Effect of Ce Content on Microstructure-Toughness Relationship in the Simulated Coarse-Grained Heat-Affected Zone of High-Strength Low-Alloy Steels
Abstract
:1. Introduction
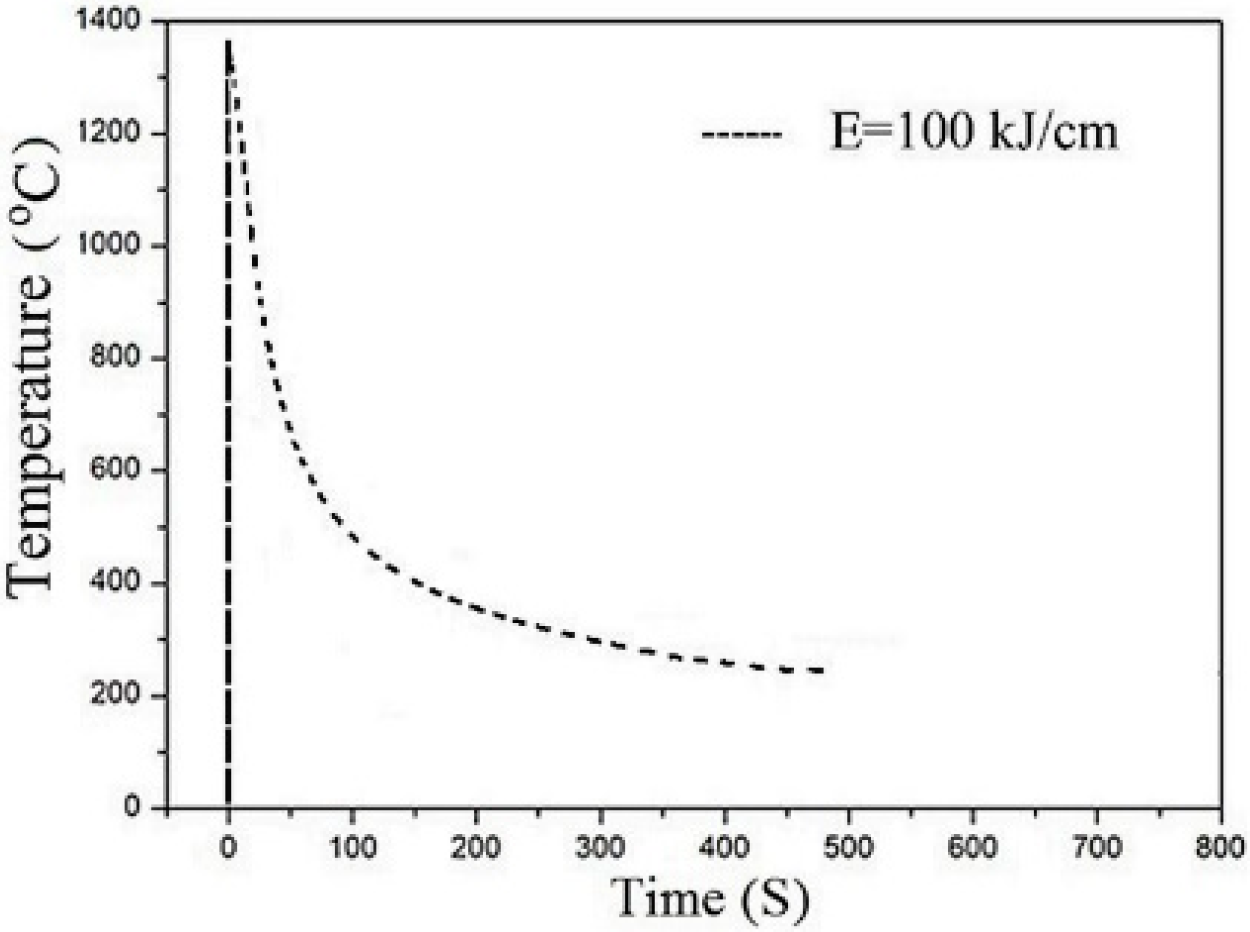
2. Materials and Methods
3. Results
