Effects of Induction Heating in the ESP Line on Microstructural Evolution and Nb Dissolution Behavior of Nb-Ti Micro-Alloyed Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructure
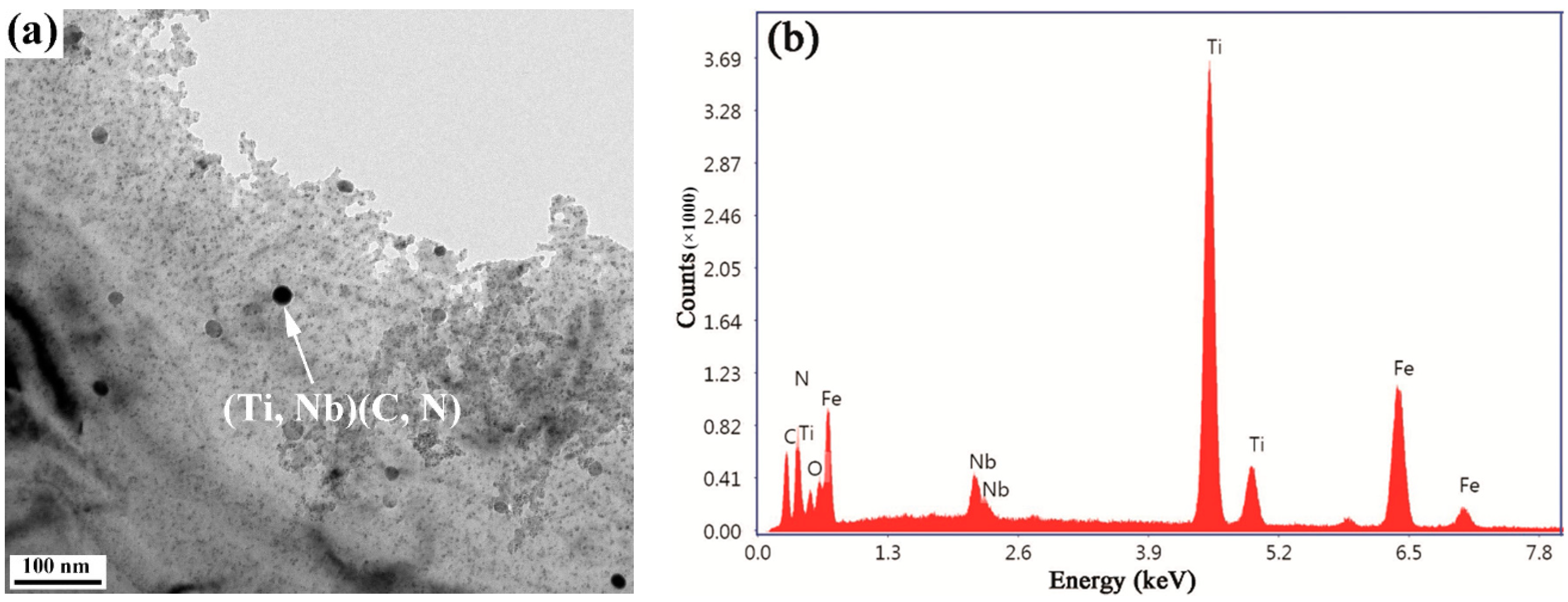
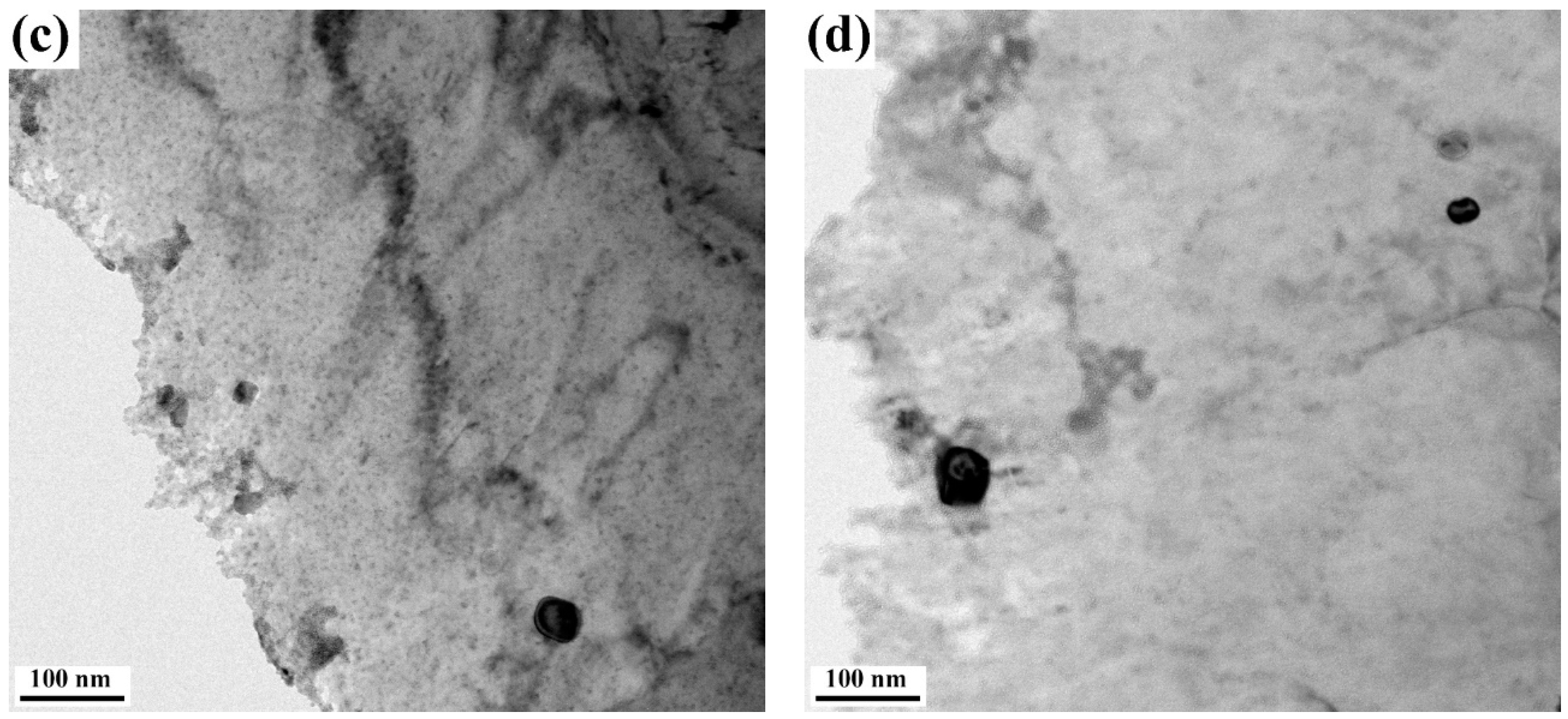
3.2. Dissolution Behavior
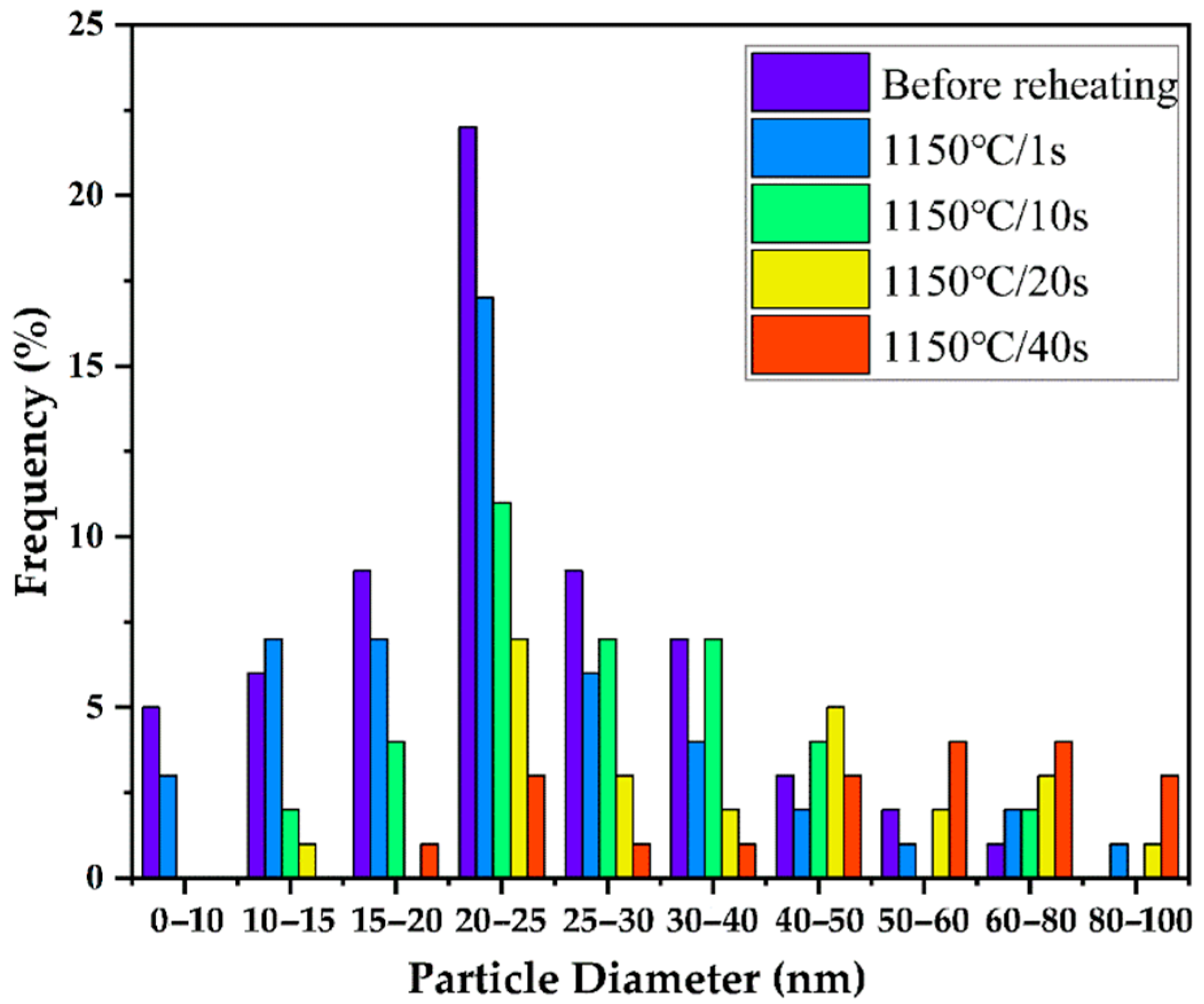
3.3. Dissolution of Niobium
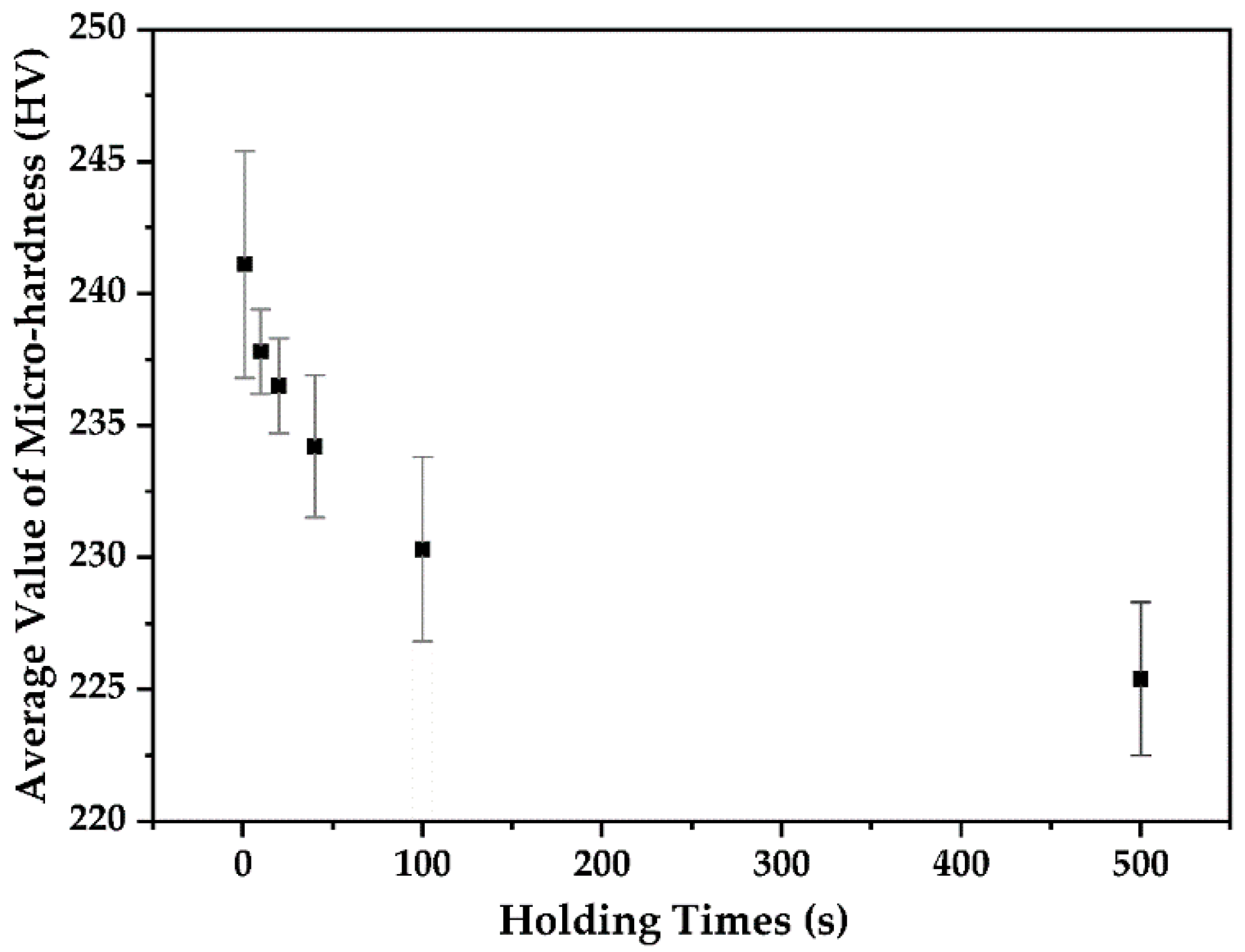
3.4. Micro-Hardness
4. Discussion
4.1. Coarsening of Austenite Grains during the Heating Process
4.2. Dissolution Behavior of Nb during the Heating Process
4.3. Effects of the Heating Process on DIFT
5. Conclusions
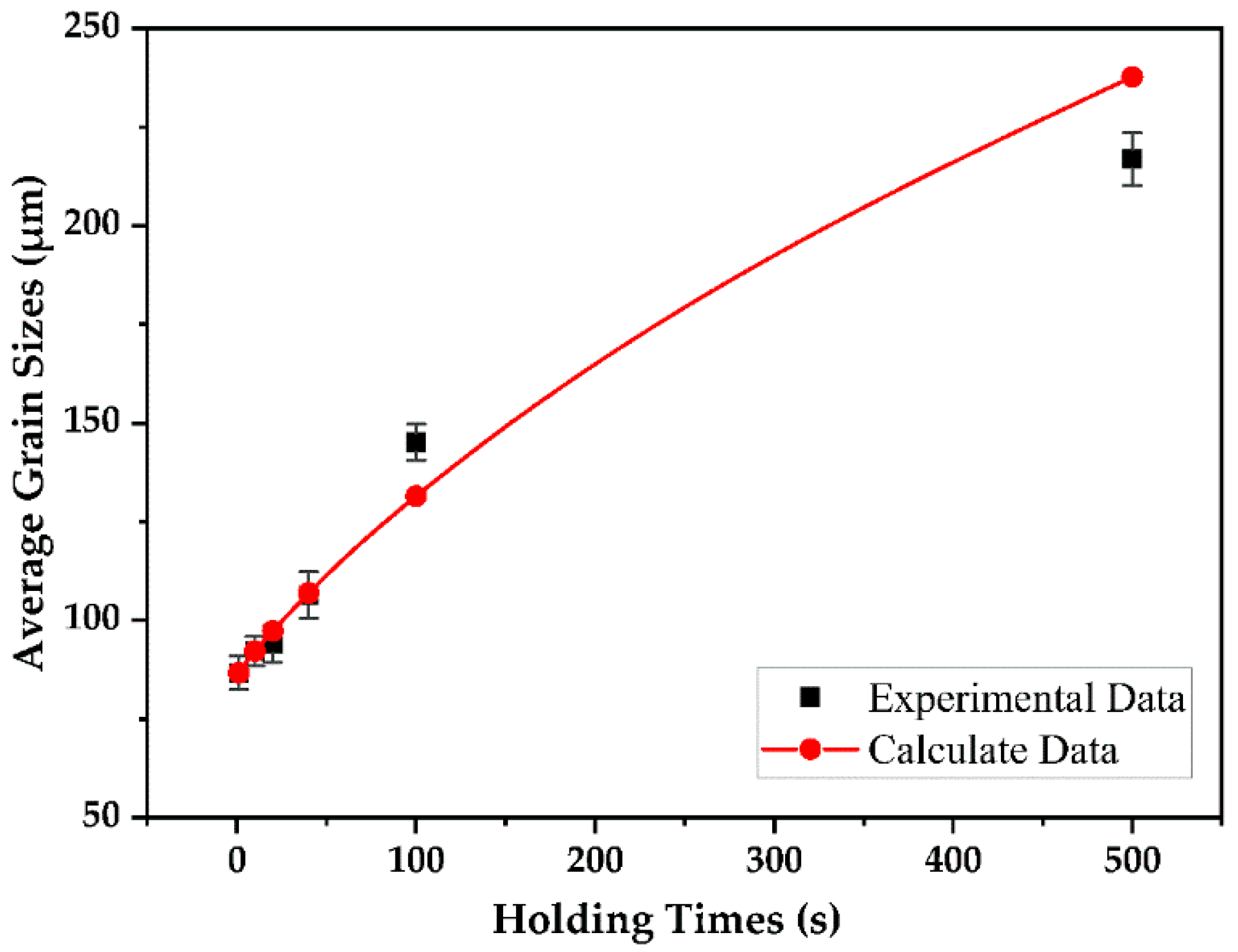
- The average sizes of austenite grains were 92.2 ± 3.8, 94.1 ± 4.7, 106.4 ± 5.9, 145.1 ± 4.6, and 216.9 ± 6.7 µm after holding for 10, 20, 40, 100, and 500 s, respectively, and the predicted austenite grain sizes after 20, 40, 100, and 500 s were 97.3, 106.9, 131.5, and 237.7 µm, respectively. Therefore, the coarsening behavior of austenite predicted by Feltham’s theory was consistent with the obtained experimental data.
- Round precipitates of (Ti, Nb) (C, N) and square precipitates of Ti (C, N) gradually dissolved into the austenite matrix with the holding time. The amounts of Nb in the solution were 0.0137, 0.0221, 0.0282, and 0.0299 wt% after holding for 1, 10, 20, and 40 s, respectively. The amounts of Nb in the solution accounting for the total amount of added Nb increased from 27.4% to 59.8% as the holding time increased from 1 to 40 s.
