin on AZ91D magnesium alloy. Corros. Sci. 2012, 63, 367–378. [Google Scholar] [CrossRef]
- Huang, D.; Hu, J.; Song, G.L.; Guo, X. Inhibition effect of inorganic and organic inhibitors on the corrosion of Mg–10Gd–3Y–0.5Zr alloy in an ethylene glycol solution at ambient and elevated temperatures. Electrochim. Acta 2011, 56, 10166–10178. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, F.; Song, G.-L.; Liu, M.; Atrens, A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with Mg(OH)2 of as-cast and solution heat-treated binary Mg–RE alloys: RE = Ce, La, Nd, Y, Gd. Corros. Sci. 2013, 76, 98–118. [Google Scholar] [CrossRef]
- Cao, F.Y.; Shi, Z.M.; Song, G.L.; Liu, M.; Atrens, A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with Mg(OH)2 of as-cast and solution heat-treated binary Mg-X alloys: X = Mn, Sn, Ca, Zn, Al, Zr, Si, Sr. Corros. Sci. 2013, 76, 60–97. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; Huang, G.; Liu, K.; Jiang, B.; Wang, G.; Xia, D.; Atrens, A.; Pan, F. Influence of microalloying with Ca and Ce on the corrosion behavior of extruded Mg-3Al-1Zn. J. Electrochem. Soc. 2019, 166, C445–C453. [Google Scholar] [CrossRef]
- Zhong, L.P.; Wang, Y.J.; Lu, H.; Cui, X.J.; Zhang, Y.J.; Dou, B.J.; Peng, J. Influence of aging prior to extrusion on the microstructure and corrosion resistance of Mg-8Sn-2Zn-0.2Mn alloy. J. Alloys Compd. 2019, 780, 783–791. [Google Scholar] [CrossRef]
- Silva, C.L.P.; Oliveira, A.C.; Costa, C.G.F.; Figueiredo, R.B.; de Fátima Leite, M.; Pereira, M.M.; Lins, V.F.C.; Langdon, T.G. Effect of severe plastic deformation on the biocompatibility and corrosion rate of pure magnesium. J. Mater. Sci. 2017, 52, 5992–6003. [Google Scholar] [CrossRef]
- Gu, M.Y.; Wei, G.L.; Liu, W.C.; Wu, G.H. Influence of neodymium on microstructure and corrosion behavior of Mg-8Li-3Al-2Zn alloy. Mater. Corros. 2017, 68, 436–443. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, C.; Feng, Y.; Wang, R.; Wang, N. Enhancement of discharge properties of an extruded Mg-Al-Pb anode for seawater-activated battery by lanthanum addition. J. Alloys Compd. 2017, 721, 392–404. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H.; He, Y.; Wang, X. Comparison of corrosion behavior of Mg–1.5Zn–0.6Zr and AZ91D alloys in a NaCl solution. Mater. Corros. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, C.; Xu, S.; Gao, Y.; Jiang, S. Accelerated degradation rate of AZ31 magnesium alloy by copper additions. Mater. Corros. 2018, 69, 760–769. [Google Scholar] [CrossRef]