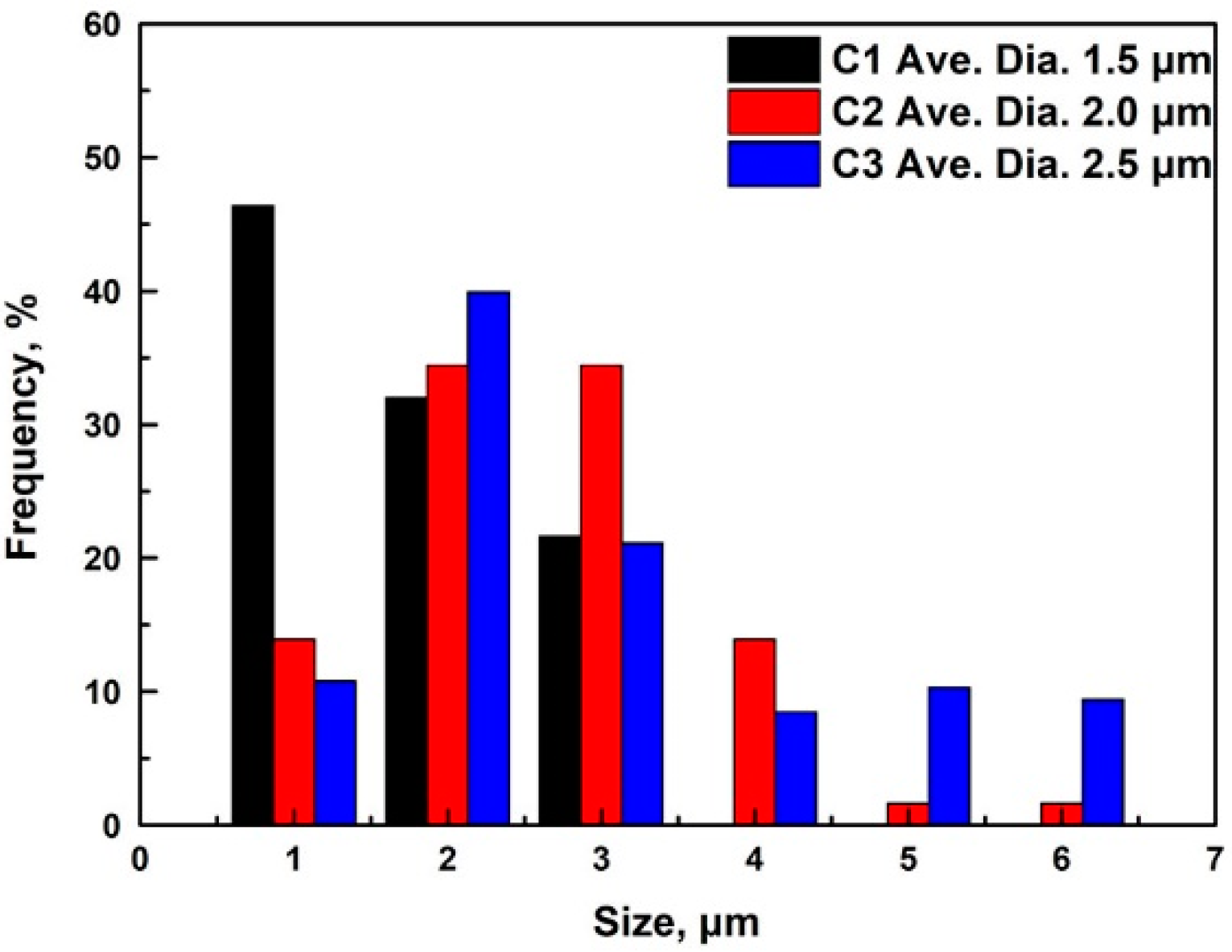
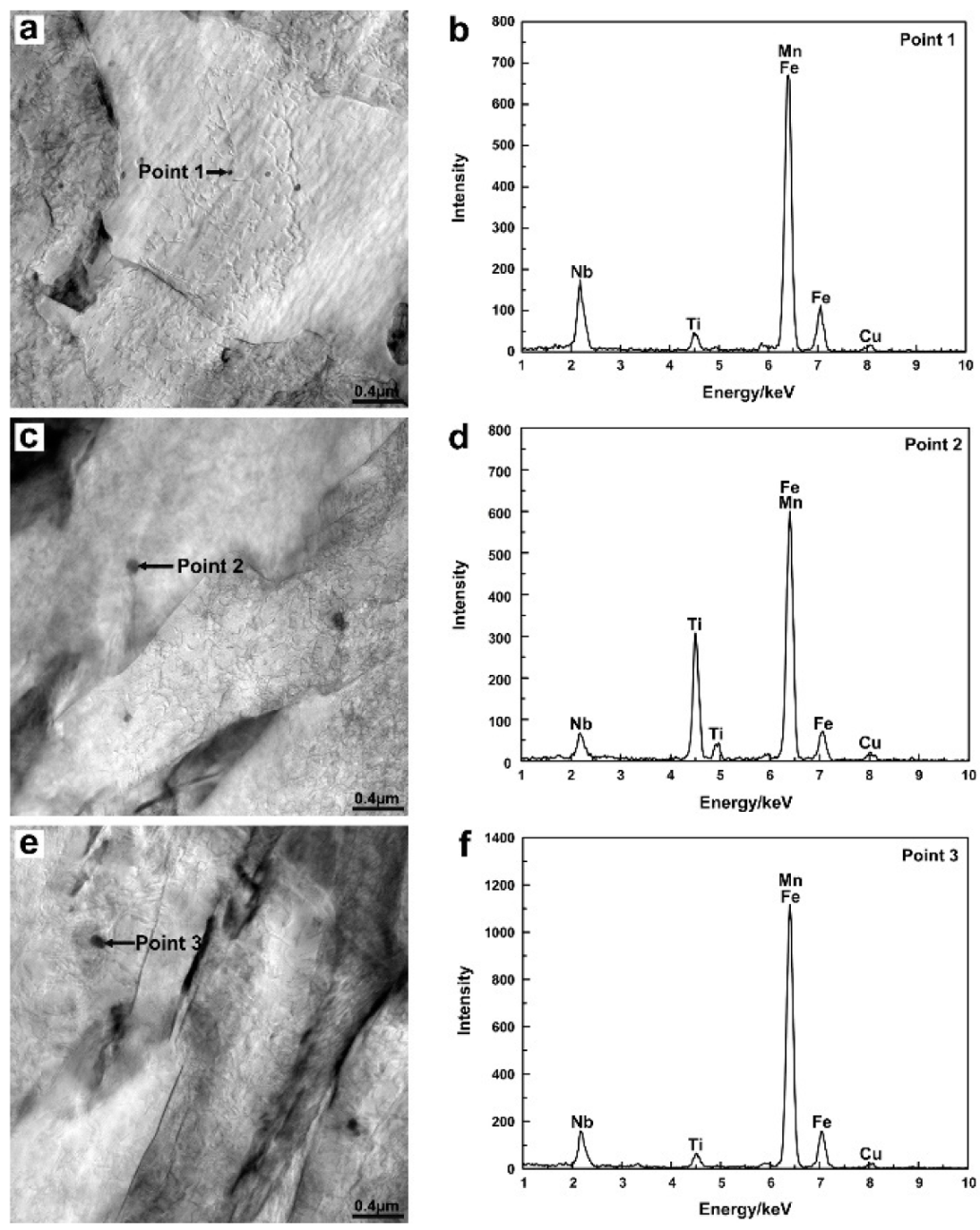
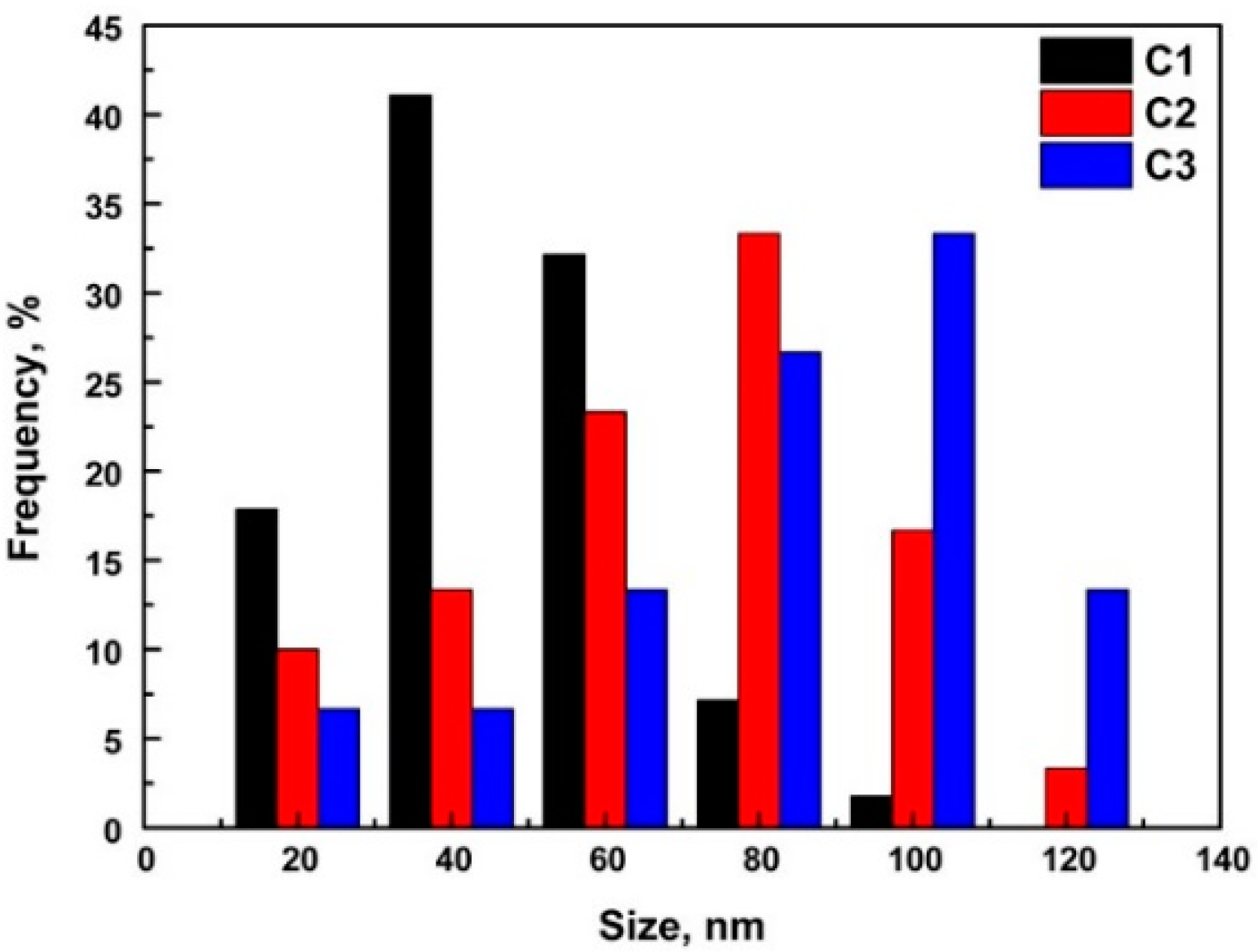
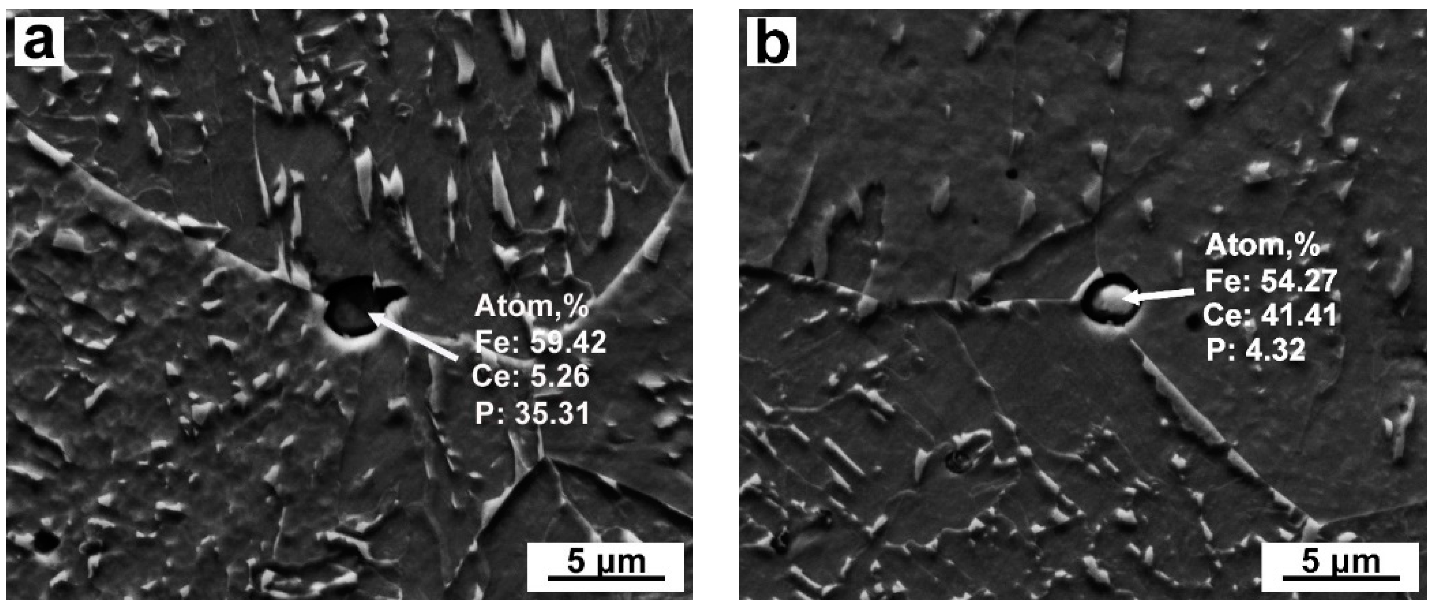
3.1. Particle Analysis
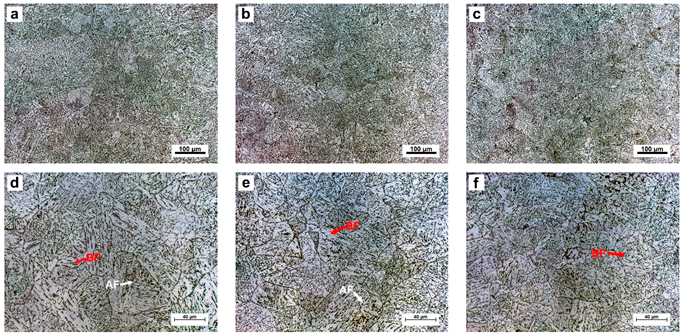
