Do Titanium Mini-Implants Have the Same Quality of Finishing and Degree of Contamination before and after Different Manipulations? An In Vitro Study
Abstract
1. Introduction
2. Material and Methods
2.1. Sample
2.2. Study Design
2.3. Statistical Analyses
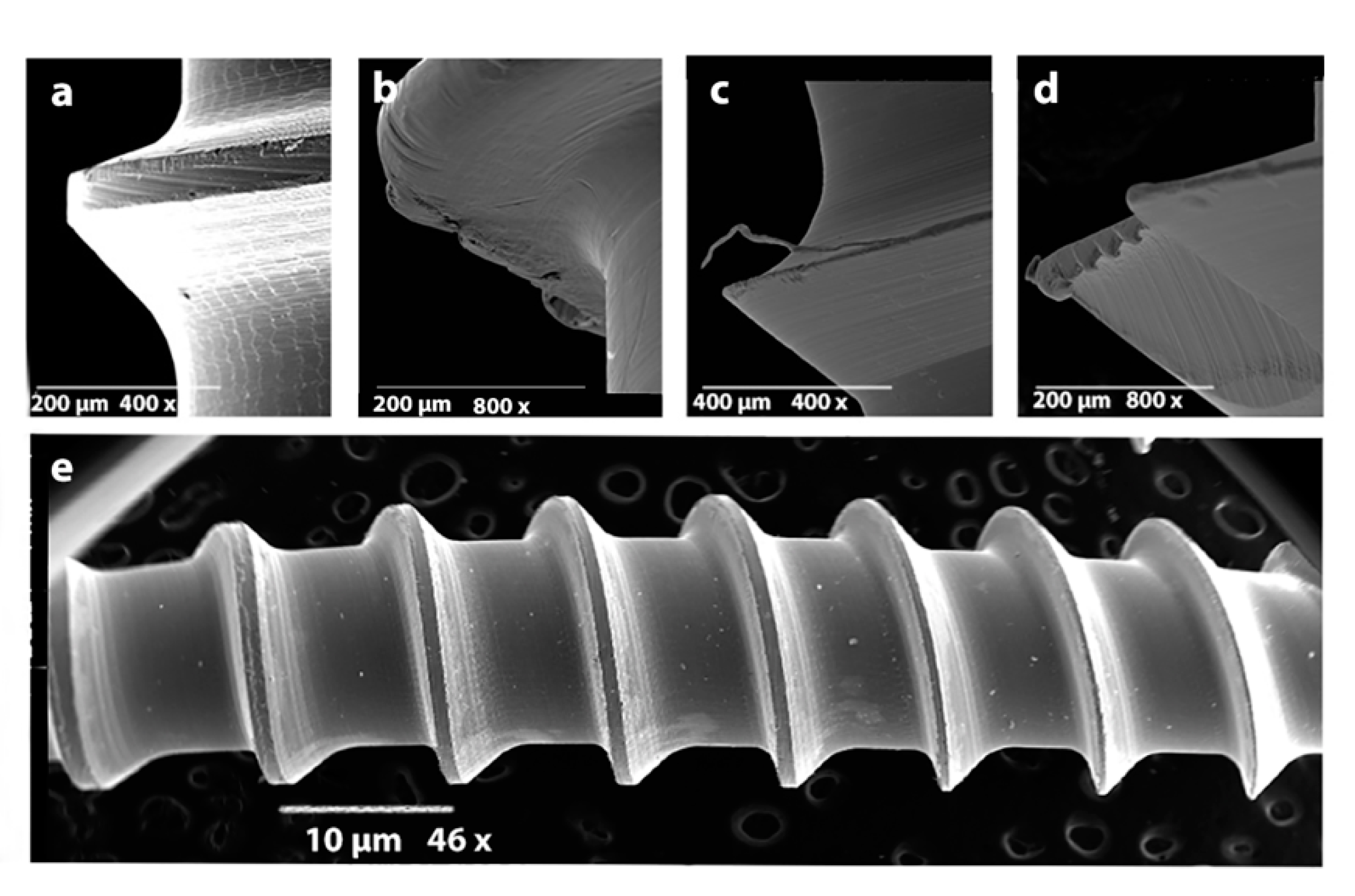
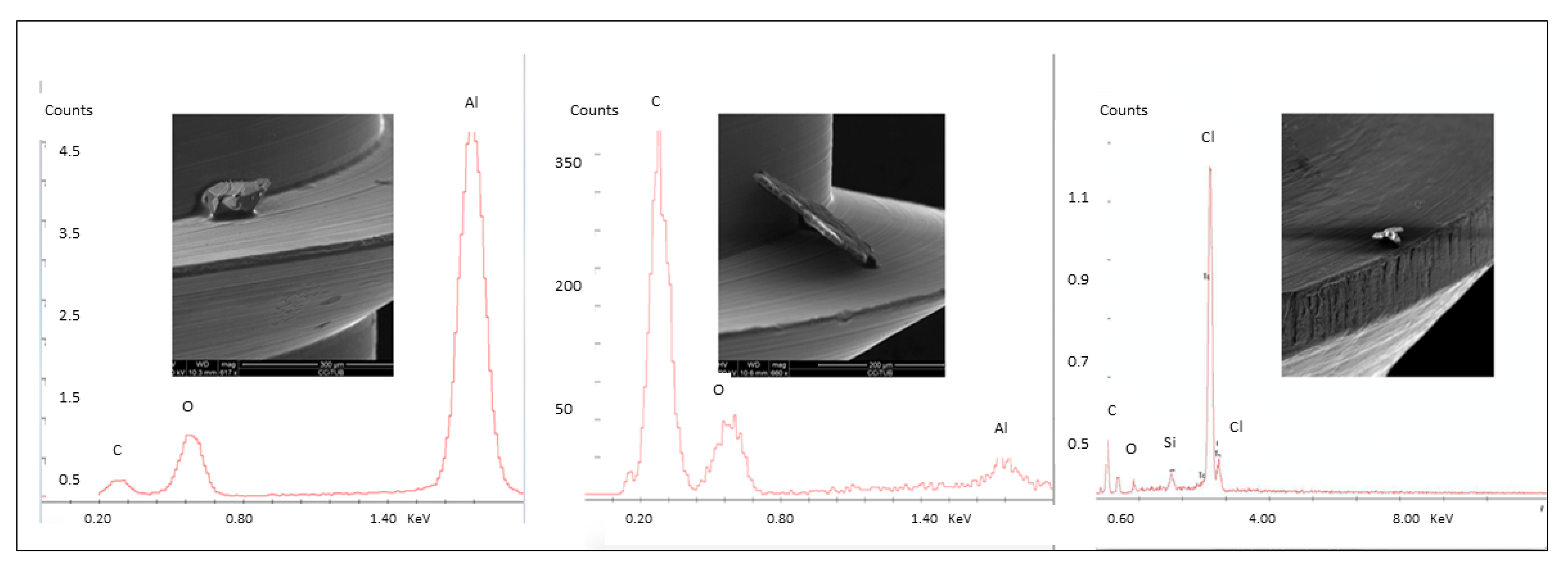
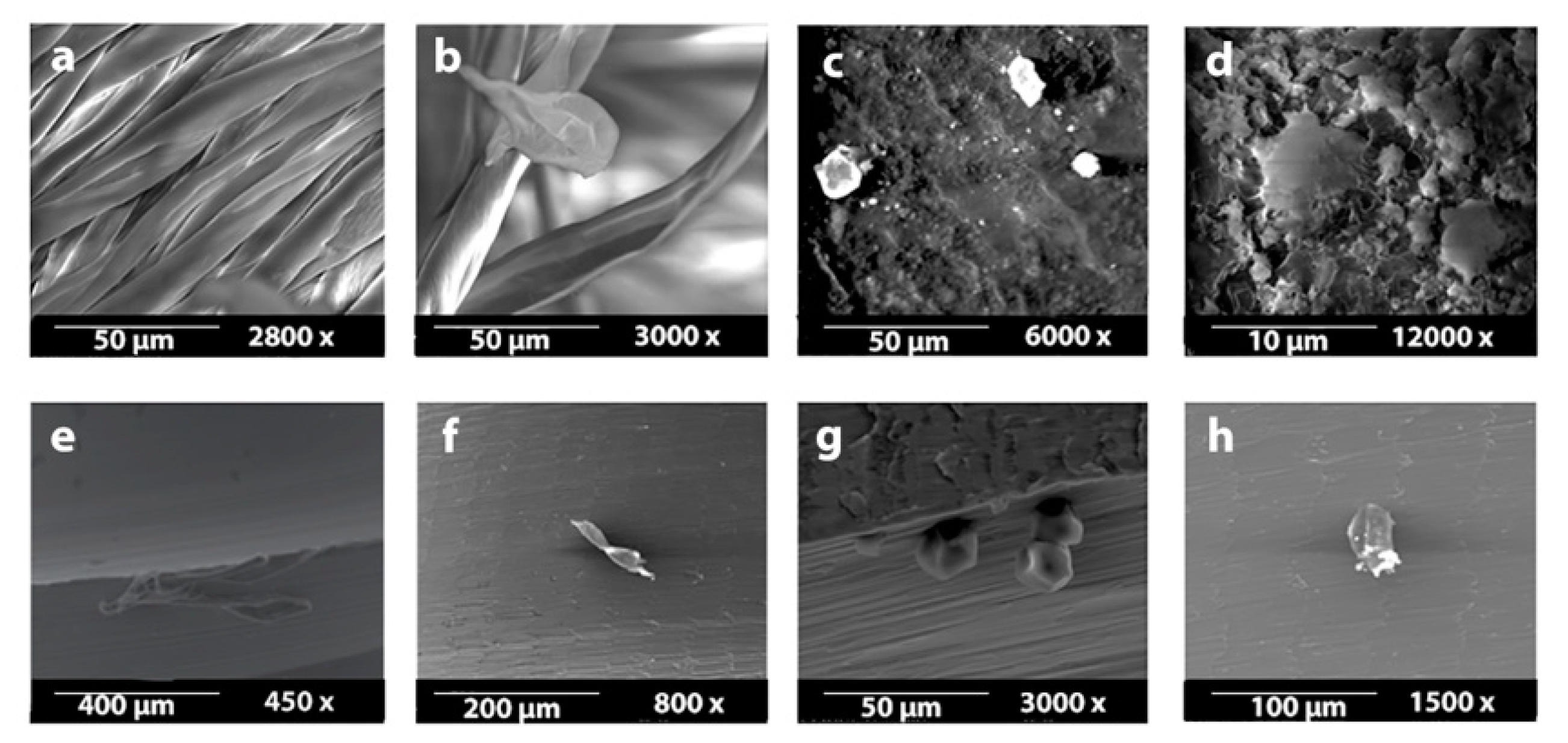
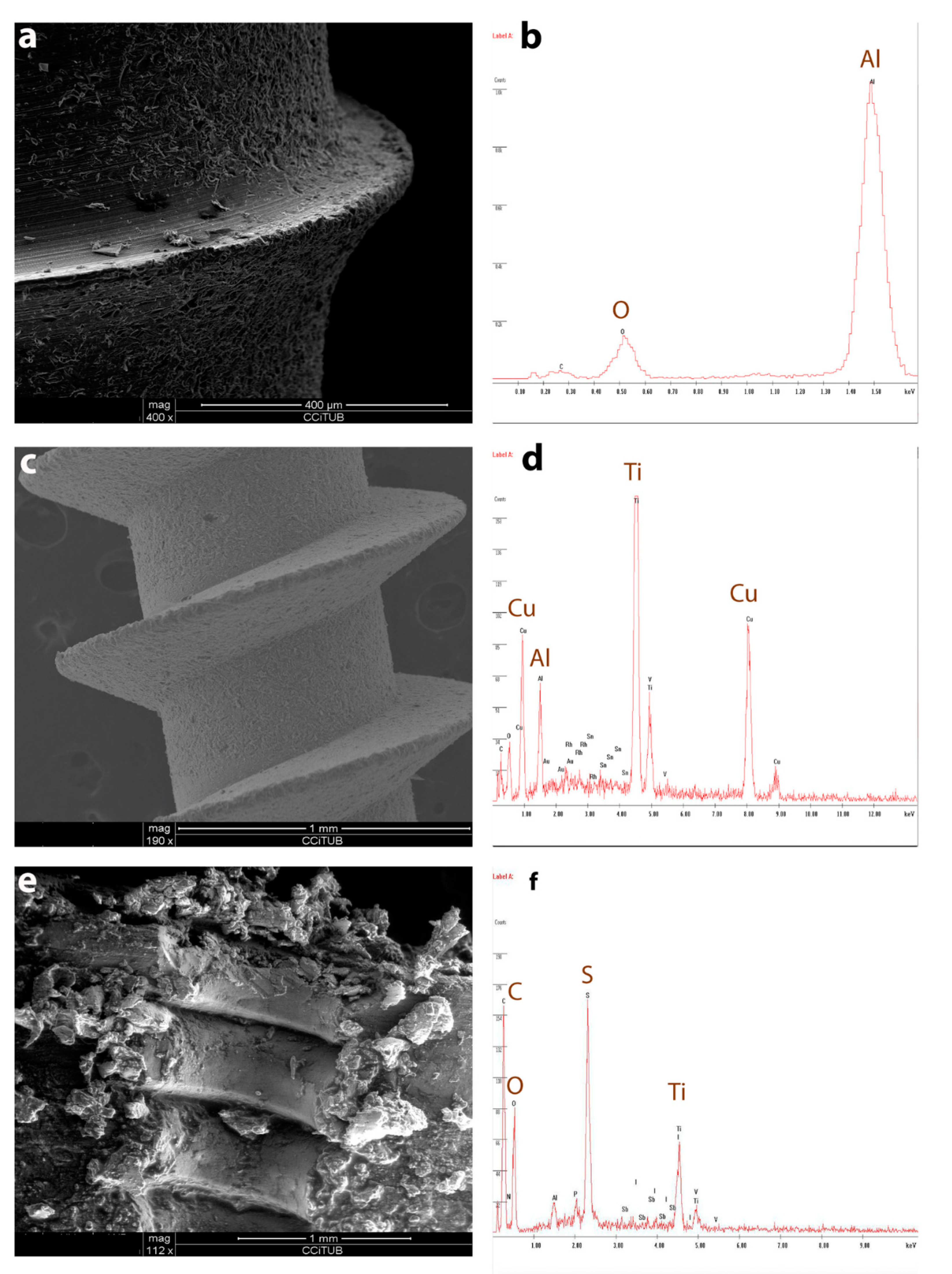
3. Results
3.1. Part 1
3.2. Part 2
3.3. Part 3
3.4. Part 4
4. Discussion
5. Conclusions
- OMIs have surface contaminants and irregularities from the manufacturer; the amount depends on the surface finishing.
- After clinical handling, OMIs surfaces are additionally contaminated; therefore, when handling is needed, the use of a gauze soaked in chlorhexidine is recommended.
- OMIs leave titanium particles in the bone during the procedure of insertion-removal, especially when surface-treated.
- Sandblasted and sandblasted with anodic oxidation produce an increasing of roughness and hydrophobic character of the surfaces.
