The Influence of Temperature on the Microstructure and Properties of Nb-V-Ti-Mo Complex Microalloyed High-Strength Fire-Resistant Steel
Abstract
:1. Introduction
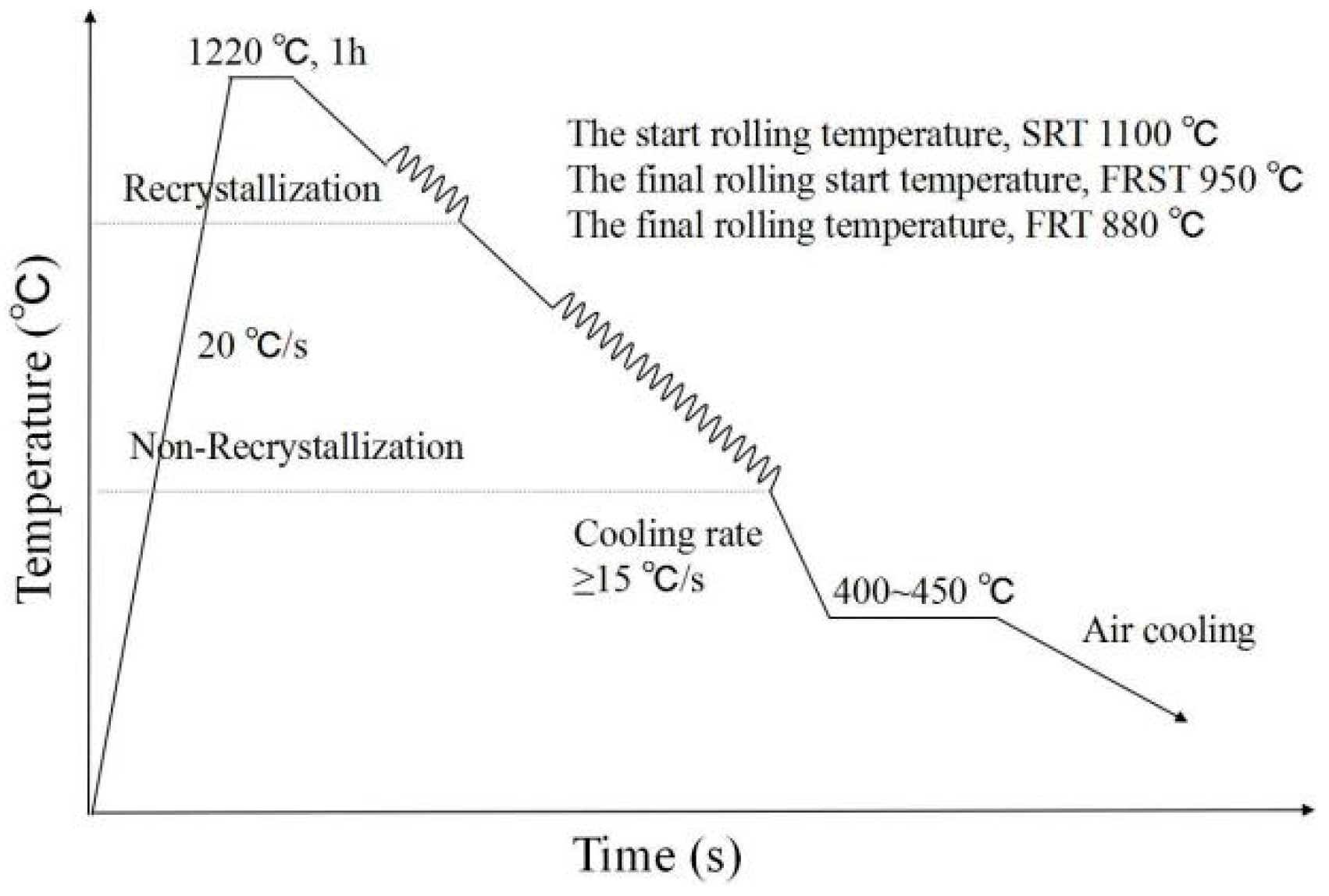
2. Experimental Materials and Methods
3. Results
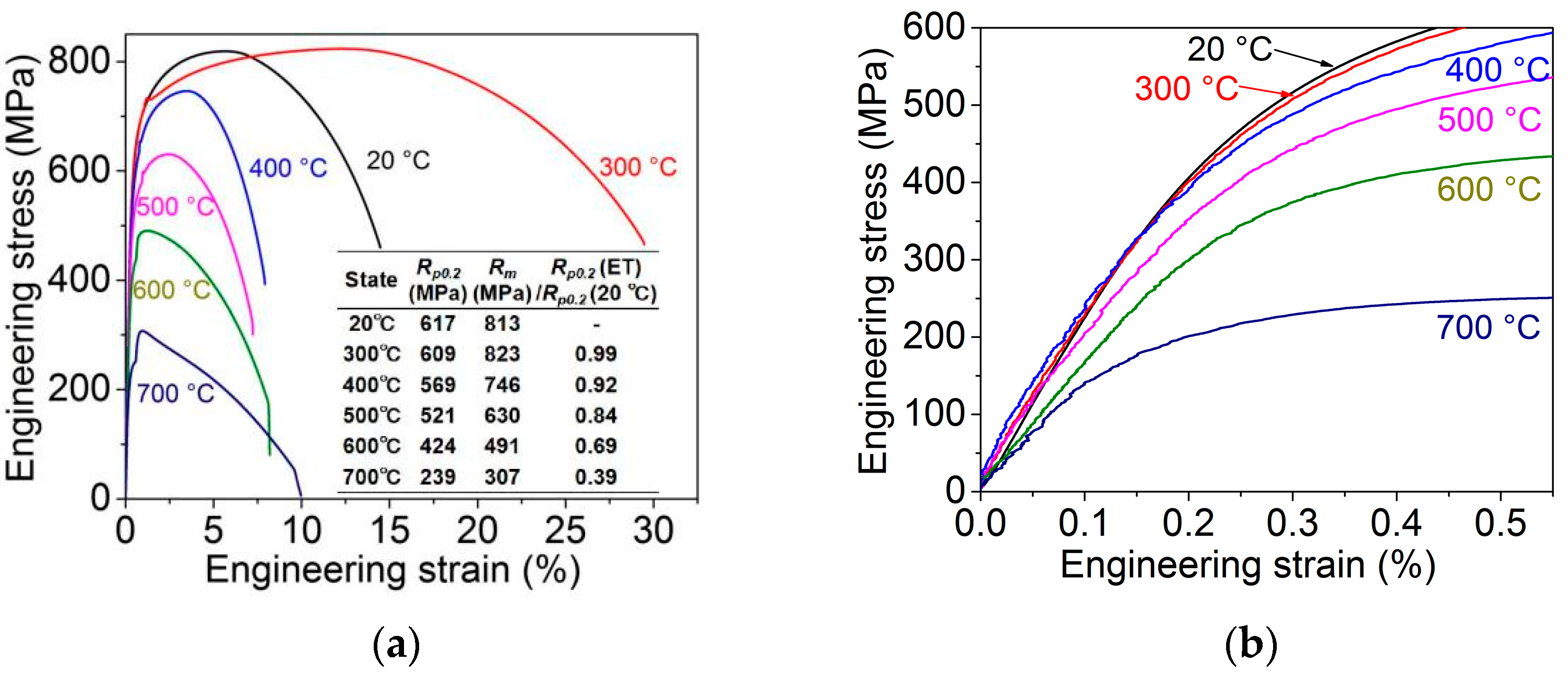