3.2. Microstructural Characteristic in the Simulated CGHAZ
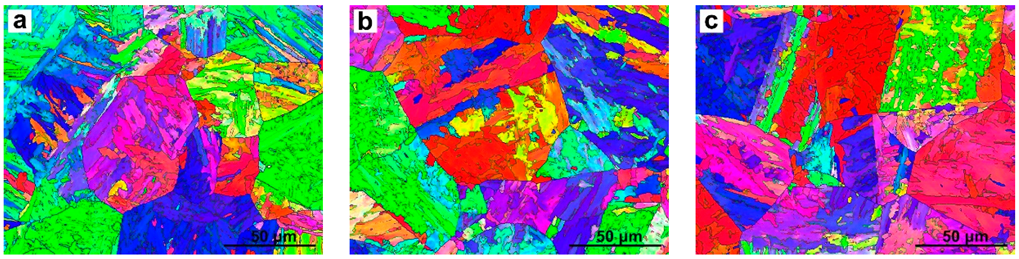
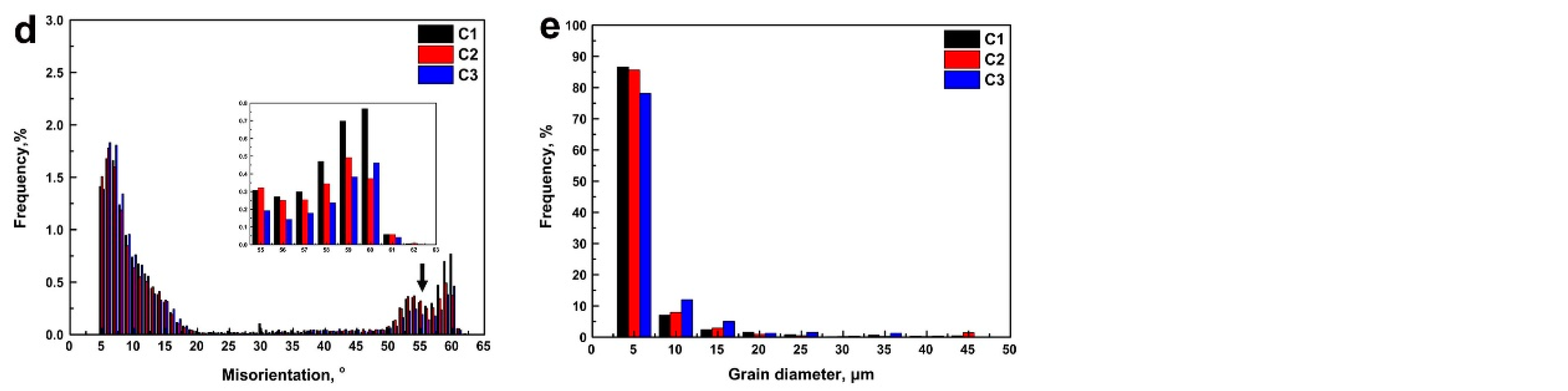
3.3. EBSD Analysis in the Simulated CGHAZ
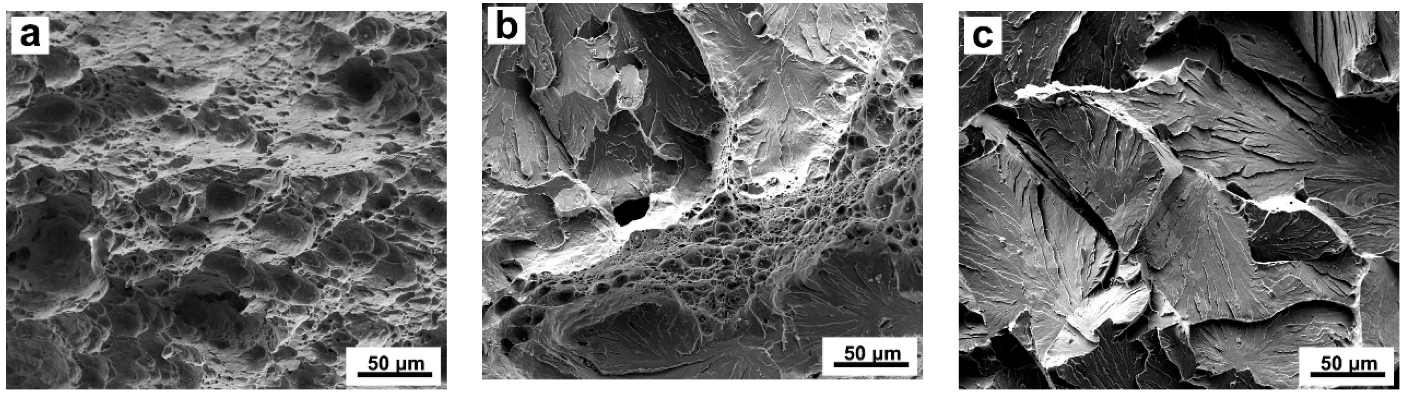
3.4. Impact Toughness and Fractured Surface Characteristics in the Simulated CGHAZ
4. Discussion
4.1. The Effect of Ce Content on Grain Refinement
4.2. Effect of Ce Content on Impact Toughness and the Formation of Acicular Ferrite
5. Conclusions
- With the increasing of the Ce content from 0.012 wt.% to 0.086 wt.%, the Ce2O2S inclusion compounds in the C1 steel were gradually modified to CexSy-CeP in the C2 steel and CeP in the C3 steel. The number and density of inclusion compounds also increased with the increasing of the Ce content.
