Corrosion Behavior and Osteogenic Activity of a Biodegradable Orthopedic Implant Mg–Si Alloy with a Gradient Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Bio-Corrosion Tests
2.3.1. Electrochemical Measurements
2.3.2. Immersion Test
2.4. Biological Test
2.4.1. Cytotoxicity Test
2.4.2. Osteogenesis Test
3. Results
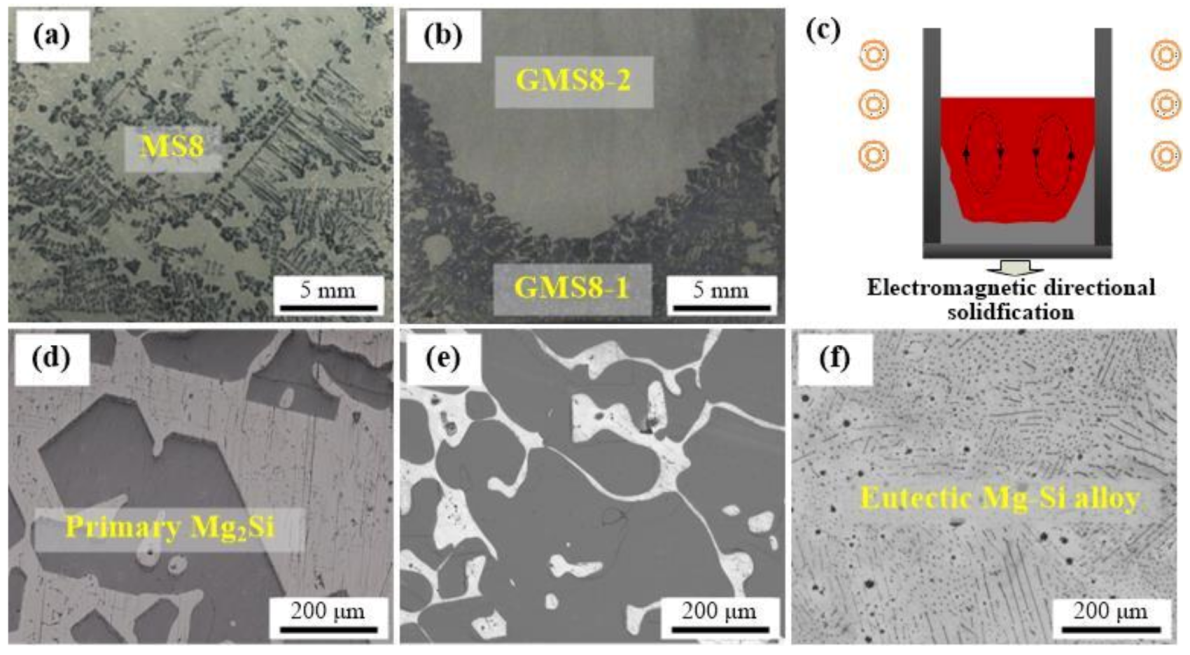
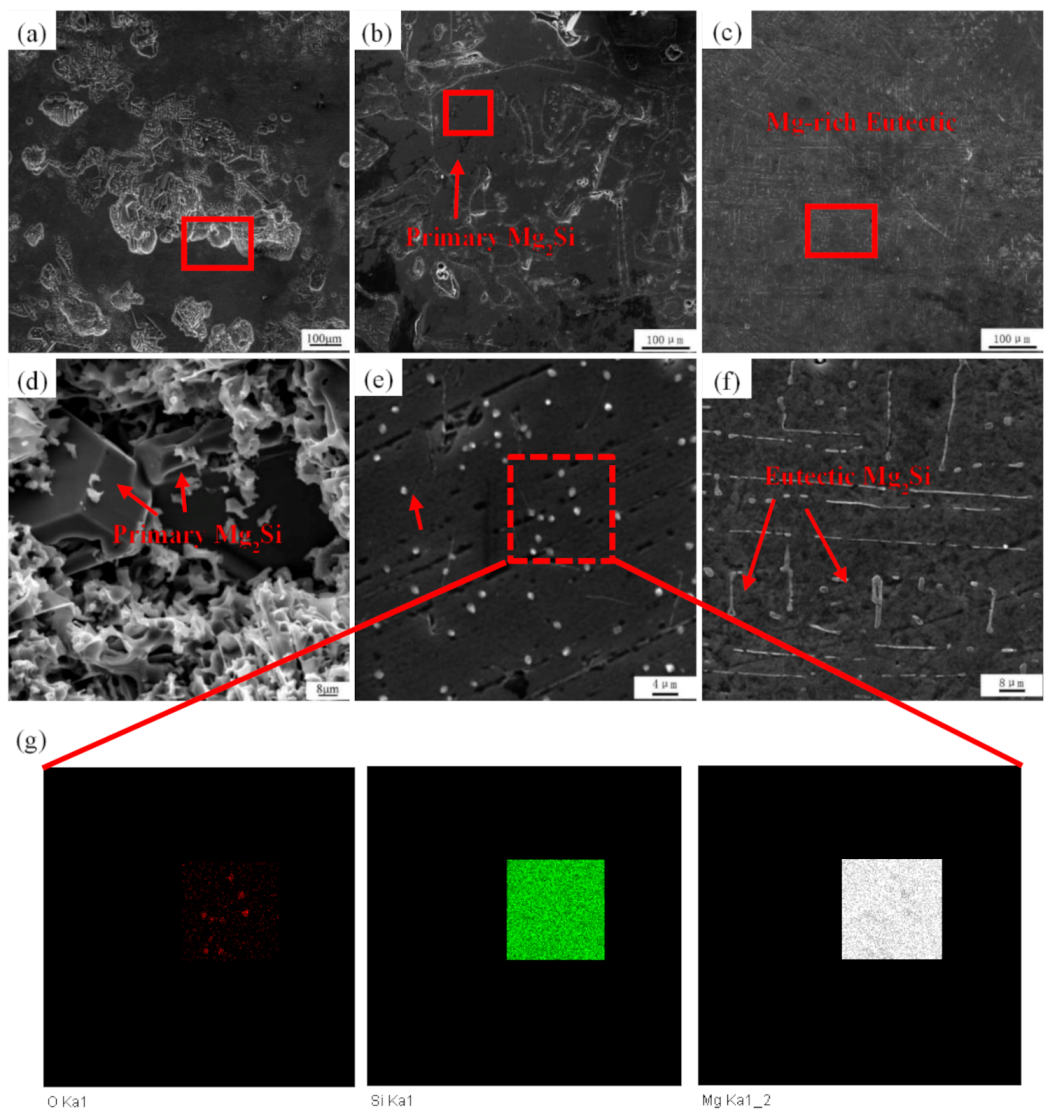
3.1. Macro Appearance and Microstructures
3.2. Bio-Corrosion Behavior
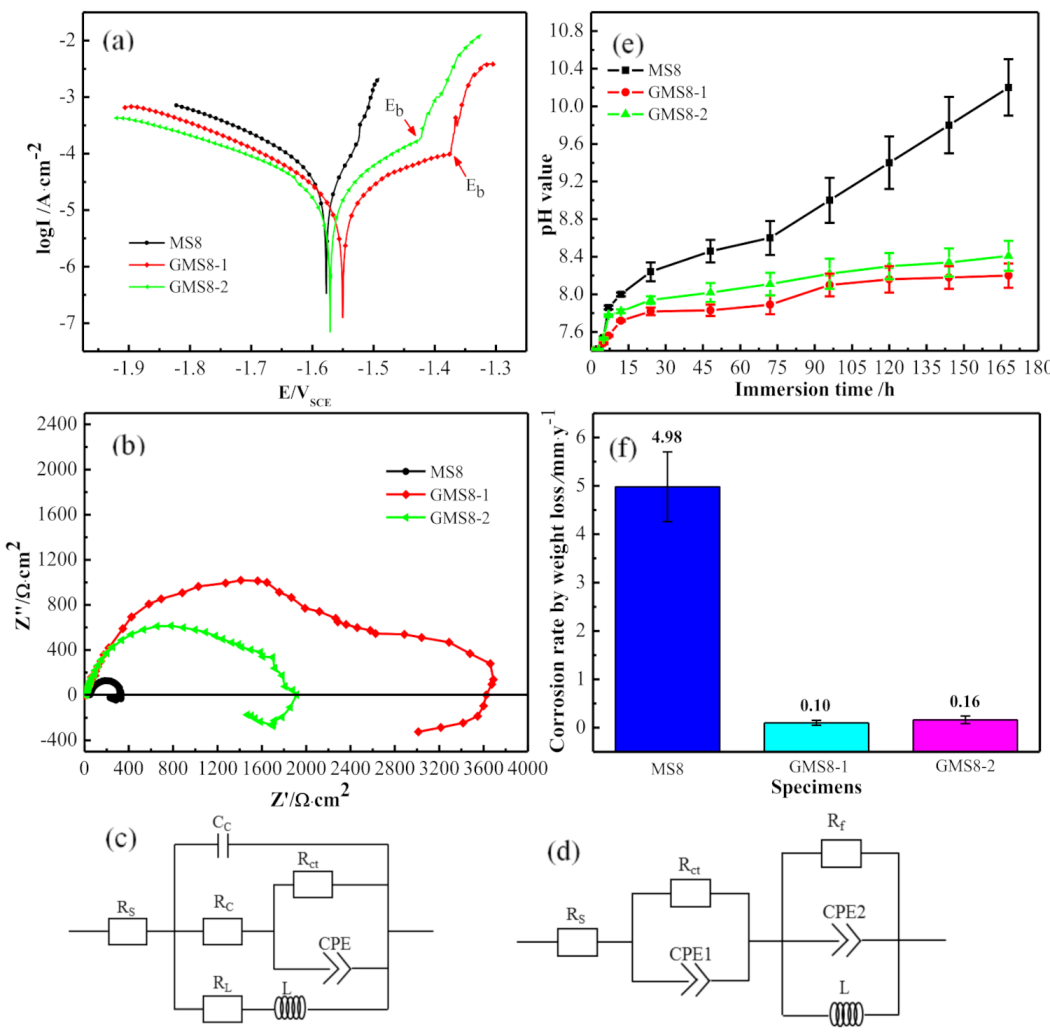
3.2.1. Electrochemical Corrosion Behavior
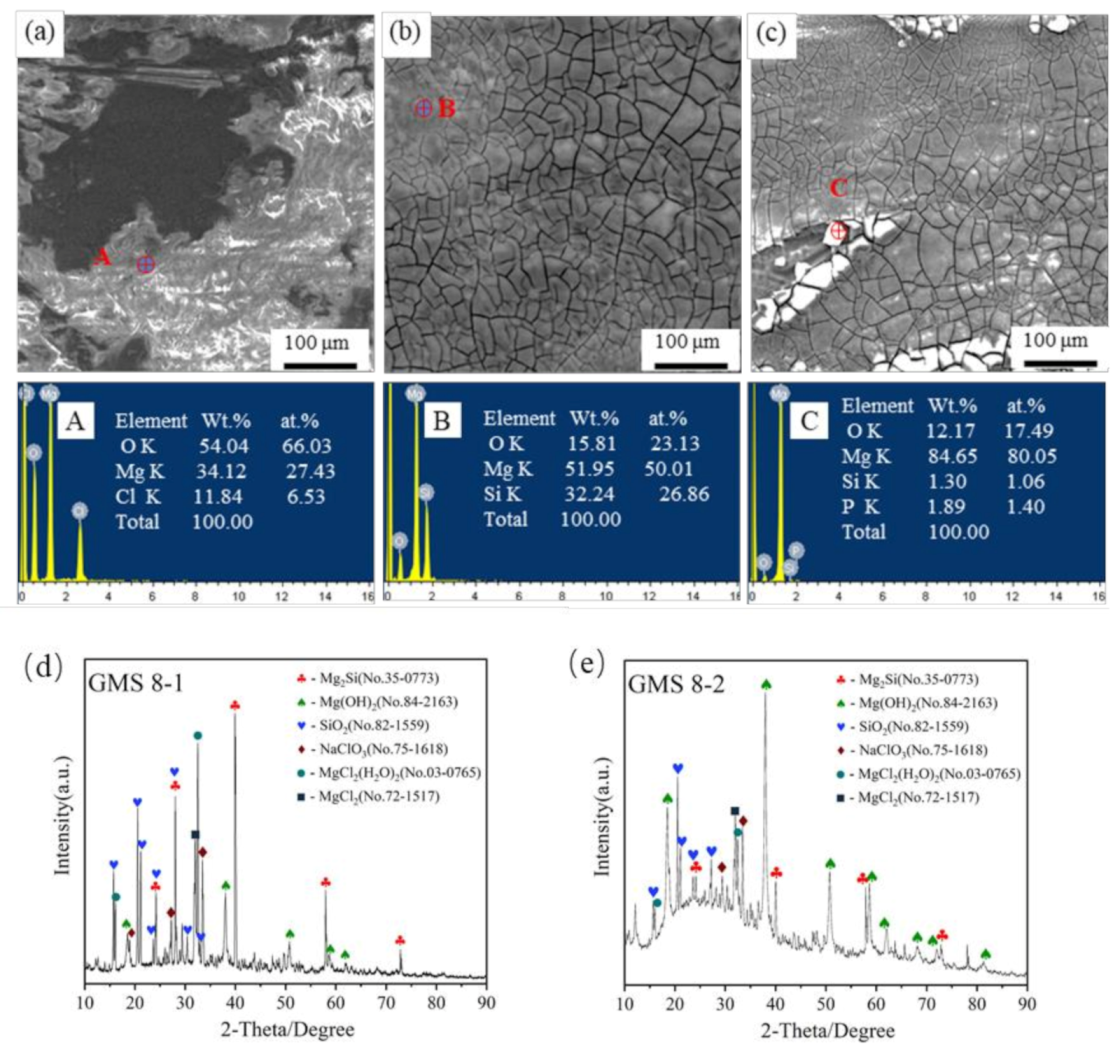
3.2.2. Static Immersion Corrosion Behavior
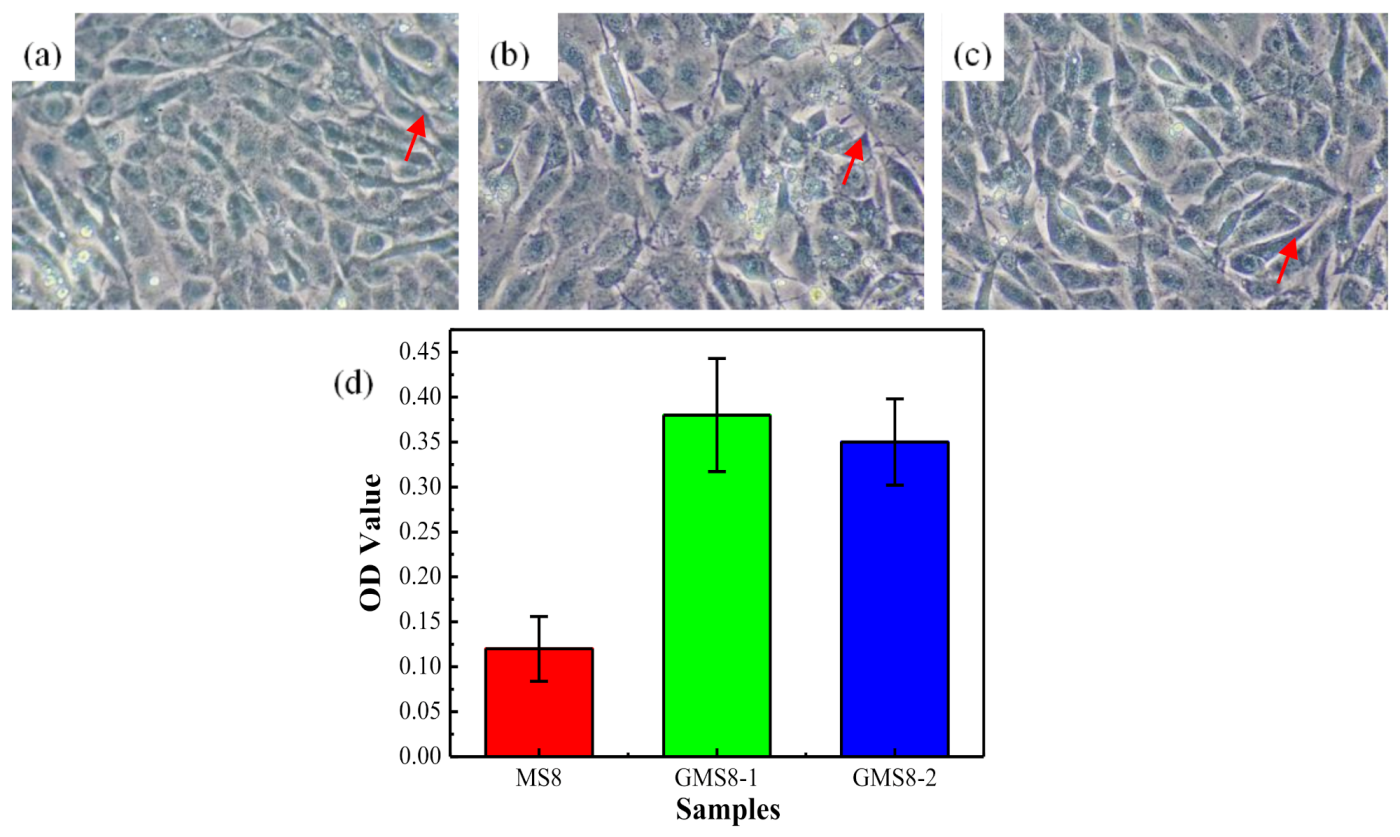
3.3. Cytotoxicity Evaluation
3.4. Osteogenic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, S.; Gowtham, S.; Arun, S.; Ashok, M.; Rameshbabu, N. Fabrication of duplex coatings on biodegradable AZ31 magnesium alloy by integrating cerium conversion (CC) and plasma electrolytic oxidation (PEO) processes. J. Alloys Compd. 2017, 722, 698–715. [Google Scholar]
- Rakoch, A.G.; Monakhova, E.P.; Khabibullina, Z.V.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Gladkova, A.A. Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys: Comparison of coatings formation mechanism. J. Alloys Compd. 2020, 8, 587–600. [Google Scholar] [CrossRef]