3.1. Tensile Properties at Different Temperatures
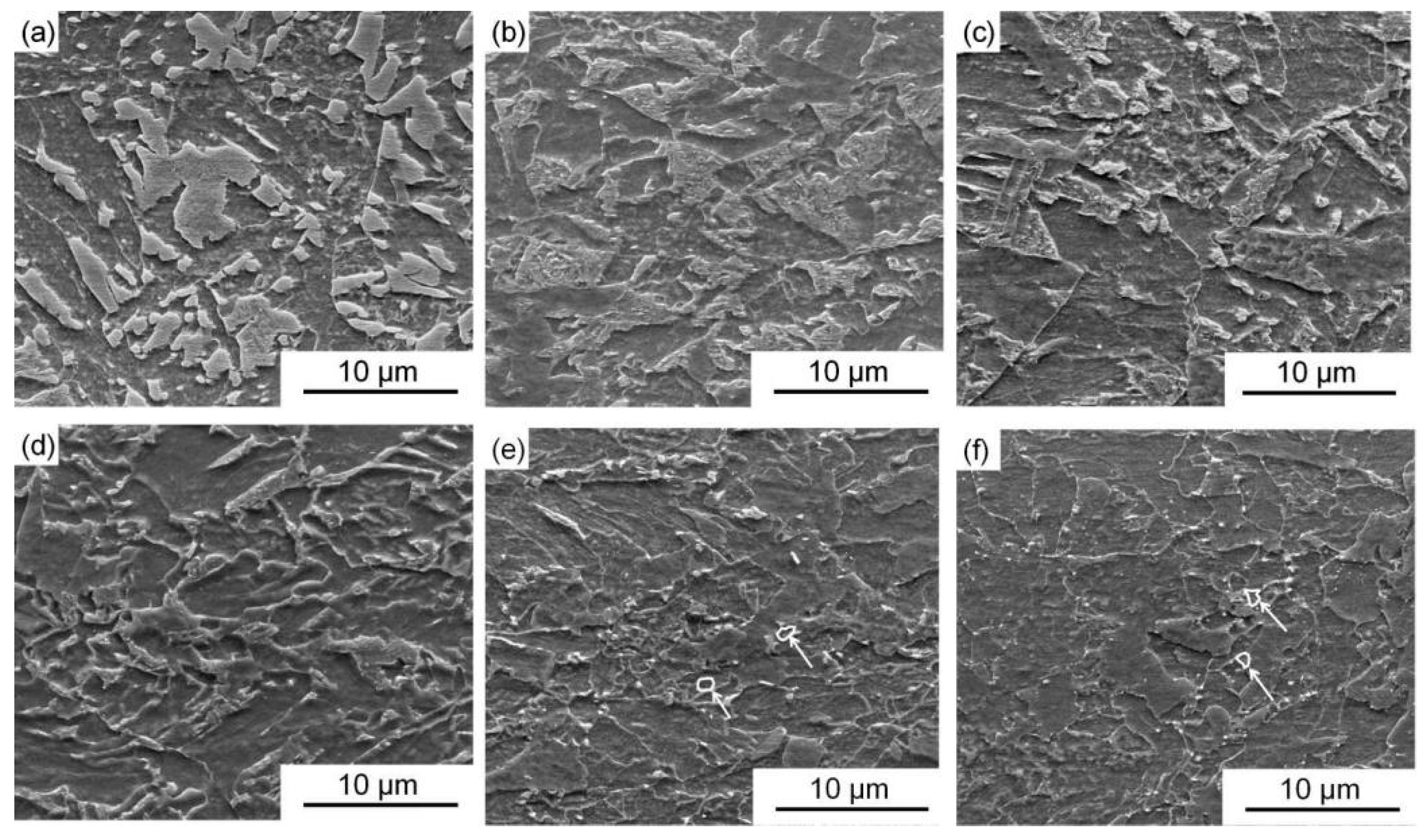
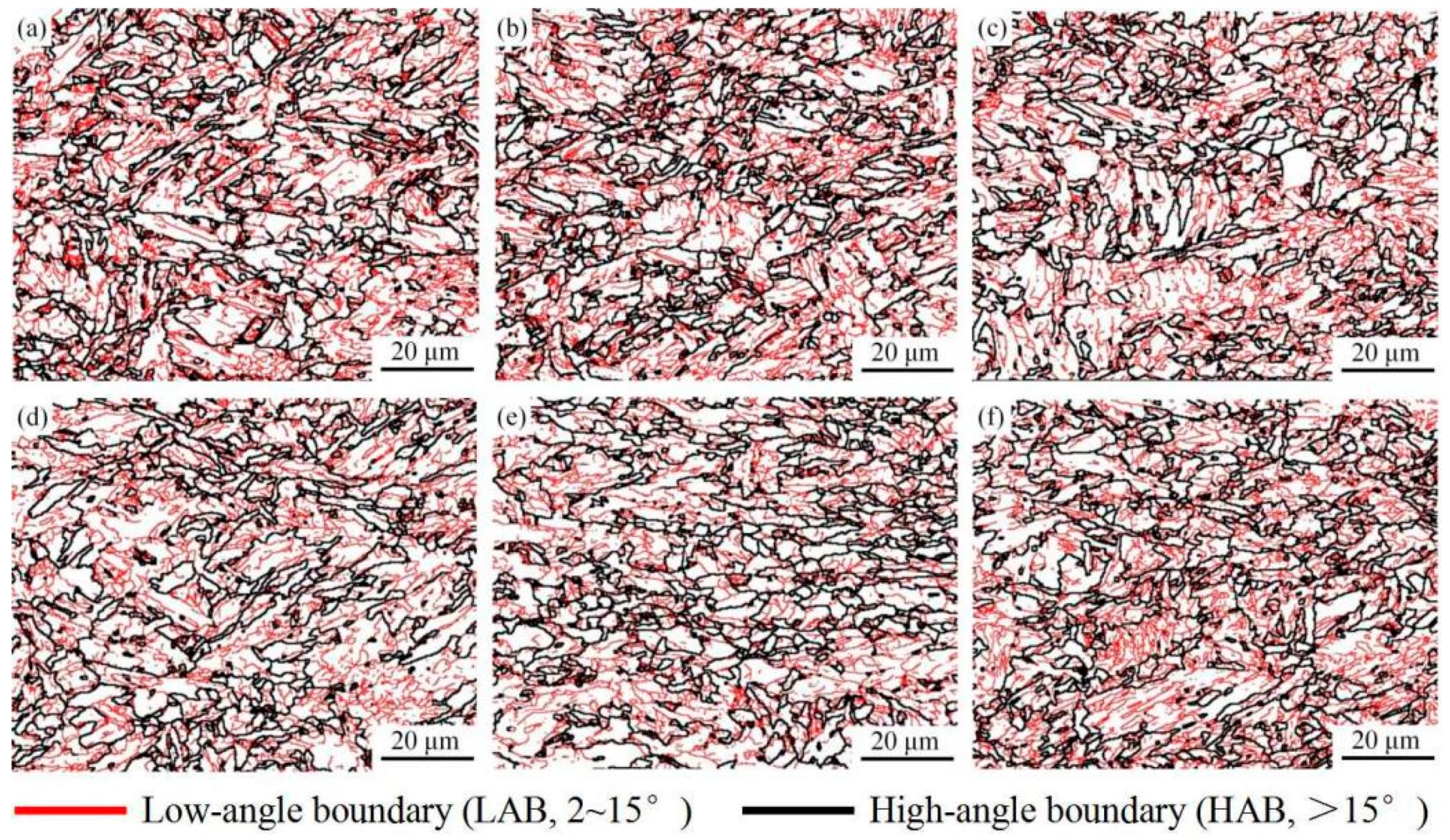
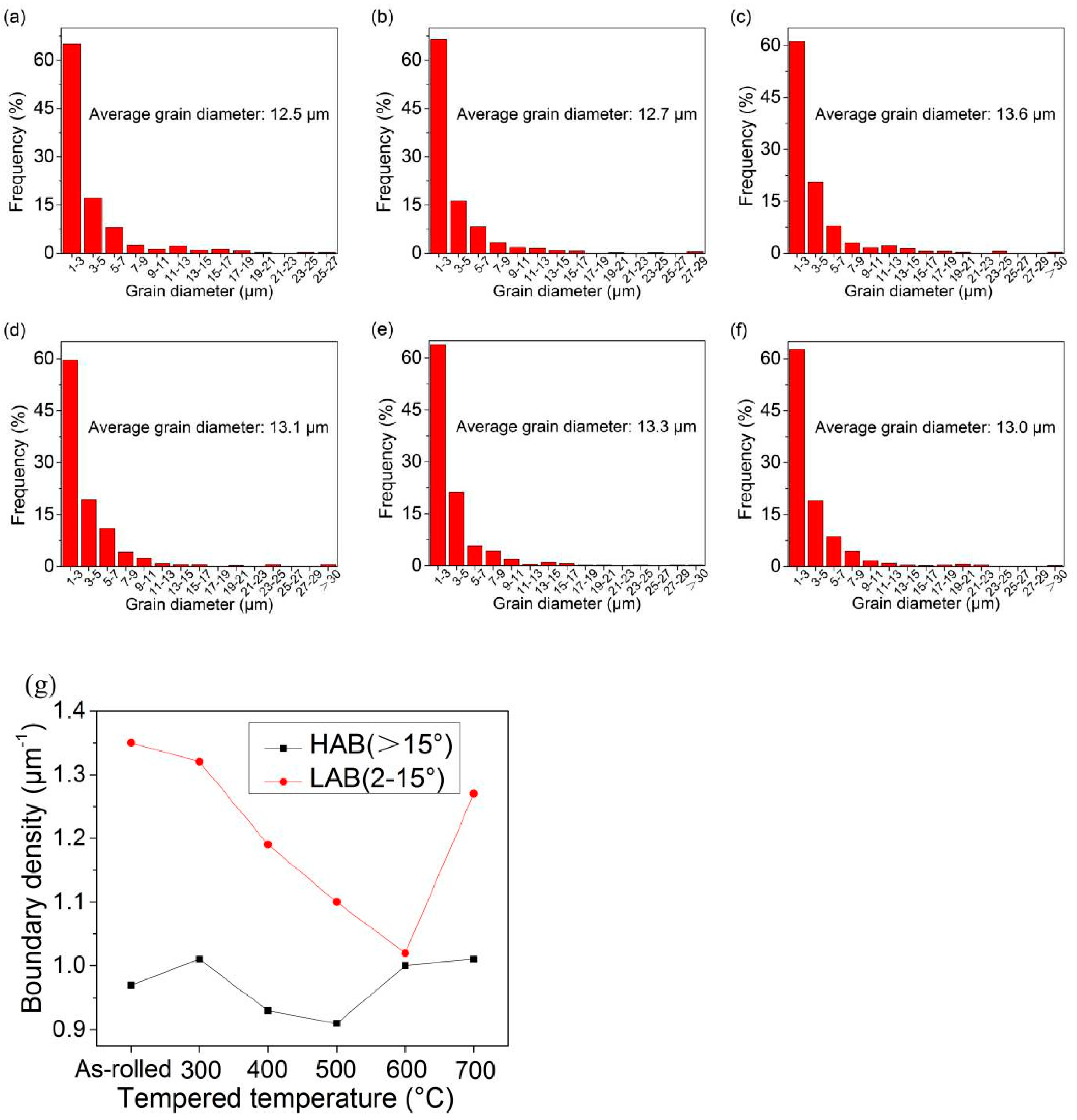
3.2. Microstructures
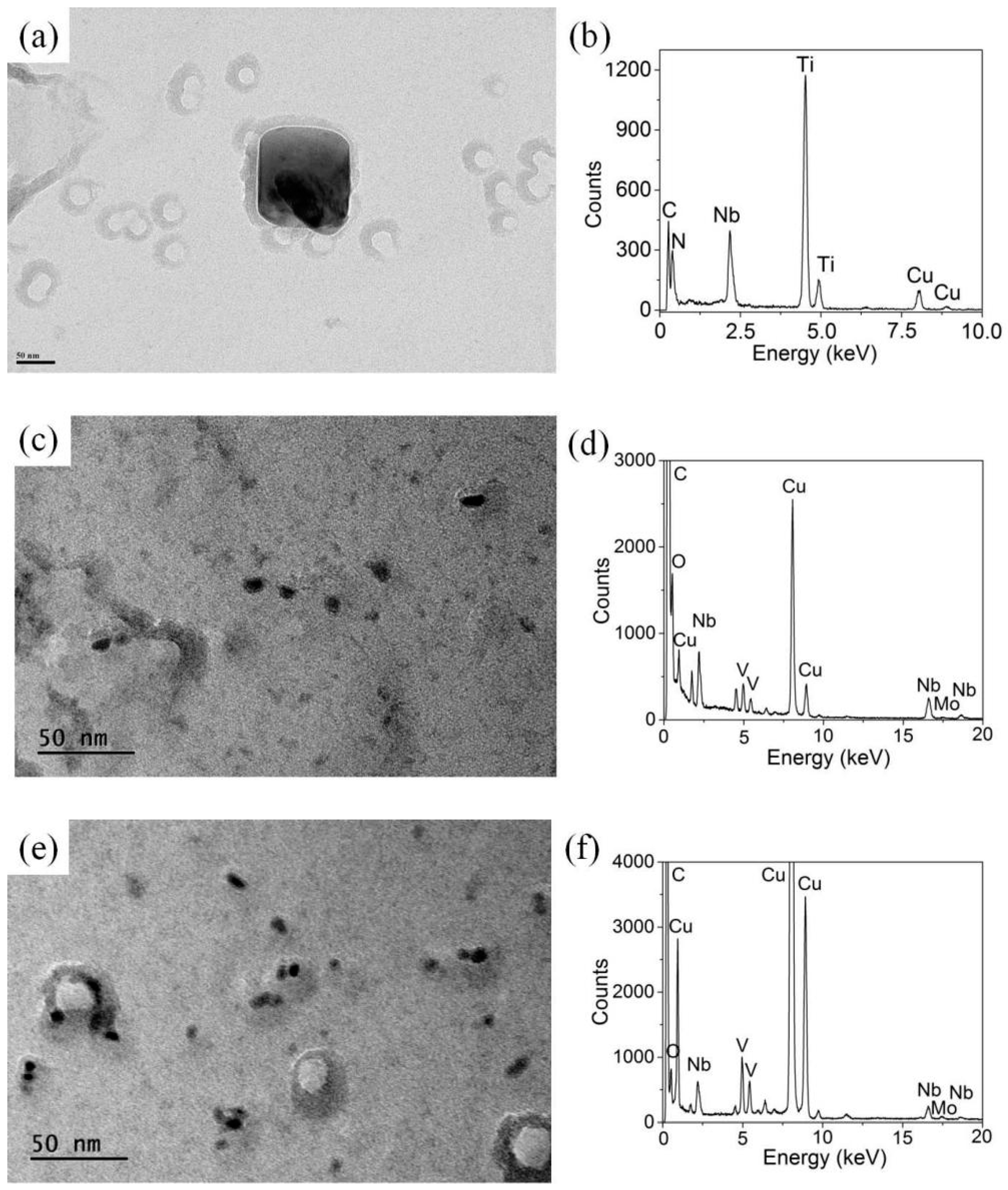
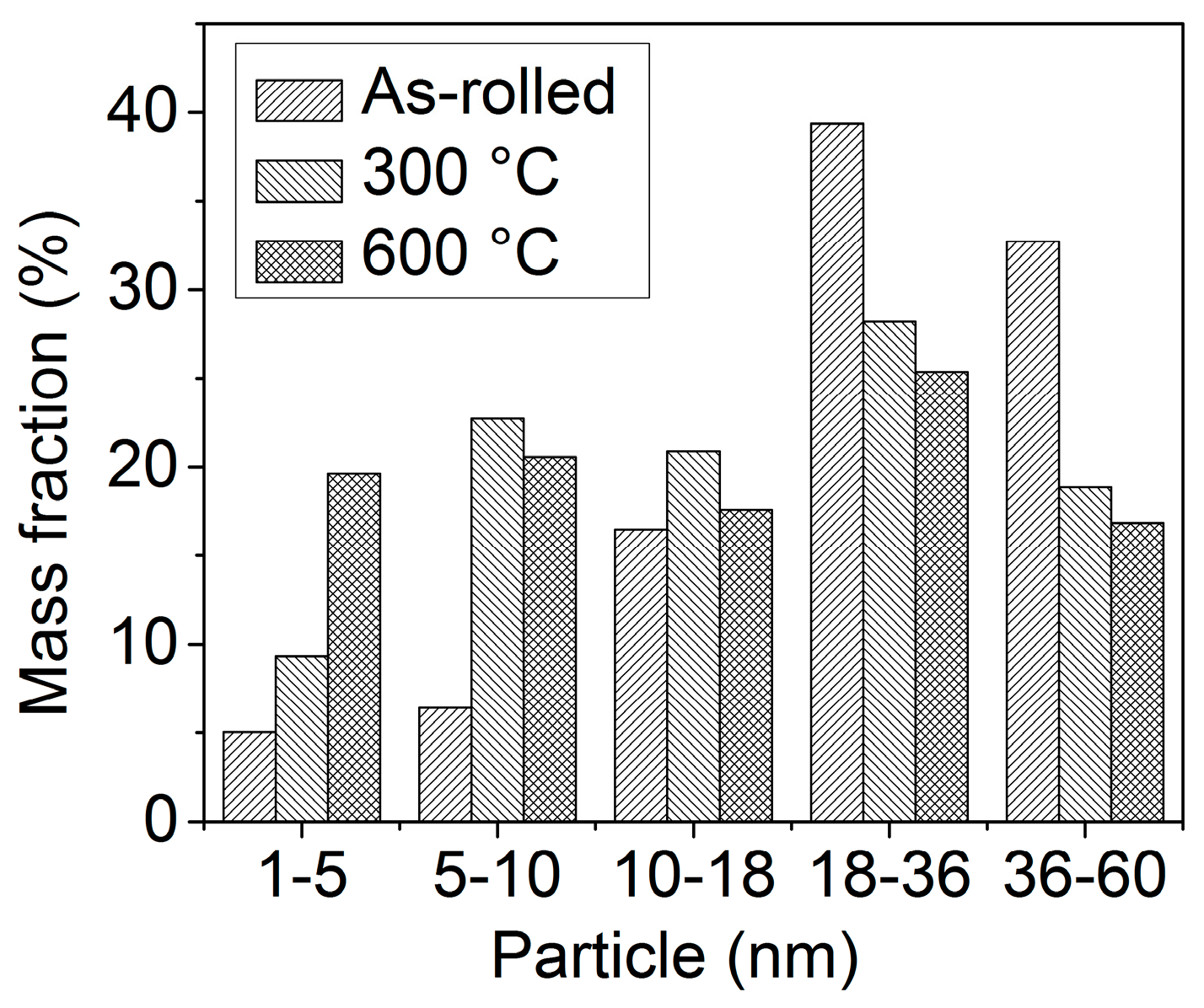
3.3. Nanoprecipitation
4. Discussion
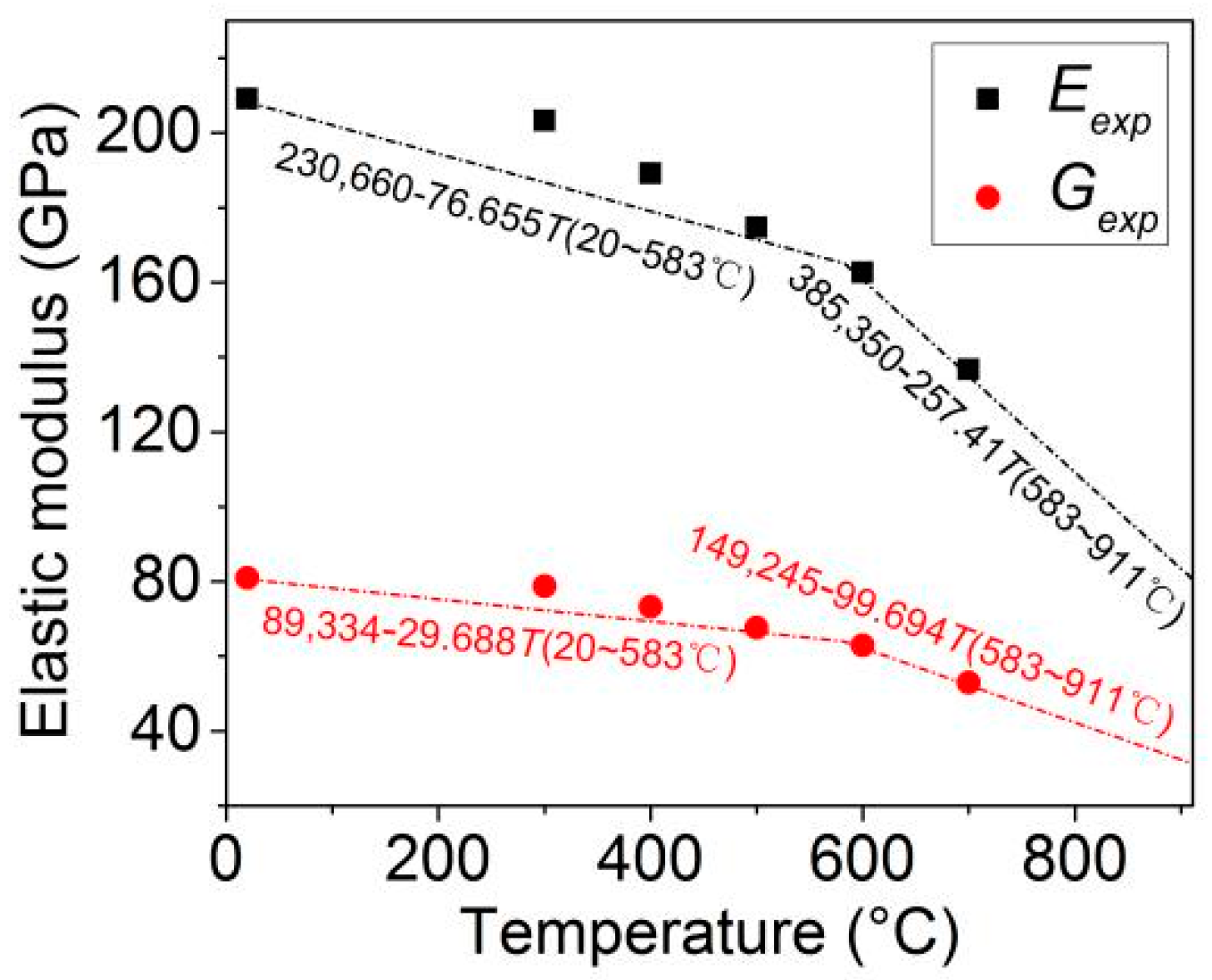
4.1. Tensile Properties at Different Temperatures
4.2. Work Hardening Behavior
4.3. Comparison with Mild Steels
5. Conclusions
- The yield strength of experimental steel is 617 MPa and the tensile strength is 813 MPa. As the experimental temperature increases, the high-temperature yield strength gradually decreases to 239 MPa at 700 °C. The effective grain size does not increase significantly at different temperatures and slightly fluctuates within the range of 12.5–13.6 μm. The matrix micromorphology does not change significantly, and recrystallized grains begin to appear at the grain boundaries at 600 °C.