Author Contributions
Funding
Conflicts of Interest
References
- Costa, A.; Raffainl, M.; Melsen, B. Miniscrews as orthodontic anchorage: A preliminary report. Int. J. Adult Orthod. Orthognath. Surg. 1998, 13, 201–209. [Google Scholar]
- Kanomi, R. Mini-implant for orthodontic anchorage. J. Clin. Orthod. JCO 1997, 31, 763–767. [Google Scholar] [PubMed]
- Park, H.S. The skeletal cortical anchorage using titanium microscrew implants. Korea J. Orthod. 1999, 29, 699–706. [Google Scholar]
- Baumgaertel-Sebastian, G.H.M.; Razavi Mohammad, R. Mini-implant anchorage for the orthodontic practi-tioner. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 621–627. [Google Scholar] [CrossRef]
- Kuroda, S.; Yamada, K.; Deguchi, T.; Hashimoto, T.; Kyung, H.-M.; Yamamoto, T.T. Root proximity is a major factor for screw failure in orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2007, 131, S68–S73. [Google Scholar] [CrossRef]
- Watanabe, H.; Deguchi, T.; Hasegawa, M.; Ito, M.; Kim, S. Orthodontic miniscrew failure rate and root proximi-ty, insertion angle, bone contact length, and bone density. Orthod. Craniofac. Res. 2013, 16, 44–55. [Google Scholar] [CrossRef]
- Sebbar, M.; Bourzgui, F.; Aazzab, B.; Elquars, F. Anchorage miniscrews: A surface characterization study using optical microscopy. Int. Orthod. 2011, 9, 325–338. [Google Scholar] [CrossRef]
- Iijima, M.; Muguruma, T.; Kawaguchi, M.; Yasuda, Y.; Mizoguchi, I. In vivo degradation of orthodontic miniscrew implants: Surface analysis of as-received and retrieved specimens. J. Mater. Sci. Mater. Electron. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Yucesoy, T.; Seker, D.E.; Arslan, E. Comparison of surface roughness and elemental analysis of different mini implant systems. Pak. Oral Dent. J. 2017, 30, 383–393. [Google Scholar]
- Stokes, D.J. Principles and Practice of Variable Pressure/Environmental Scanning Electron Microscopy (VP-ESEM); John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Park, Y.-C.; Lee, S.-Y.; Kim, D.-H.; Jee, S.-H. Intrusion of posterior teeth using mini-screw implants. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 690–694. [Google Scholar] [CrossRef]
- Huang, L.-H.; Shotwell, J.L.; Wang, H.-L. Dental implants for orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Chaddad, K.; Ferreira, A.H.; Geurs, N.; Reddy, M.S. Influence of Surface Characteristics on Survival Rates of Mini-Implants. Angle Orthod. 2008, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Oshida, Y.; Andres, C.J.; Barco, M.T. Surface characterizations of variously treated titanium materials. Int. J. Oral Maxillofac. Implant. 2001, 16, 333–342. [Google Scholar]
- Yamagami, A.; Yoshihara, Y.; Suwa, F. Mechanical and histologic examination of titanium alloy material treat-ed by sandblasting and anodic oxidization. Int. J. Oral Maxillofac. Implants 2005, 20, 48–53. [Google Scholar] [PubMed]
- Shayganpour, A.; Rebaudi, A.; Cortella, P.; Diaspro, A.; Salerno, M. Electrochemical coating of dental implants with anodic porous titania for enhanced osteointegration. Beilstein J. Nanotechnol. 2015, 6, 2183–2192. [Google Scholar] [CrossRef]
- Annarelli, C.; Fornazero, J.; Cohen, R.; Bert, J.; Besse, J.-L. Colloidal Protein Solutions as a New Standard Sensor for Adhesive Wettability Measurements. J. Colloid Interface Sci. 1999, 213, 386–394. [Google Scholar] [CrossRef]
- Sharma, P.; Rao, K.H. Analysis of different approaches for evaluation of surface energy of microbial cells by contact angle goniometry. Adv. Colloid Interface Sci. 2002, 98, 341–463. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface to-pography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Bürgers, R.; Gerlach, T.; Hahnel, S.; Schwarz, F.; Handel, G.; Gosau, M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin. Oral Implants Res. 2010, 21, 156–164. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A.; Padrós, A. Fracture and fatigue behaviour of shot blasted titanium dental implants. Implant Dent. 2002, 11, 28–32. [Google Scholar] [CrossRef]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar]
- Walter, A.; Winsauer, H.; Marcé-Nogué, J.; Mojal, S.; Puigdollers, A. Design characteristics, primary stability and risk of fracture of orthodontic mini-implants: Pilot scan electron microscope and mechanical studies. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e804. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Benedicenti, S.; Itri, A. Hydro air abrasion on dental glass-ceramics: A direct 3D analysis by stylus profilometry. J. Mech. Behav. Biomed. Mater. 2019, 93, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Metrological Characteristics of Phase Correct Filters; ISO 11562:1996; ISO: Geneva, Switzerland, 1996.