- Li, W.; Guan, S.; Chen, J.; Hu, J.; Chen, S.; Wang, L.; Zhu, S. Preparation and in vitro degradation of the composite coating with high adhesion strength on biodegradable Mg-Zn-Ca alloy. Mater. Charact. 2011, 62, 1158–1165. [Google Scholar] [CrossRef]
- Liu, P.; Pan, X.; Yang, W.; Cai, K.; Chen, Y. Improved anticorrosion of magnesium alloy via layer-by-layer self-assembly technique combined with micro-arc oxidation. Mater. Lett. 2012, 75, 118–121. [Google Scholar] [CrossRef]
- Hamid, A.; Baviththira, S.; Sama, G.; Hitesh, H.; Ramaraja, P.R. A multifunctional polymeric coating incorporating lawsone with corrosion resistance and antibacterial activity for biomedical Mg alloys. Prog. Org. Coat. 2021, 153, 106157–106171. [Google Scholar]
- Jesslyn, K.; Balan, P.; Birbilis, N. Advances in LDH coatings on Mg alloys for biomedical applications: A corrosion perspective. Appl. Clay Sci. 2021, 202, 105948–105969. [Google Scholar]
- Mostafizur, R.; Yuncang, L.; Cuie, W. HA coating on Mg alloys for biomedical applications: A review. JMA 2020, 8, 929–943. [Google Scholar]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. JMA 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Keeting, P.E.; Oursler, M.J.; Wiegand, K.E.; Bonde, S.K.; Spelsberg, T.C.; Riggs, B.L. Zeolite A increases proliferation, differentiation, and transforming growth factor beta production in normal adult human osteoblast-like cells in vitro. JBMR 1992, 7, 1281–1289. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Hampson Orthosilicic acid stimulates collagen type I synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, Y.F.; Cheng, Y.; Zhong, S.P.; Xi, T.F. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S. The role of Si and Ca on new wrought Mg-Zn-Mn based alloy. MSE A 2007, 447, 35–43. [Google Scholar] [CrossRef]
- Lu, Y.; Bradshaw, A.; Chiu, Y.L.; Jones, I. Effects of secondary phase and grain size on the corrosion of biodegradable Mg-Zn-Ca alloys. MSE C 2015, 48, 480–486. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Amirnejad, M.; Rajabi, M.; Motavalli, A. Effect of addition of Si on microstructure, mechanical properties, bio-corrosion and cytotoxicity of Mg-6Al-1Zn alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1755–1762. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S. The role of Mg2Si on the corrosion behavior of wrought Mg-Zn-Mn alloy. Intermetallics 2008, 16, 860–867. [Google Scholar] [CrossRef]
- Srinivasan, A.; Ningshen, S.; Mudali, U.; Pillai, U.T.S.; Pai, B.C. Influence of Si and Sb addition on the corrosion behavior of AZ91 magnesium alloy. Intermetallics 2007, 15, 1511–1517. [Google Scholar] [CrossRef]
- Zhang, E.L.; Yang, L.; Xu, J.W.; Chen, H.Y. Microstructure, mechanical properties and bio-corrosion properties of Mg-Si(-Ca, Zn) alloy for biomedical application. Acta Biomater. 2010, 6, 1756–1762. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.M.; Wu, S.L.; Yeung, K.W.K.; Zheng, Y.F.