- A small number of nanoprecipitates are distributed in the as-rolled specimen, with an average diameter of 29.2 nm. Upon increasing the temperature, the number of fine nanoprecipitates gradually increases, especially those less than 10 nm, which gradually decreases the average diameter, reaching 19.4 nm at 600 °C. The calculated precipitation strengthening value is consistent with the experimental precipitation strengthening value, and the Orowan equation explains well the precipitation strengthening effect of nanoprecipitates formed at elevated temperatures.
- The work hardening rate decreases significantly with the temperature, and necking is more likely to occur. At room temperature and 300 °C, the Ashby work hardening theoretical curves are consistent with the experimental true stress-strain curves.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.Y.; Yong, Q.L.; Sun, X.J.; Li, Z.D.; Kang, J.Y.; Wang, G.D. Microstructure and mechanical properties of precipitation strengthened fire resistant steel containing high Nb and low Mo. J. Iron Steel Res. Int. 2015, 22, 337–343. [Google Scholar] [CrossRef]
- Sim, J.H.; Kim, T.Y.; Kim, J.Y.; Kim, C.W.; Chung, J.H.; Moon, J.; Lee, C.H.; Hong, H.U. On the strengthening effects affecting tensile and low cycle fatigue properties of low-alloyed seismic/fire-resistant structural steels. Met. Mater. Int. 2020. [Google Scholar] [CrossRef]
- Chijiiwa, R.; Tamehiro, H.; Yoshida, Y.; Funato, K.; Uemori, R.; Hoeii, Y. Development and practical application of fire-resistant steel for buildings. Nippon Steel Tech. Rep. 1993, 58, 47–55. [Google Scholar]
- Fujino, H.; Hitomi, K.; Hashimoto, J.; Umezawa, S. Fire-resistant steel for building structures. Kawasaki Steel Tech. Rep. 1993, 29, 89–93. [Google Scholar]
- Bjorhovde, R. Performance and design issues for high strength steel in structures. Adv. Struct. Eng. 2010, 13, 403–411. [Google Scholar] [CrossRef]
- Li, G.Q.; Song, L.X. Mechanical properties of TMCP Q690 high strength structural steel at elevated temperatures. Fire Saf. J. 2020, 116, 103190. [Google Scholar] [CrossRef]
- Bennetts, I.D.; Moinuddin, K.A.M.; Goh, C.C.; Thomas, I.R. Testing and factors relevant to the evaluation of the structural adequacy of steel members within fire-resistant elevator shafts. Fire Saf. J. 2005, 40, 698–727. [Google Scholar] [CrossRef]
- Assefpour-Dezfuly, M.; Hugass, B.A.; Brownrigg, A. Fire resistant high strength low alloy steels. Mater. Sci. Technol. 1990, 6, 1210–1214. [Google Scholar] [CrossRef]
- Lee, W.B.; Hong, S.G.; Park, C.G.; Park, S.H. Carbide precipitation and high-temperature strength of hot-rolled high-strength, low-alloy steels containing Nb and Mo. Metall. Mater. Trans. A 2002, 33, 1689–1698. [Google Scholar] [CrossRef] [Green Version]
- Sha, W.; Kelly, F.S.; Guo, Z.X. Microstructure and properties of Nippon fire-resistant steels. J. Mater. Eng. Perform. 1999, 8, 606–612. [Google Scholar] [CrossRef]
- Sha, W.; Kirby, B.R.; Kelly, F.S. The behavior of structural steels at elevated temperatures and the design of fire resistant steels. Mater. Trans. 2001, 42, 1913–1927. [Google Scholar] [CrossRef] [Green Version]
- Sha, W.; Kelly, F.S.; Browne, P.; Blackmore, S.P.O.; Long, A.E. Development of structural steels with fire resistant microstructures. Mater. Sci. Technol. 2002, 18, 319–325. [Google Scholar] [CrossRef]