- Salerno, M.; Itri, A.; Frezzato, M.; Rebaudi, A. Surface Microstructure of Dental Implants Before and After Insertion: An in vitro study by means of scanning probe microscopy. Implant Dent. 2015, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, M.; Gil, F.; Planell, J.; Aparicio, C. The influence of blasting and sterilization on static and time-related wettability and surface-energy properties of titanium surfaces. Surf. Coat. Technol. 2008, 202, 3470–3479. [Google Scholar] [CrossRef]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Pegueroles, M.; Tonda-Turo, C.; Planell, J.A.; Gil, F.J.; Aparicio, C. Adsorption of fibronectin, fibrinogen, and albu-min on TiO2, Time-resolved kinetics, structural changes, and competition study. Biointerphases 2012, 7, 48. [Google Scholar] [CrossRef]
- Guillem-Marti, J.; Delgado, L.M.; Godoy-Gallardo, M.; Pegueroles, M.; Herrero, M.; Gil, F.J.; Herrero-Climent, M. Fibroblast adhesion and activation onto micro-machined titanium surfaces. Clin. Oral Implant. Res. 2012, 24, 770–780. [Google Scholar] [CrossRef]
- Manero, J.M.; Gil, F.; Padrós, E.; Planell, J. Applications of environmental scanning electron microscopy (ESEM) in biomaterials field. Microsc. Res. Tech. 2003, 61, 469–480. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Espinar-Escalona, E.; Bravo-Gonzalez, L.A.; Pegueroles, M.; Gil, F.J. Roughness and wettability effect on histologi-cal and mechanical response of self-drilling orthodontic mini-implants. Clin. Oral Investig. 2016, 20, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.A.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F.J. Relevant aspects in the surface properties in titanium dental implants for the cellular vi-ability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Ghosal, P.; Muraleedharan, K.; Nandy, T.K.; Ray, K.K. Effect of boron and carbon addition on micro-structure and mechanical properties of Ti-15-3 alloy. Mater. Sci. Eng. A 2011, 528, 4819–4829. [Google Scholar] [CrossRef]
- Burmann, P.F.P.; Ruschel, H.C.; Vargas, I.A.; De Verney, J.C.K.; Kramer, P.F. Titanium alloy orthodontic mini-implants: Scanning electron microscopic and metallographic analyses. Acta Odontol. Lat. 2015, 28, 42–47. [Google Scholar]
- Patil, P.; Kharbanda, O.P.; Duggal, R.; Das, T.K.; Kalyanasundaram, D. Surface deterioration and elemental compo-sition of retrieved orthodontic miniscrews. Am. J. Orthod. Dentofac. Orthop. 2015, 147, S88–S100. [Google Scholar] [CrossRef] [PubMed]
- Könönen, M.H.; Lavonius, E.T.; Kivilahti, J.K. SEM observations on stress corrosion cracking of commercially pure titanium in a topical fluoride solution. Dent. Mater. 1995, 11, 269–272. [Google Scholar] [CrossRef]
- Chung, K.R.; Choo, H.; Kim, S.H.; Ngan, P. Timely relocation of mini-implants for uninterrupted full-arch distali-zation. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 839–849. [Google Scholar] [CrossRef]
- Baek, S.-H.; Kim, B.-M.; Kyung, S.-H.; Lim, J.K.; Kim, Y.H. Success Rate and Risk Factors Associated with Mini-Implants Reinstalled in the Maxilla. Angle Orthod. 2008, 78, 895–901. [Google Scholar] [CrossRef]