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thouas, G.A. Metallic implant biomaterials. MSE R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Zhao, T.G. Improvement of corrosion and biological properties of microarc oxidized coatings on Mg-Zn-Zr alloy by optimizing negative power density parameters. Colloid Surf. B 2014, 113, 421–428. [Google Scholar] [CrossRef]
- Kaseem, M.; Choi, K.; Ko, Y.G. A highly compact coating responsible for enhancing corrosion properties of Al-Mg-Si alloy. Mater. Lett. 2017, 196, 316–319. [Google Scholar] [CrossRef]
- Chen, S.S.; Tu, J.X.; Hu, Q.; Xiong, X.B.; Wu, J.J. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non-Cryst. Solids 2017, 456, 125–131. [Google Scholar] [CrossRef]
- Jiang, W.Y.; Wang, J.F.; Yu, W.Z.; Ma, Y.; Guo, S.F. In-situ formation of a gradient Mg2Si/Mg composite with good biocompatibility. Surf. Coat. Technol. 2019, 361, 255–262. [Google Scholar] [CrossRef]
- ASTM-G31-72: Standard Practice for Laboratory Immersion Corrosion Testing of Metals. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2004.
- Yu, W.Z.; Ma, W.H.; Lv, G.Q.; Xue, H.Y.; Li, S.Y.; Dai, Y.N. Effect of electromagnetic stirring on the enrichment of primary silicon from Al-Si melt. J. Cryst. Growth 2015, 405, 23–28. [Google Scholar] [CrossRef]
- Kania, A.; Nowosielski, R.; Gawlas-Mucha, A.; Babilas, R. Mechanical and corrosion properties of Mg-Based alloys with Gd addition. Materials 2019, 12, 1775. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir, M.R.A.; Izman, S.; E-Kahrizsangi, R. Fabrication, bio-corrosion behavior and mechanical properties of a Mg/HA/MgO nanocomposite for. biomedical applications. Mater. Des. 2015, 88, 1223–1233. [Google Scholar] [CrossRef]