- The contents of Nb in the solution calculated by the JMAK equation were 0.0122, 0.0245, 0.0291, and 0.0322 wt% after holding for 1, 10, 20, and 40 s, respectively. The Nb dissolution behavior with the isothermal time simulated by the Johnson–Mehl theory was consistent with the measured data when the isothermal time was less than 20 s; however, the measured data gradually became smaller than the calculated values with the holding time; thus, the addition of Ti inhibited the dissolution of Nb.
- Nb-Ti micro-alloyed steel with an amount of 0.05 wt% Nb was industrially produced on an ESP line. The microstructure of the test steel contains mainly polygonal ferrite and a small content of quasi-polygonal ferrite, with an average grain size of 2.97 μm. The test steel displayed a yield strength and tensile strength of 684 and 745 MPa, respectively, with an elongation of 18.9%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timokhina, I.; Hodgson, P.; Ringer, S.P.; Zheng, R.; Pereloma, E. Precipitate characterisation of an advanced high-strength low-alloy (HSLA) steel using atom probe tomography. Scr. Mater. 2007, 56, 601–604. [Google Scholar] [CrossRef]
- Charleux, M.; Poole, W.J.; Militzer, M.; Deschamps, A. Precipitation behavior and its effect on strengthening of an HSLA-Nb/Ti steel. Met. Mater. Trans. A 2001, 32, 1635–1647. [Google Scholar] [CrossRef]