- Wan, R.C.; Sun, F.; Zhang, L.T.; Shan, A.D. Effects of Mo on high-temperature strength of fire-resistant steel. Mater. Des. 2012, 35, 335–341. [Google Scholar] [CrossRef]
- Dere, E.G.; Sharma, H.; Petrov, R.H.; Sietsma, J.; Offerman, S.E. Effect of niobium and grain boundary density on the fire resistance of Fe–C–Mn steel. Scr. Mater. 2013, 68, 651–654. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Sun, X.J.; Wang, Z.Q.; Li, Z.D.; Yong, Q.L.; Wang, G.D. Carbide precipitation in austenite of Nb-Mo-bearing low-carbon steel during stress relaxation. Mater. Lett. 2015, 159, 249–252. [Google Scholar] [CrossRef]
- Wan, R.C.; Sun, F.; Zhang, L.T.; Shan, A.D. Development and study of high-strength low-Mo fire-resistant steel. Mater. Des. 2012, 36, 227–232. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.D.; Zhou, S.T.; Wang, W.T.; Yong, Q.L.; Yang, Z.M.; Shen, J.C.; Shang, C.J.; Liu, Q.Y. Precipitation behavior of Ti-Nb-V-Mo quaternary microalloyed high strength fire-resistant steel and its influence on mechanical properties. Mater. Sci. Eng. A 2021, 807, 140865. [Google Scholar] [CrossRef]
- Gross, C.T.; Isheim, D.; Vaynman, S.; Fine, M.E.; Chung, Y.W. Design and development of lightly alloyed ferritic fire-resistant structural steels. Metall. Mater. Trans. A 2019, 50, 209–219. [Google Scholar] [CrossRef]
- Cong, J.H.; Li, J.W.; Fan, J.J.; Liu, P.C.; Misra, R.D.K.; Shang, C.J.; Wang, X.M. The impact of interphase precipitation on the mechanical behavior of fire-resistant steels at an elevated temperature. Materials 2020, 13, 4294. [Google Scholar] [CrossRef]
- Ghosh, S.; Mula, S.; Mondal, D.K. Development of ultrahigh strength cast-grade microalloyed steel by simple innovative heat treatment techniques for industrial applications. Mater. Sci. Eng. A 2017, 700, 667–680. [Google Scholar] [CrossRef]
- Jarvenpaa, A.; Ghosh, S.; Khosravifard, A.; Jaskari, M.; Hamada, A. A new processing route to develop the nano-grained structure of a TRIP-aided austenitic stainless-steel using double reversion fast-heating annealing. Mater. Sci. Eng. A 2021, 808, 140917. [Google Scholar] [CrossRef]
- Xie, Z.J.; Song, Z.D.; Chen, K.; Jiang, M.H.; Tao, Y.; Wang, X.M.; Shang, C.J. Study of Nanometer-Sized Precipitation and Properties of Fire Resistant Hot-Rolled Steel. Metals 2019, 9, 1230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y. Effect of Mo on the Nanometer-Sized Precipitates, Microstructure and Properties of High Nb Low Carbon Steel. Ph.D. Dissertation, Northeastern University, Shenyang, China, 2015. [Google Scholar]
- Zhang, Z.Y.; Li, Z.D.; Yong, Q.L.; Sun, X.J.; Wang, Z.Q.; Wang, G.D. Precipitation behavior of carbide during heating process in Nb and Nb-Mo micro-alloyed steels. Acta Metall. Sin. 2015, 51, 315–324. [Google Scholar]
- Zhang, Z.Y.; Sun, X.J.; Yong, Q.L.; Li, Z.D.; Wang, Z.Q.; Wang, G.D. Precipitation behavior of nanometer-sized carbides in Nb-Mo microalloyed high strengh steel and its strengthening mechanism. Acta Metall. Sin. 2016, 52, 410–418. [Google Scholar]
- Jo, H.H.; Shin, C.S.; Moon, J.O.; Jae, J.H.; Ha, H.Y.; Park, S.J.; Lee, T.H.; Lee, B.H.; Chung, J.H.; Speer, J.G.; et al. Mechanisms for improving tensile properties at elevated temperature in fire-resistant steel with Mo and Nb. Mater. Des. 2020, 194, 108882. [Google Scholar] [CrossRef]