- Tompa, G.S.; Li, Y.B.; Agassi, D.; Kim, S.I.; Hong, S.K. Mg2Si buffer layers on Si(1 0 0)prepared by a simple evaporation method. J. Electron. Mater. 1996, 25, 925–929. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.Y.; Li, H.; Liu, X.F. Morphological evolution and growth mechanism of primary Mg2Si phase in Al-Mg2Si alloys. Acta Mater. 2011, 59, 1058–1067. [Google Scholar] [CrossRef]
- Stathokostopoulos, D.; Chaliampalias, D.; Stefanaki, E.; Polymeris, G.; Pavlidou, E.; Chrissafis, K.; Hatzikraniotis, E.; Paraskevopoulos, K.; Vourlias, G. Structure, morphology and electrical properties of Mg2Si layers deposited by pack cementation. Appl. Surf. Sci. 2013, 285P, 417–424. [Google Scholar] [CrossRef]
- Mandal, M.; Moon, A.P.; Deo, G.; Mendis, C.L.; Mondal, K. Corrosion behavior of Mg–2.4Zn alloy micro-alloyed with Ag and Ca. Corros. Sci. 2014, 78, 172–182. [Google Scholar] [CrossRef]
- Song, G.L. Effect of tin modification on corrosion of AM70 magnesium alloy. Corros. Sci. 2009, 51, 2063–2070. [Google Scholar] [CrossRef]
- Yamasaki, M.; Izumi, S.; Kawamura, Y.; Habazaki, H. Corrosion and passivation behavior of Mg-Zn-Y-Al alloys prepared by cooling rate-controlled solidification. Appl. Surf. Sci. 2011, 257, 8258–8267. [Google Scholar] [CrossRef]