- Patel, J.; Wilshire, B. The challenge to produce consistent mechanical properties in Nb-HSLA strip steels. J. Mater. Process. Technol. 2002, 120, 316–321. [Google Scholar] [CrossRef]
- Show, B.; Veerababu, R.; Balamuralikrishnan, R.; Malakondaiah, G. Effect of vanadium and titanium modification on the microstructure and mechanical properties of a microalloyed HSLA steel. Mater. Sci. Eng. A 2010, 527, 1595–1604. [Google Scholar] [CrossRef]
- Park, D.B.; Huh, M.Y.; Shim, J.-H.; Suh, J.Y.; Lee, K.H.; Jung, W.-S. Strengthening mechanism of hot rolled Ti and Nb microalloyed HSLA steels containing Mo and W with various coiling temperature. Mater. Sci. Eng. A 2013, 560, 528–534. [Google Scholar] [CrossRef]
- Karmakar, A.; Kundu, S.; Roy, S.; Neogy, S.; Srivastava, D.; Chakrabarti, D. Effect of microalloying elements on austenite grain growth in Nb–Ti and Nb–V steels. Mater. Sci. Technol. 2014, 30, 653–664. [Google Scholar] [CrossRef]
- Hong, S.G.; Jun, H.J.; Kang, K.B.; Park, C.G. Evolution of precipitates in the Nb–Ti–V microalloyed HSLA steels during heating. Scr. Mater. 2003, 48, 1201–1206. [Google Scholar] [CrossRef]
- Zhou, C.; Priestner, R. The evolution of precipitates in Nb-Ti microalloyed steels during solidification and post-solidification cooling. ISIJ Int. 1996, 36, 1397–1405. [Google Scholar] [CrossRef]