- Ferreira, D.; Alves, A.; Neto, R.; Martins, T.; Brandi, S. A new approach to simulate HSLA steel multipass welding through distributed point heat sources model. Metals 2018, 8, 951. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.T.; Yang, S.W.; Gao, K.W.; He, X.L. Corrosion resistance of low alloying weather steels in environment containing chloride ion. Trans. Mater. Heat Treat. 2008, 4, 170–175. [Google Scholar]
- Jia, J.H.; Cheng, X.Q.; Yang, X.J.; Li, X.G.; Li, W. A study for corrosion behavior of a new-type weathering steel used in harsh marine environment. Constr. Build. Mater. 2020, 259, 119760. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Xu, X.X.; Li, Y.; Lv, X.W. The function of Cr on the rust formed on weathering steel performed in a simulated tropical marine atmosphere environment. Constr. Build. Mater. 2021, 277, 122298. [Google Scholar] [CrossRef]
- Ghosh, S.; Komi, J.; Mula, S. Flow stress characteristics and design of innovative 3-steps multiphase control thermomechanical processing to produce ultrafine-grained bulk steels. Mater. Des. 2020, 186, 108297. [Google Scholar] [CrossRef]
- Ghosh, S.; Mula, S.; Malakar, A.; Somani, M.; Komi, J. High cycle fatigue performance, crack growth, and failure mechanisms of an ultrafine-grained Nb+Ti stabilized, low-C microalloyed steel processed by multiphase controlled rolling and forging. Mater. Sci. Eng. A 2021, 825, 141883. [Google Scholar] [CrossRef]
- Wang, W.Y.; Liu, B.; Kodur, V. Effect of Temperature on Strength and Elastic Modulus of High-Strength Steel. J. Mater. Civil. Eng. 2013, 25, 174–782. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phase in Steels; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]
- Yang, H.Y.; Li, K.Q.; Bu, Y.Q.; Wu, J.M.; Fang, Y.T.; Meng, L.; Liu, J.B.; Wang, H.T. Nanoprecipitates induced dislocation pinning and multiplication strategy for designing high strength, plasticity and conductivity Cu alloys. Scr. Mater. 2021, 195, 113741. [Google Scholar] [CrossRef]
- Ghosh, S.; Bibhanshu, N.; Suwas, S.; Chatterjee, K. Surface mechanical attrition treatment of additively manufactured 316L stainless steel yields gradient nanostructure with superior strength and ductility. Mater. Sci. Eng. A 2021, 820, 141540. [Google Scholar] [CrossRef]
- Kim, Y.W.; Song, S.W.; Seo, S.J.; Hong, S.G.; Lee, C.S. Development of Ti and Mo micro-alloyed hot-rolled high strength sheet steel by controlling thermomechanical controlled processing schedule. Mater. Sci. Eng. A 2013, 565, 430–438. [Google Scholar] [CrossRef]
- Cohen, M. Dislocation in Metals; AIME: New York, NY, USA, 1954; Volume 69. [Google Scholar]
- Argon, A.S. Physical of Strength and Plasticity; MIT Press: Cambridge, MA, USA, 1969; Volume 113. [Google Scholar]
- Dieter, G.E. Mechanical Metallurgy; McGraw-Hill Book Company: London, UK, 1988; Volume 289. [Google Scholar]
- Kamikawa, N.; Abe, Y.; Miyamoto, G.; Funakawa, Y.; Furuhara, T. Tensile behavior of Ti,Mo-added low carbon steels with interphase precipitation. ISIJ Int. 2014, 54, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Ashby, M.F. Work hardening of dispersion hardened crystals. Philos. Mag. 1966, 14, 1157. [Google Scholar] [CrossRef]