- Cesarz-Andraczke, K.; Kazek-Kęsik, A. PEO layers on Mg-based metallic glass to control hydrogen evolution rate. Bull. Pol. Acad. Sci. Tech. Sci. 2020, 68, 119–124. [Google Scholar]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef]
- Grillo, C.A.; Alvarez, F.; de Mele, M.A.F.L. Cellular response to rare earth mixtures (La and Gd) as components of degradable Mg alloys for medical applications. Colloid Surf. B 2014, 117, 312–321. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, J.; Yang, K.; Zhang, B.; Yao, Z.; Wang, H. Study of bio-corrosion of pure magnesium. Acta Metall. Sin. 2005, 41, 1228–1232. [Google Scholar]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar] [CrossRef]
- Bahl, S.; Aleti, B.T.; Suwas, S.; Chatterjee, K. Surface nanostructuring of titanium imparts multifunctional properties for orthopedic and cardiovascular applications. Mater. Des. 2018, 144, 169–181. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, Y.K.; Kim, K.S.; Lee, K.B.; Lee, M.H. Enhancement of bone formation on LBL-coated Mg alloy depending on the different concentration of BMP-2. Colloid Surf. B 2019, 173, 437–446. [Google Scholar] [CrossRef]


| Specimen | Ecorr(V) | Icorr (10−5A·cm−2) | Eb(V) | Rp (Ω·cm2) | Refs. |
|---|---|---|---|---|---|
| MS8 | −1.58 | 28.1 | 254.5 | This work | |
| GMS8-1 | −1.55 | 0.17 | −1.37 | 1610 | This work |
| GMS8-2 | −1.57 | 2.56 | −1.42 | 1291 | This work |