- Kostryzhev, A.G.; Al Shahrani, A.; Zhu, C.; Ringer, S.P.; Pereloma, E. Effect of austenitising and deformation temperatures on dynamic recrystallisation in Nb-Ti microalloyed steel. Mater. Sci. Forum 2013, 753, 431–434. [Google Scholar] [CrossRef]
- Jia, Z.; Misra, R.; O’Malley, R.J.; Jansto, S. Fine-scale precipitation and mechanical properties of thin slab processed titanium–niobium bearing high strength steels. Mater. Sci. Eng. A 2011, 528, 7077–7083. [Google Scholar] [CrossRef]
- Shukla, R.; Ghosh, S.; Chakrabarti, D.J.; Chatterjee, S.K. Microstructure, texture, property relationship in thermo-mechanically processed ultra-low carbon microalloyed steel for pipeline application. Mater. Sci. Eng. A 2013, 587, 201–208. [Google Scholar] [CrossRef]
- Jahazi, M.; Egbali, B. The influence of hot rolling parameters on the microstructure and mechanical properties of an ultra-high strength steel. J. Mater. Process. Technol. 2000, 103, 276–279. [Google Scholar] [CrossRef]
- Zhang, L.J.; Amp, E.A.; Limited, I. Production technology on hot endless rolling of ultrathin strips and long products. Metals 2018, 222, 3–7. [Google Scholar]
- He, Y.P.; Xiao, Y.W.; Dai, Z.B. Development and application of endless hot rolling strip production technology. China Heavy Equip. 2017, 04, 11–16. [Google Scholar]
- Linzer, B.; Jungbauer, A. Arvedi ESP for high-quality hot-strip production at rizhao steel in materials science forum. Trans. Tech. Publ. 2016, 854, 207–214. [Google Scholar]