- Compared with C2 and C3 steels, the Ce2O2S inclusion compounds in C1 steel were more conducive to inducing the formation of AF due to their lower mismatch with α-Fe, which contributed to the improvement of the toughness in the CGHAZ.
- Smaller prior austenite grain was observed in the C3 steel because of the formation of CeP at the grain boundary. However, the impact toughness in the CGHAZ of the C3 steel decreased due to the larger size and density of the CeP.
- The superior toughness in the simulated CGHAZ of the C1 steel was attributed to the formation of a fine-grained microstructure with a higher density of acicular ferrite grains and a lower fraction of M-A constituents.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hashemi, S.G.; Eghbali, B. Analysis of the formation conditions and characteristics of interphase and random vanadium precipitation in a low-carbon steel during isothermal heat treatment. Int. J. Miner. Metall. Mater. 2018, 3, 339–349. [Google Scholar] [CrossRef]
- Xie, Z.; Shang, C.J.; Wang, X.; Wang, X.; Han, G.; Misra, R. Recent progress in third-generation low alloy steels developed under M3 microstructure control. Int. J. Miner. Metall. Mater. 2020, 27, 1–9. [Google Scholar] [CrossRef]
- Gan, X.L.; Wan, X.L.; Zhang, Y.J.; Wang, H.H.; Li, G.Q.; Xu, G.; Wu, K.M. Investigation of characteristic and evolution of fine-grained bainitic microstructure in the coarse-grained heat-affected zone of super-high strength steel for offshore structure. Mater. Charact. 2019, 157, 109893. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Liu, D.; Pan, X.Q.; Xu, L.Y. Improvement of impact toughness of the welding heat-affected zone in high-strength low-alloy steels through Ca deoxidation. Metall. Mater. Trans. A 2021, 52, 668–679. [Google Scholar] [CrossRef]
- Tervo, H.; Kaijalainen, A.; Pallaspuro, S.; Anttila, S.; Mehtonen, S.; Porter, D.; Kömi, J. Low-temperature toughness properties of 500 MPa offshore steels and their simulated coarse-grained heat-affected zones. Mater. Sci. Eng. A 2020, 773, 138719. [Google Scholar] [CrossRef]
- Lin, C.K.; Su, Y.H.; Hwang, W.S.; Lin, G.R.; Kuo, J.C. On pinning effect of austenite grain growth in Mg-containing low-carbon steel. Mater. Sci. Technol. 2018, 34, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.Q.; Wan, X.L.; Zhang, X.G.; Shen, Y.; Wu, K.M. Toughness improvement by Zr addition in the simulated coarse-grained heat-affected zone of high strength low-alloy steels. Ironmak. Steelmak. 2019, 46, 113–123. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.Q.; Wan, X.L.; Wang, H.H.; Wu, K.M.; Misra, R.D.K. The role of Cu and Al addition on the microstructure and fracture characteristics in the simulated coarse-grained heat-affected zone of high-strength low-alloy steels with superior toughness. Mater. Sci. Technol. 2017, 33, 1750–1764. [Google Scholar] [CrossRef]
- Xiong, Z.H.; Liu, S.L.; Wang, X.M.; Shang, C.J.; Li, X.C.; Misra, R.D.K. The contribution of intragranular acicular ferrite microstructural constituent on impact toughness and impeding crack initiation and propagation in the heat-affected zone (HAZ) of low-carbon steels. Mater. Sci. Eng. A 2015, 636, 117–123. [Google Scholar] [CrossRef]
- Shim, J.; Cho, Y. Nucleation of intragranular ferrite at Ti2O3 particle in low carbon steel. Acta Mater. 1999, 47, 2751–2760. [Google Scholar] [CrossRef]
- Li, X.; Fan, Y.; Ma, X.; Subramanian, S.V.; Shang, C.J. Influence of Martensite-Austenite constituents formed at different intercritical temperatures on toughness. Mater. Des. 2015, 67, 457–463. [Google Scholar] [CrossRef]