- Dinodi, N.; Nityananda, S. Alkyl carboxylates as efficient and green inhibitors of magnesium alloy ZE41 corrosion in aqueous salt solution. Corros. Sci. 2014, 85, 411–427. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, W.J. The improvement of corrosion resistance of AZ91 magnesium alloy through development of dense and tight network structure of Al-rich α phase by addition of a trace amount of Ti. J. Alloys Compd. 2017, 696, 736–745. [Google Scholar] [CrossRef]
- Duffó, G.S.; Farina, S.B.; Rodríguez, F.M. Corrosion behaviour of non-ferrous metals embedded in mortar. Constr. Build. Mater. 2019, 210, 548–554. [Google Scholar] [CrossRef]
- Pardo, A.; Feliu, S., Jr.; Merino, M.C.; Arrabal, R.; Matykina, E. Electrochemical estimation of the corrosion rate of Magnesium/Aluminium alloys. Int. J. Corros. 2010, 2010, 953850. [Google Scholar] [CrossRef]



| Element | Mg | Al | Zn | Mn |
|---|---|---|---|---|
| wt.% | 95.8 | 3.0 | 1.0 | 0.2 |
| NaCl | KCl | KH2PO4 | Na2HPO4·12H2O | MgSO4·7H2O | CaCl2 | NaHCO3 | MgCl2·6H2O | C6H12O6 | |
|---|---|---|---|---|---|---|---|---|---|
| Ringer’s (g/L) [27] | 8.36 | 0.3 | - | - | - | 0.15 | - | - | - |
| PBS (g/L) [2] | 8.0 | 0.2 | 0.2 | 2.89 | - | - | - | - | - |
| Hank’s (g/l) [2] | 8.0 | 0.4 | 0.06 | 0.06 | 0.06 | 0.14 | 0.35 | 0.1 | 1.0 |
| SBF (g/L) [2] | 8.0 | 0.4 | 0.06 | 0.12 | 0.2 | 0.14 | 0.35 | – | – |
| Electrolyte Solution | O | Mg | P | Ca | Ca/P |
|---|---|---|---|---|---|
| Ringer’s | 75 | 25 | 0 | 0 | - |
| PBS | 66 | 19 | 15 | 0 | 0 |
| Hank’s | 56 | 10 | 13 | 21 | 1.6 |
| SBF | 57 | 8 | 14 | 21 | 1.5 |
| Solution Time | OCP (VSCC) | ||||
|---|---|---|---|---|---|
| 1 h | 1 Day | 2 Days | 3 Days | 4 Days | |
| Ringer’s | −1.533 | −1.505 | −1.506 | −1.494 | −1.502 |
| PBS | −1.534 | −1.494 | −1.502 | −1.495 | −1.494 |
| Hank’s | −1.526 | −1.514 | −1.501 | −1.508 | −1.507 |
| SBF | −1.522 | −1.506 | −1.495 | −1.501 | −1.507 |
| Ringer’s | PBS | Hank’s | SBF | |
|---|---|---|---|---|
| Ecorr (VSCC) | −1.48 | −1.45 | −1.38 | −1.47 |
| icorr (µA cm−2) | 37 | 18 | 4.3 | 5.1 |
| βa (mV/dec) | 68.1 | 73.7 | 33.5 | 60.2 |
| βc (mV/dec) | 173.9 | 216.7 | 204.2 | 195.9 |
| B (mV/dec) | 21.3 | 23.9 | 12.5 | 20.0 |
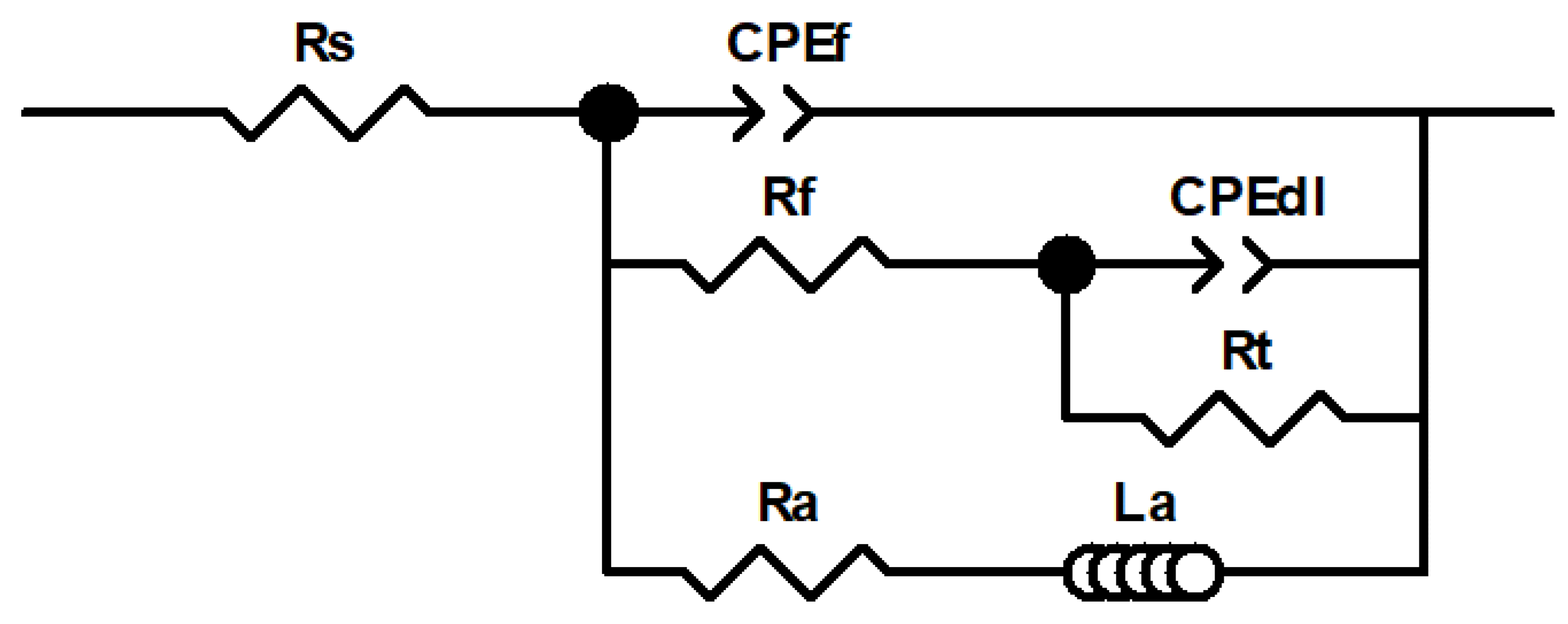
| Electrolyte | Rs | CPEf | Cefff | n1 | Rf | CPEdl | Ceffdl | n2 | Rt | Ra | La | Rp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | (Ω) | (µS cm−2 sn1) | (µF cm−2) | (Ω cm2) | (µS cm−2 sn2) | (µF cm−2) | (Ω cm2) | (Ω cm2) | (H cm2) | (Ω cm2) | ||