- Lei, G.Z.; Zhao, H.G.; Shun, S.H. ESP super-thin strip endless rolling equipment & process flow. CFHI Technol. 2016, 4, 27–29. [Google Scholar]
- Mao, Y. A new generation of high efficiency and energy saving hot-rolled strip line-ESP line. Metall. Equip. 2014, 5, 45–49. [Google Scholar]
- Yu, Y.; Zheng, X.T. Endless strip production technology in Rizhao Steel Company. Contin. Cast. 2016, 41, 1–4. [Google Scholar]
- Jung, J.; Lee, S.-J.; Kim, S.; De Cooman, B.C. Effect of Ti additions on micro-alloyed Nb TRIP steel. Steel Res. Int. 2011, 82, 857–865. [Google Scholar] [CrossRef]
- Zhuo, X.-J.; Woo, D.-H.; Wang, X.H.; Lee, H.G. Formation and thermal stability of large precipitates and oxides in titanium and niobium microalloyed steel. J. Iron Steel Res. Int. 2008, 15, 70–77. [Google Scholar] [CrossRef]
- Cao, Y.B.; Xiao, F.R.; Qiao, G.Y.; Liao, B. Quantitative analysis and application of soluble Nb and undissolved Nb in Nb-bearing steels. J. Yanshan Univ. 2011, 35, 319–322. [Google Scholar]
- Cao, Y.B.; Xiao, F.R.; Qiao, G.Y.; Zhang, J.; Liao, B. Quantitative research on effects of Nb on hot deformation behaviors of high-Nb microalloyed steels. Mater. Sci. Eng. A 2011, 530, 277–284. [Google Scholar] [CrossRef]
- Cao, Y.B.; Xiao, F.R.; Qiao, G.Y.; Liao, B. Quantitative Research on Dissolving of Nb in High Nb Microalloyed Steels during Heating. J. Iron Steel Res. 2014, 21, 596–599. [Google Scholar] [CrossRef]