- Cui, J.J.; Zhu, W.T.; Chen, Z.Y.; Chen, L.Q. Microstructural Characteristics and Impact Fracture Behaviors of a Novel High-Strength Low-Carbon Bainitic Steel with Different Reheated Coarse-Grained Heat-Affected Zones. Metall. Mater. Trans. A 2020, 51, 1–11. [Google Scholar] [CrossRef]
- Zou, X.D.; Sun, J.C.; Matsuura, H.; Wang, C. In situ observation of the nucleation and growth of ferrite laths in the heat-affected zone of EH36-Mg shipbuilding steel subjected to different heat inputs. Metall. Mater. Trans. B 2018, 49, 2168–2173. [Google Scholar] [CrossRef]
- Shen, Y.; Wan, X.L.; Liu, Y.; Zheng, Z.L.; Wu, K.M. The significant impact of Ti content on microstructure-toughness relationship in the simulated coarse-grained heated-affected zone of high-strength low-alloy steels. Ironmak. Steelmak. 2018, 46, 584–596. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, X.L.; Li, G.Q.; Wang, Y.; Zheng, W.; Hou, Y.H. Grain refinement in coarse-grained heat-affected zone of Al-Ti-Mg complex deoxidised steel. Sci. Technol. Weld. Join. 2019, 24, 43–51. [Google Scholar] [CrossRef]
- Liu, D.K.; Yang, J.; Zhang, Y.H.; Xu, L.Y. Effect of Mo content on nano-scaled particles, prior austenite grains and impact toughness of CGHAZ in offshore engineering steels. J. Iron Steel Res. Int. 2021. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Song, M.M.; Song, B.; Xin, W.B.; Sun, G.L.; Song, G.Y.; Hu, C.L. Effects of rare earth addition on microstructure of C-Mn steel. Ironmak. Steelmak. 2015, 42, 594–599. [Google Scholar] [CrossRef]
- Guo, F.; Lin, Q. Study on interaction between lanthanum and phosphor in purity steel. J. Rare Earth 2006, 24, 409–412. [Google Scholar]
- Cao, Y.X.; Wan, X.L.; Hou, Y.H.; Liu, Y.; Song, M.M.; Li, G.Q. Comparative study on the effect of Y content on grain refinement in the simulated coarse-grained heat-affected zone of X70 pipeline steels. Micron 2019, 127, 102758. [Google Scholar] [CrossRef]
- Yan, N.; Yu, S.; Ying, C. In situ observation of austenite grain growth and transformation temperature in coarse grain heat affected zone of Ce-alloyed weld metal. J. Rare Earth 2017, 35, 203–210. [Google Scholar] [CrossRef]
- Cao, Y.X.; Wan, X.L.; Zhou, F.; Dong, H.Y.; Wu, K.M.; Li, G.Q. The critical influence of La content on the microstructure-toughness relationship in the simulated coarse-grained heat-affected zone of high-strength low-alloy steels. Metall. Res. Technol. 2021, 118, 212. [Google Scholar] [CrossRef]
- Dere, G.E.; Sharma, H.; Petrov, R.H.; Sietsma, J.; Offerman, S.E. Effect of niobium and grain boundary density on the fire resistance of Fe-C-Mn steel. Scr. Mater. 2013, 68, 651–654. [Google Scholar] [CrossRef]
- Wan, X.L.; Wu, K.M.; Nune, K.C.; Li, Y.; Cheng, L. In situ observation of acicular ferrite formation and grain refinement in simulated heat affected zone of high strength low alloy steel. Sci. Technol. Weld. Join. 2015, 20, 254–263. [Google Scholar] [CrossRef]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five decades of the Zener equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.W.; Zhou, S.B.; Huang, J.H. Austenite grain size prediction in the coarse-grained heat-affected zone of the developed Cu-free high-strength low-alloy hull structure steel. Metall. Mater. Trans. A 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Seah, M.P.; Spencer, P.J.; Hondros, E.D. Additive remedy for temper brittleness. Mater. Sci. Technol. 2013, 13, 307–314. [Google Scholar] [CrossRef]
- Shim, J.H.; Byun, J.S.; Cho, Y.W.; Oh, Y.J.; Lee, D.N. Mn absorption characteristics of Ti2O3 inclusions in low carbon steel. Scr. Mater. 2001, 44, 49–54. [Google Scholar] [CrossRef]