- Hepner, D.L.; Castells, M.C. Latex Allergy: An Update. Anesth. Analg. 2003, 96, 1219–1229. [Google Scholar] [CrossRef]
- Neugut, A.I.; Ghatak, A.T.; Miller, R.L. Anaphylaxis in the United States: An investigation into its epidemiology. Arch. Intern. Med. 2001, 161, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R. Perioperative care of patients with latex allergy. AORN J. 2000, 72, 47–54. [Google Scholar] [CrossRef]
- de Morais, L.S.; Serra, G.; Pelero, G.F.A.; Rodrigues, L.; Muller, C.; Meyers, A.; Elias, C.N. Systemic levels of metallic ions released from orthodontic mini-implants. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Kim, S.K.; Chang, Y.I.; Baek, S.H. In vitro and in vivo mechanical stability of orthodontic mini-implants: Effect of sandblasted, large-grit, and anodic-oxidation vs. sandblasted, large-grit, and acid-etching. Angle Orthod. 2012, 82, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Baumgaertel, S.; Hans, M.G. Buccal cortical bone thickness for mini-implant placement. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T.; Takano-Yamamoto, T.; Kanomi, R.; Hartsfield, J.K.J.; Roberts, W.E.; Garetto, L.P. The use of small titani-um screws for orthodontic anchorage. J. Dent. Res. 2003, 82, 377–381. [Google Scholar] [CrossRef]
- Hitchon, P.W.; Brenton, M.D.; Coppes, J.K.; From, A.M.; Torner, J.C. Factors Affecting the Pullout Strength of Self-Drilling and Self-Tapping Anterior Cervical Screws. Spine 2003, 28, 9–13. [Google Scholar] [CrossRef]
- Sevilla, P.; Gil, J.; Aparicio, C. Relevant Properties for Immobilizing Short Peptides on Biosurfaces. IRBM 2017, 38, 256–265. [Google Scholar] [CrossRef]
- Giner, L.; Mercadé, M.; Torrent, S.; Punset, M.; Perez, R.; Delgado, L.M.; Gil, F.J. Double acid etching treatment of dental implants for enhanced biological properties. J. Appl. Biomater. Funct. Mater. 2017, 16, 83–89. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Román, J.; Padilla, S.; Doadrio, J.; Gil, F.J. Bioactivity and mechanical properties of SiO2-CaO-P2O5 glass-ceramics. J. Mater. Chem. 2005, 15, 1353–1359. [Google Scholar] [CrossRef]
- Fernández, E.; Gil, F.J.; Best, S.; Ginebra, M.P.; Driessens, F.C.; Planell, J.A. The cement setting reaction in the Ca-HPO4-alpha-Ca3(PO4)2 system: An X-ray diffraction study. J. Biomed. Mater. Res. 1998, 42, 403–406. [Google Scholar] [CrossRef]
- Roberts, W.; Smith, R.K.; Zilberman, Y.; Mozsary, P.G.; Smith, R.S. Osseous adaptation to continuous loading of rigid endosseous implants. Am. J. Orthod. 1984, 86, 95–111. [Google Scholar] [CrossRef]
- Lee, S.J.; Chung, K.R. The effect of early loading on the direct bone-to-implant surface contact of the orthodontic osseointegrated titanium implant. Korean J. Orthod. 2001, 31, 173–185. [Google Scholar]
- Arciniegas, M.P.; Casals, J.; Manero, J.M.; Pena, J.; Gil, F.J. Study of hardness and wear behaviour of NiTi shape memory alloys. J. Alloy. Compd. 2008, 460, 213–219. [Google Scholar] [CrossRef]