| Mg-6Al-1Zn-1Si | −1.65 | 8.61 | [17] | ||
| Mg-0.6Si | −1.72 | 3.06 | 711 | [20] |
| Specimen | MS8 | GMS8-1 | GMS8-2 |
|---|---|---|---|
| Rs (Ωcm2) | 26.48 | 8.15 | 7.17 |
| Rct (Ω/cm2) | 266.3 | 1335 | 1072 |
| CPE1-T (F/cm2) | 1.22 × 10−5 | 3.38 × 10−5 | |
| CPE1-P (F/cm2) | 0.83884 | 0.83241 | |
| Rf (Ω/cm2) | 511.4 | 271.2 | |
| Rc | 26.3 | ||
| Cc | 4.44 × 10−8 | ||
| CPE2-T (F/cm2) | 6.72 × 10−5 | 4.43 × 10−4 | 1.99 × 10−4 |
| CPE2-P (F/cm2) | 0.9133 | 0.9687 | 0.9409 |
| L (H/cm2) | 846.1 | 185.3 | 73.88 |
| Chisq | 9.013 × 10−4 | 7.120 × 10−4 | 7.938 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Yu, W. Corrosion Behavior and Osteogenic Activity of a Biodegradable Orthopedic Implant Mg–Si Alloy with a Gradient Structure. Metals 2021, 11, 781. https://doi.org/10.3390/met11050781
Jiang W, Yu W. Corrosion Behavior and Osteogenic Activity of a Biodegradable Orthopedic Implant Mg–Si Alloy with a Gradient Structure. Metals. 2021; 11(5):781. https://doi.org/10.3390/met11050781
Chicago/Turabian StyleJiang, Weiyan, and Wenzhou Yu. 2021. "Corrosion Behavior and Osteogenic Activity of a Biodegradable Orthopedic Implant Mg–Si Alloy with a Gradient Structure" Metals 11, no. 5: 781. https://doi.org/10.3390/met11050781
APA StyleJiang, W., & Yu, W. (2021). Corrosion Behavior and Osteogenic Activity of a Biodegradable Orthopedic Implant Mg–Si Alloy with a Gradient Structure. Metals, 11(5), 781. https://doi.org/10.3390/met11050781