- Hou, Y.; Zheng, W.; Wu, Z.; Li, G.Q.; Moelans, N.; Guo, M.X.; Khan, B.S. Study of Mn absorption by complex oxide inclusions in Al-Ti-Mg killed steels. Acta Mater. 2016, 118, 8–16. [Google Scholar] [CrossRef]
- Koseki, T.; Thewlis, G. Overview inclusion assisted microstructure control in C-Mn and low alloy steel welds. Mater. Sci. Technol. 2013, 21, 867–879. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous hucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Mohseni, P.; Solberg, J.K.; Karlsen, M.; Akselsen, O.M.; Østby, E. Cleavage fracture initiation at M–A constituents in intercritically coarse-grained heat-affected zone. Metall. Mater. Trans. A 2014, 45, 384–394. [Google Scholar] [CrossRef]
- Yang, L.Y.; Wang, B.F.; Li, C.L.; Wang, Q. Effect of rare earth on microstructure and phase transformations point. J. Baotou Univ. Iron Steel Technol. 2004, 23, 45–47. [Google Scholar]


| Samples | C | Mn | Al | Si | Ti | Nb | Cr | Ce | P | S | O | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 0.05 | 1.62 | 0.038 | 0.26 | 0.023 | 0.065 | 0.25 | 0.012 | 0.012 | 0.0019 | 0.0015 | 0.0028 |
| C2 | 0.05 | 1.58 | 0.041 | 0.28 | 0.019 | 0.064 | 0.26 | 0.050 | 0.011 | 0.0013 | 0.0019 | 0.0031 |
| C3 | 0.05 | 1.63 | 0.053 | 0.28 | 0.021 | 0.063 | 0.25 | 0.086 | 0.011 | 0.0010 | 0.0020 | 0.0035 |
| Samples | Inclusion Compound Size, μm | Inclusion Compound Density, /mm2 | Precipitate Size, nm | Precipitate Density, /mm2 |
|---|---|---|---|---|
| C1 | 1.5 ± 0.5 | 91 | 36 ± 8 | 1.1 × 106 |
| C2 | 2.0 ± 0.4 | 124 | 51 ± 5 | 3.7 × 105 |
| C3 | 2.5 ± 0.3 | 265 | 80 ± 7 | 3.3 × 105 |
| Samples | Mean Length of Prior Austenite Grain, μm | Mean Length of AF, μm | Fraction of AF, % | Fraction of M-A, % |
|---|---|---|---|---|
| C1 | 51.2 ± 10 | 6.1 ± 1.3 | 4.3 | 5.3 |
| C2 | 40.4 ± 9 | 4.2 ± 0.8 | 1.2 | 6.8 |
| C3 | 35.2 ± 11 | 3.9 ± 0.6 | 0.2 | 8.1 |
| Samples | −20 °C Absorbed Energy, J | Crystallographic Grain Size, μm |
|---|---|---|
| C1 | 105 ± 10 | 3.5 |
| C2 | 80 ± 15 | 3.6 |
| C3 | 24 ± 6 | 4.5 |
| Inclusion | Lattice Type | Lattice Constant d/nm | d/dα-Fe | δ/% |
|---|---|---|---|---|
| Ce2O2S | Cubic | 0.4004 | 1.395 | 0.2 |
| Ce2S3 | Cubic | 0.8749 | 3.048 | 1.7 |
| CeS | Cubic | 0.5766 | 2.013 | 0.5 |
| Ce3S4 | Cubic | 0.8630 | 3.007 | 0.4 |
| CeP | Cubic | 0.5920 | 2.063 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Wan, X.; Zhou, F.; Wang, Y.; Liu, X.; Wu, K.; Li, G. Effect of Ce Content on Microstructure-Toughness Relationship in the Simulated Coarse-Grained Heat-Affected Zone of High-Strength Low-Alloy Steels. Metals 2021, 11, 2003. https://doi.org/10.3390/met11122003
Cao Y, Wan X, Zhou F, Wang Y, Liu X, Wu K, Li G. Effect of Ce Content on Microstructure-Toughness Relationship in the Simulated Coarse-Grained Heat-Affected Zone of High-Strength Low-Alloy Steels. Metals. 2021; 11(12):2003. https://doi.org/10.3390/met11122003
Chicago/Turabian StyleCao, Yuxin, Xiangliang Wan, Feng Zhou, Yong Wang, Xinbin Liu, Kaiming Wu, and Guangqiang Li. 2021. "Effect of Ce Content on Microstructure-Toughness Relationship in the Simulated Coarse-Grained Heat-Affected Zone of High-Strength Low-Alloy Steels" Metals 11, no. 12: 2003. https://doi.org/10.3390/met11122003
APA StyleCao, Y., Wan, X., Zhou, F., Wang, Y., Liu, X., Wu, K., & Li, G. (2021). Effect of Ce Content on Microstructure-Toughness Relationship in the Simulated Coarse-Grained Heat-Affected Zone of High-Strength Low-Alloy Steels. Metals, 11(12), 2003. https://doi.org/10.3390/met11122003