- Niu, G.; Tang, Q.; Wu, H.-B.; Gong, N.; Yin, Y.; Tang, D. Achieving high strength and high ductility of austenitic steel by cold rolling and reverse annealing. Materialia 2019, 6, 100–264. [Google Scholar] [CrossRef]
- Feltham, P. Grain growth in metals. Acta Met. 1957, 5, 97–105. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Pereloma, E.; Crawford, B.; Hodgson, P. Strain-induced precipitation behaviour in hot rolled strip steel. Mater. Sci. Eng. A 2001, 299, 27–37. [Google Scholar] [CrossRef]
- Xiang, L.; Tang, B.; Xue, X.; Kou, H.; Li, J. Characteristics of the dynamic recrystallization behavior of Ti-45Al-8.5Nb-0.2W-0.2B-0.3Y alloy during high temperature deformation. Metals 2017, 7, 261. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Liu, Z.Y.; Tang, S.; Wang, G.D. Influence of deformation temperature on γ-α phase transformation in Nb–Ti microalloyed steel during continuous cooling. ISIJ Int. 2013, 53, 1070–1075. [Google Scholar] [CrossRef]
- Imandoust, A.; Zarei-Hanzaki, A.; Abedi, H.R. Low-temperature strain-induced ferrite transformation in twinning-induced plas-ticity steel. Scr. Mater. 2012, 67, 995–998. [Google Scholar] [CrossRef]







| Elements | C | Mn | Si | Als | Ti | Nb | P | S |
|---|---|---|---|---|---|---|---|---|
| wt% | 0.05 | 1.65 | 0.25 | 0.025 | 0.06 | 0.05 | 0.012 | 0.0008 |
| Holding Time/s | Before Heating | 1 | 10 | 20 | 40 |
|---|---|---|---|---|---|