- Ashby, M.F. The deformation of plastically non-homogeneous materials. Philos. Mag. 1970, 21, 399. [Google Scholar] [CrossRef]
- Ohmori, A.; Torizuka, S.; Nagai, K. Strain-hardening due to dispersed cementite for low carbon ultrafine-grained steels. ISIJ Int. 2004, 44, 1063. [Google Scholar] [CrossRef] [Green Version]
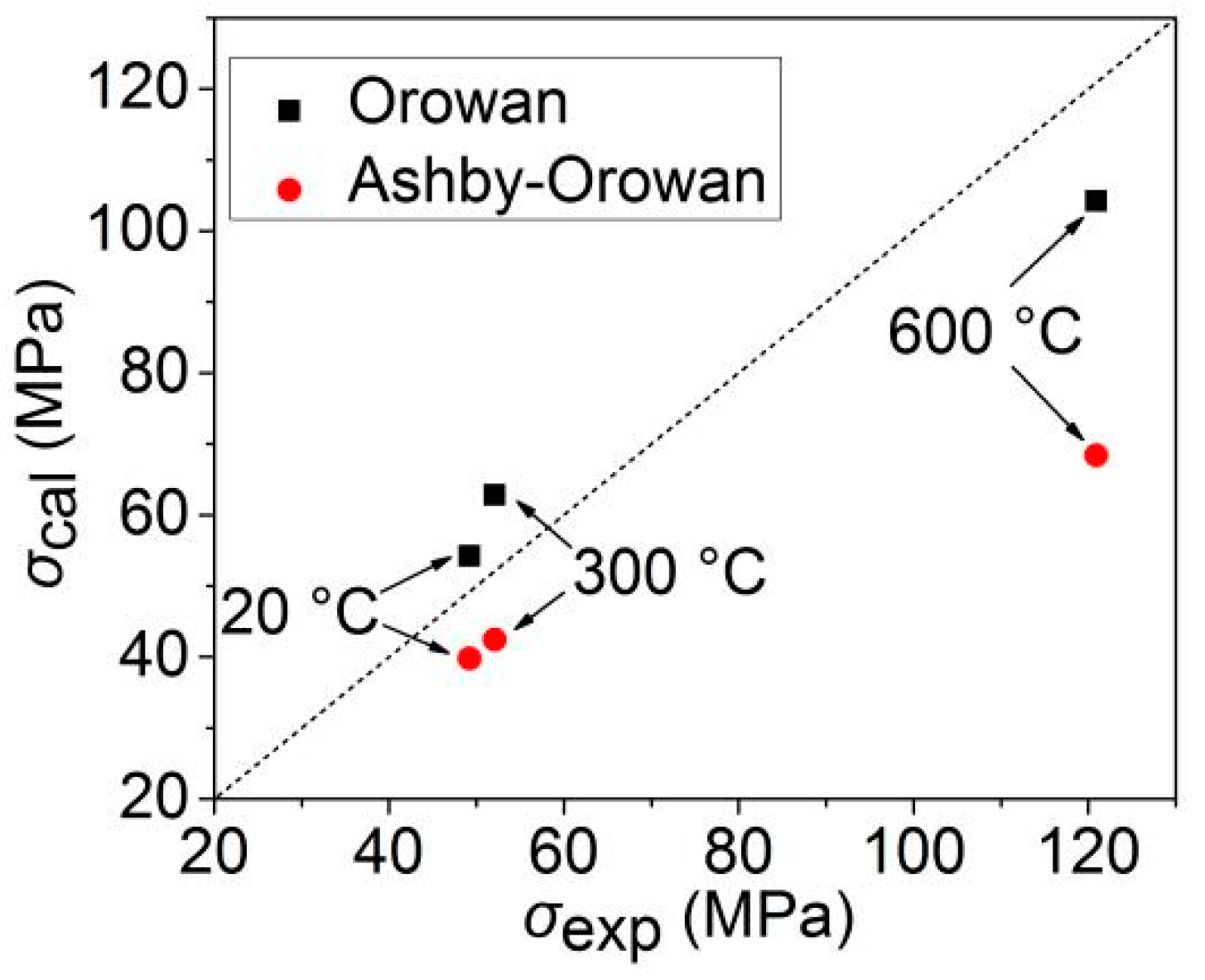

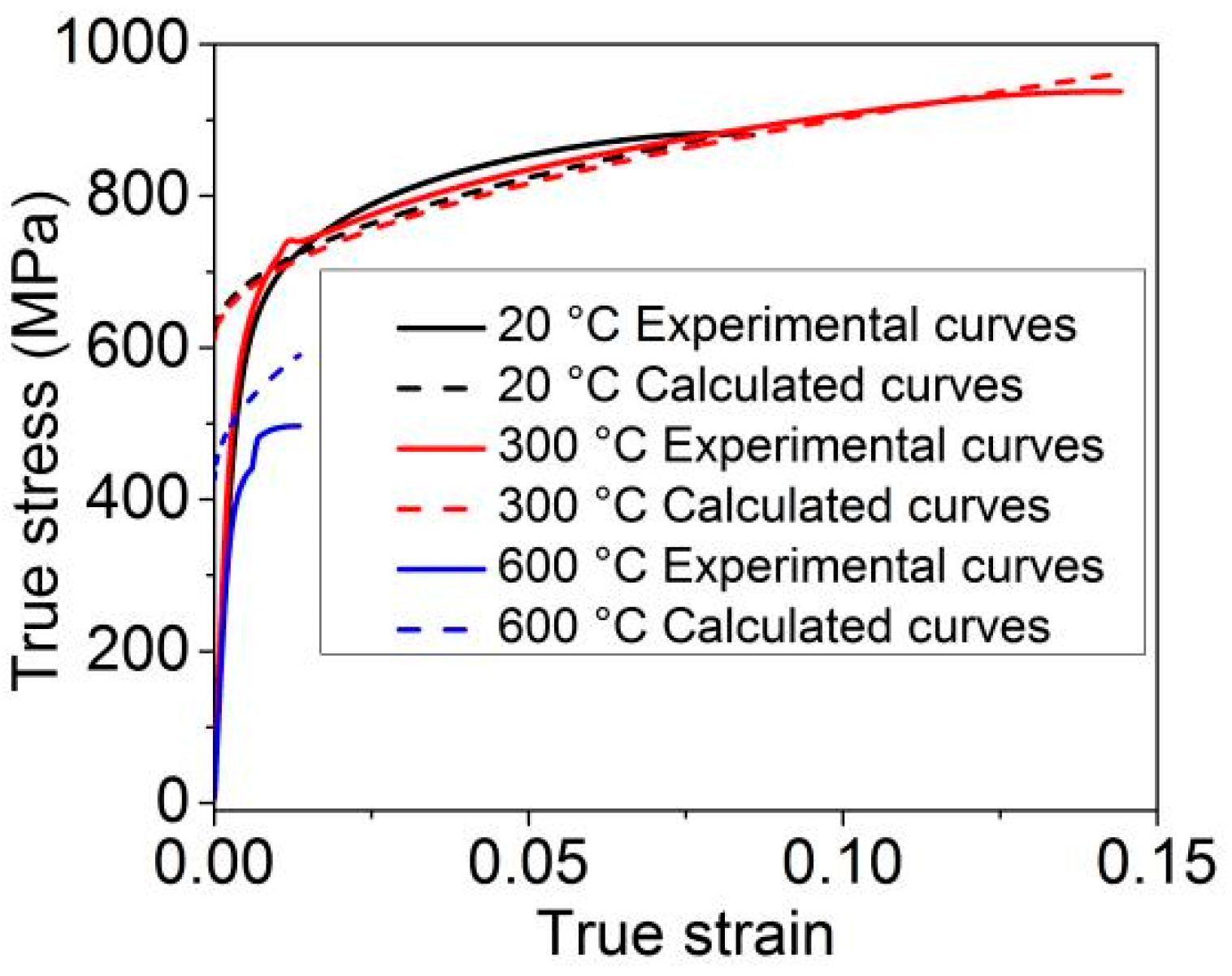
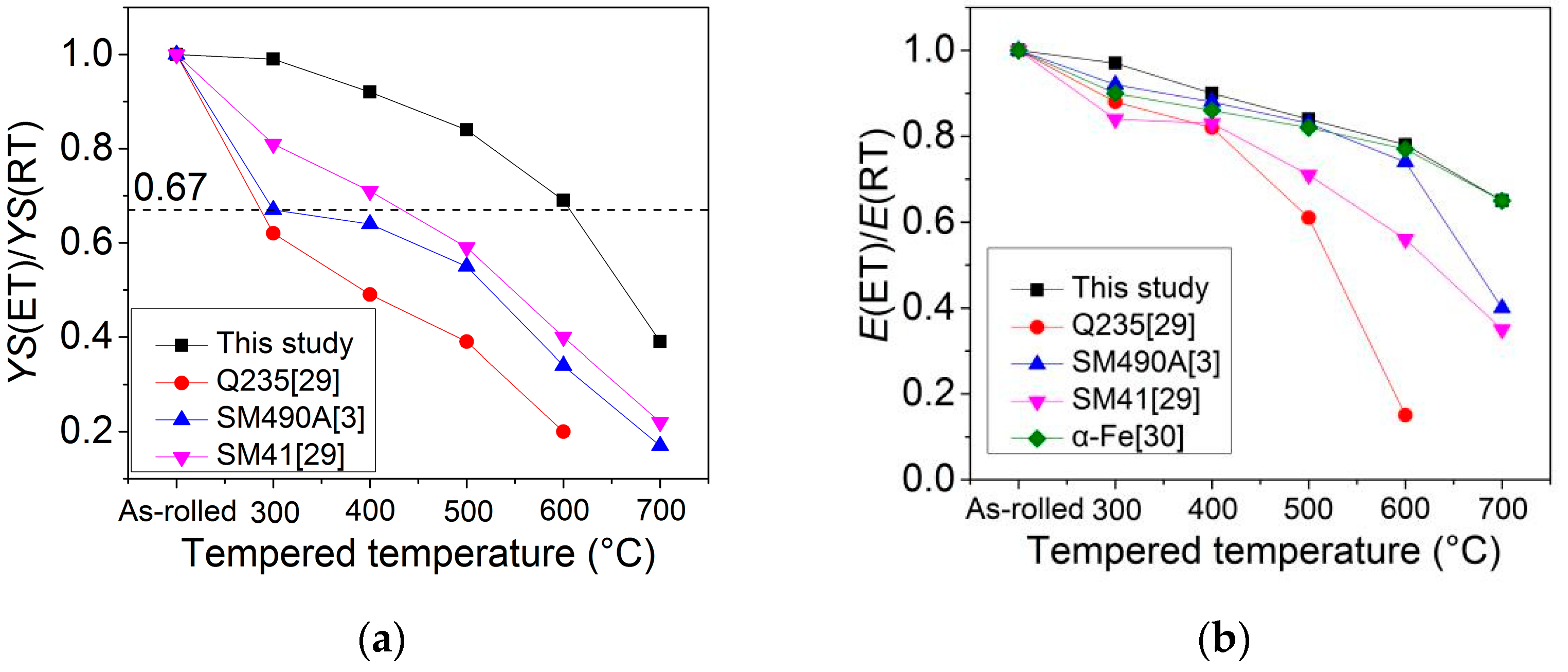
| C | Si | Mn + C r + Ni + Cu | P | S | Mo | Ti | Nb | V | N |
|---|---|---|---|---|---|---|---|---|---|
| 0.056 | 0.32 | ≤2.5 | 0.007 | 0.004 | 0.21 | 0.014 | 0.093 | 0.03 | 0.0035 |
| State | Mass Fraction in Alloy of Elements in M(C, N) Phase (%) | M(C, N) Phase Composition | ||||||
|---|---|---|---|---|---|---|---|---|
| Nb | Ti | V | Mo | N | C * | Σ | ||
| As-rolled | 0.021 | 0.011 | 0.0022 | 0.0080 | 0.0030 | 0.0044 | 0.050 | (Nb0.388Ti0.395V0.074Mo0.143) (C0.632N0.368) |
| 300 °C | 0.018 | 0.013 | 0.0011 | 0.0050 | 0.0025 | 0.0041 | 0.043 | (Nb0.362Ti0.512V0.037Mo0.088) (C0.655N0.345) |
| 600 °C | 0.055 | 0.013 | 0.0074 | 0.027 | 0.0032 | 0.013 | 0.119 | (Nb0.459Ti0.211V0.112Mo0.218) (C0.823N0.177) |
| State | Mass Fraction in Alloy of Elements in M3C Phase(%) | M3C Phase Composition | |||||
|---|---|---|---|---|---|---|---|
| Fe | Cr | Mn | Mo | C * | Σ | ||
| As-rolled | 0.084 | 0.0089 | 0.0039 | 0.003 | 0.0071 | 0.107 | (Fe0.846Cr0.096Mn0.040Mo0.017)3C |
| 300 °C | 0.128 | 0.0050 | 0.0040 | 0.002 | 0.010 | 0.147 | (Fe0.931Cr0.039Mn0.030Mo0.005)3C |
| 600 °C | 0.537 | 0.034 | 0.0096 | 0.010 | 0.042 | 0.633 | (Fe0.912Cr0.062Mn0.016Mo0.010)3C |
| Experimental temperature (°C) | YS (MPa) | YS0 (MPa) | YSs (MPa) | YSg (MPa) | YSd (MPa) | YSp (MPa) |
|---|---|---|---|---|---|---|
| As-rolled | 617 | 57.0 | 246.2 | 153.8 | 110.8 | 49.2 |
| 300 °C | 609 | 55.4 | 230.8 | 164.2 | 106.5 | 52.1 |
| 600 °C | 424 | 44.3 | 45 | 138.8 | 75.0 | 120.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, Z.; Zhou, S.; Chen, R.; Da, G.; Yong, Q.; Yang, Z.; Shen, J.; Shang, C.; Liu, Q. The Influence of Temperature on the Microstructure and Properties of Nb-V-Ti-Mo Complex Microalloyed High-Strength Fire-Resistant Steel. Metals 2021, 11, 1670. https://doi.org/10.3390/met11111670
Wang X, Li Z, Zhou S, Chen R, Da G, Yong Q, Yang Z, Shen J, Shang C, Liu Q. The Influence of Temperature on the Microstructure and Properties of Nb-V-Ti-Mo Complex Microalloyed High-Strength Fire-Resistant Steel. Metals. 2021; 11(11):1670. https://doi.org/10.3390/met11111670
Chicago/Turabian StyleWang, Xin, Zhaodong Li, Shitong Zhou, Runnong Chen, Guangjie Da, Qilong Yong, Zhongmin Yang, Junchang Shen, Chengjia Shang, and Qingyou Liu. 2021. "The Influence of Temperature on the Microstructure and Properties of Nb-V-Ti-Mo Complex Microalloyed High-Strength Fire-Resistant Steel" Metals 11, no. 11: 1670. https://doi.org/10.3390/met11111670
APA StyleWang, X., Li, Z., Zhou, S., Chen, R., Da, G., Yong, Q., Yang, Z., Shen, J., Shang, C., & Liu, Q. (2021). The Influence of Temperature on the Microstructure and Properties of Nb-V-Ti-Mo Complex Microalloyed High-Strength Fire-Resistant Steel. Metals, 11(11), 1670. https://doi.org/10.3390/met11111670