| Ringer’s | ||||||||||||
| 1 h | 52 | 6.41 | 0.043 | 0.64 | 36 | 14.10 | 8.87 | 0.94 | 1309 | 854 | 391 | 522 |
| 1 d | 59 | 3.97 | 0.080 | 0.71 | 25 | 17.90 | 13.44 | 0.96 | 2072 | 2115 | 3090 | 1053 |
| 4 d | 58 | 8.46 | 0.133 | 0.68 | 25 | 21.80 | 8.00 | 0.87 | 1782 | 3595 | 345 | 1203 |
| PBS | ||||||||||||
| 1 h | 13 | 2.00 | 0.013 | 0.68 | 102 | 18.60 | 12.00 | 0.95 | 2324 | 1569 | 1164 | 953 |
| 1 d | 74 | 11.90 | 0.141 | 0.62 | 337 | 14.50 | 3.93 | 0.84 | 4242 | 7725 | 18,293 | 2875 |
| 4 d | 55 | 8.72 | 0.046 | 0.60 | 214 | 19.20 | 1.94 | 0.75 | 2890 | 4955 | 1117 | 1908 |
| Hank’s | ||||||||||||
| 1 h | 53 | 7.31 | 0.044 | 0.62 | 87 | 11.00 | 2.94 | 0.85 | 2320 | 1434 | 370 | 899 |
| 1 d | 69 | 2.31 | 0.029 | 0.68 | 108 | 13.30 | 1.14 | 0.74 | 7719 | 2402 | 3294 | 1838 |
| 4 d | 71 | 2.69 | 0.049 | 0.70 | 61 | 17.60 | 2.97 | 0.79 | 5717 | 2492 | 4133 | 1741 |
| SBF | ||||||||||||
| 1 h | 75 | 2.46 | 0.085 | 0.73 | 112 | 8.59 | 1.37 | 0.80 | 8535 | 4507 | 5394 | 2963 |
| 1 d | 73 | 0.27 | 0.026 | 0.83 | 116 | 13.70 | 0.71 | 0.70 | 11,310 | 7800 | 7791 | 4636 |
| 4 d | 84 | 0.18 | 0.037 | 0.88 | 118 | 22.90 | 0.90 | 0.66 | 4524 | 3323 | 1846 | 1937 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Galvan, F.R.; Fajardo, S.; Barranco, V.; Feliu, S., Jr. Experimental Apparent Stern–Geary Coefficients for AZ31B Mg Alloy in Physiological Body Fluids for Accurate Corrosion Rate Determination. Metals 2021, 11, 391. https://doi.org/10.3390/met11030391
García-Galvan FR, Fajardo S, Barranco V, Feliu S Jr. Experimental Apparent Stern–Geary Coefficients for AZ31B Mg Alloy in Physiological Body Fluids for Accurate Corrosion Rate Determination. Metals. 2021; 11(3):391. https://doi.org/10.3390/met11030391
Chicago/Turabian StyleGarcía-Galvan, Federico R., Santiago Fajardo, Violeta Barranco, and Sebastián Feliu, Jr. 2021. "Experimental Apparent Stern–Geary Coefficients for AZ31B Mg Alloy in Physiological Body Fluids for Accurate Corrosion Rate Determination" Metals 11, no. 3: 391. https://doi.org/10.3390/met11030391
APA StyleGarcía-Galvan, F. R., Fajardo, S., Barranco, V., & Feliu, S., Jr. (2021). Experimental Apparent Stern–Geary Coefficients for AZ31B Mg Alloy in Physiological Body Fluids for Accurate Corrosion Rate Determination. Metals, 11(3), 391. https://doi.org/10.3390/met11030391