| The amount of Nb in solution/wt% | 0.0051 | 0.0137 | 0.0221 | 0.0282 | 0.0299 |
| Account for total additions/% | 10.2% | 27.4% | 44.2% | 56.4% | 59.8% |
| Isothermal Time/s | Volume Fraction of Precipitates/10−5 μm−3 | The Content of Nb in the Solution/wt% |
|---|---|---|
| 1 | 63.5 ± 1.9 | 0.0122 |
| 10 | 51.8 ± 2.8 | 0.0245 |
| 20 | 37.2 ± 3.1 | 0.0291 |
| 40 | 32.7 ± 2.3 | 0.0322 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Niu, G.; Wu, H.; Xu, L.; Yuan, R. Effects of Induction Heating in the ESP Line on Microstructural Evolution and Nb Dissolution Behavior of Nb-Ti Micro-Alloyed Steel. Metals 2021, 11, 251. https://doi.org/10.3390/met11020251
Tang Q, Niu G, Wu H, Xu L, Yuan R. Effects of Induction Heating in the ESP Line on Microstructural Evolution and Nb Dissolution Behavior of Nb-Ti Micro-Alloyed Steel. Metals. 2021; 11(2):251. https://doi.org/10.3390/met11020251
Chicago/Turabian StyleTang, Qibo, Gang Niu, Huibin Wu, Lixiong Xu, and Rui Yuan. 2021. "Effects of Induction Heating in the ESP Line on Microstructural Evolution and Nb Dissolution Behavior of Nb-Ti Micro-Alloyed Steel" Metals 11, no. 2: 251. https://doi.org/10.3390/met11020251
APA StyleTang, Q., Niu, G., Wu, H., Xu, L., & Yuan, R. (2021). Effects of Induction Heating in the ESP Line on Microstructural Evolution and Nb Dissolution Behavior of Nb-Ti Micro-Alloyed Steel. Metals, 11(2), 251. https://doi.org/10.3390/met11020251