| Handling Material (A) | Other Handling Materials (B) | Mean Difference (A-B) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Dry sterile gauze | Gauze in chlorhexidine | 2.30 | 0.68 | 0.014 | 0.35 | 4.25 |
| Nitrile | −8.15 | 0.68 | 0.000 | −10.10 | −6.20 | |
| Latex | −4.80 | 0.68 | 0.000 | −6.75 | −2.85 | |
| Sterile gauze soaked | Dry gauze | −2.30 | 0.68 | 0.014 | −4.25 | −0.35 |
| in chlorhexidine | Nitrile | −10.45 | 0.68 | 0.000 | −12.40 | −8.50 |
| Latex | −7.10 | 0.68 | 0.000 | −9.05 | −5.15 | |
| Handling Material | Screw | Mean | Std. Error | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Dry sterile gauze | Dual Top | 2.40 | 0.96 | 0.48 | 4.32 |
| Spider | 4.60 | 0.96 | 2.68 | 6.52 | |
| Microdent | 3.40 | 0.96 | 1.48 | 5.32 | |
| Absoanchor | 2.60 | 0.96 | 0.68 | 4.52 | |
| Sterile gauze soaked | Dual Top | 0.80 | 0.96 | −1.12 | 2.72 |
| in chlorhexidine | Spider | 1.00 | 0.96 | −0.92 | 2.92 |
| Microdent | 1.20 | 0.96 | −0.72 | 3.12 | |
| Absoanchor | 0.80 | 0.96 | −1.12 | 2.72 | |
| Nitrile glove | Dual Top | 11.00 | 0.96 | 9.08 | 12.92 |
| Spider | 12.20 | 0.96 | 10.28 | 14.12 | |
| Microdent | 12.00 | 0.96 | 10.08 | 13.92 | |
| Absoanchor | 10.40 | 0.96 | 8.48 | 12.32 | |
| Latex glove | Dual Top | 7.20 | 0.96 | 5.28 | 9.12 |
| Spider | 9.20 | 0.96 | 2.28 | 11.12 | |
| Microdent | 9.20 | 0.96 | 7.28 | 11.12 | |
| Absoanchor | 6.60 | 0.96 | 4.68 | 8.52 | |
| Implant Surface | Sa (m) | Sm (m) | Index Area | Contact Angle (°) |
|---|---|---|---|---|
| Control | 0.3 ± 0.2 * | 36 ± 9 * | 1.12 ± 0.20 * | 64 ± 3 * |
| AO | 0.9 ± 0.4 * | 90 ± 15 * | 1.30 ± 0.11 * | 69 ± 2 * |
| SB | 3.3 ± 0.4 & | 128 ± 10 & | 1.77 ± 0.22 & | 78 ± 2 & |
| SBAO | 3.9 ± 0.2 & | 140 ± 12 & | 1.70 ± 0.19 & | 75 ± 5 & |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zogheib, T.; Walter-Solana, A.; de la Iglesia, F.; Espinar, E.; Gil, J.; Puigdollers, A. Do Titanium Mini-Implants Have the Same Quality of Finishing and Degree of Contamination before and after Different Manipulations? An In Vitro Study. Metals 2021, 11, 245. https://doi.org/10.3390/met11020245
Zogheib T, Walter-Solana A, de la Iglesia F, Espinar E, Gil J, Puigdollers A. Do Titanium Mini-Implants Have the Same Quality of Finishing and Degree of Contamination before and after Different Manipulations? An In Vitro Study. Metals. 2021; 11(2):245. https://doi.org/10.3390/met11020245
Chicago/Turabian StyleZogheib, Tatiana, André Walter-Solana, Fernando de la Iglesia, Eduardo Espinar, Javier Gil, and Andreu Puigdollers. 2021. "Do Titanium Mini-Implants Have the Same Quality of Finishing and Degree of Contamination before and after Different Manipulations? An In Vitro Study" Metals 11, no. 2: 245. https://doi.org/10.3390/met11020245
APA StyleZogheib, T., Walter-Solana, A., de la Iglesia, F., Espinar, E., Gil, J., & Puigdollers, A. (2021). Do Titanium Mini-Implants Have the Same Quality of Finishing and Degree of Contamination before and after Different Manipulations? An In Vitro Study. Metals, 11(2), 245. https://doi.org/10.3390/met11020245









