Microstructural Stability of the CoCrFe2Ni2 High Entropy Alloys with Additions of Cu and Mo
Abstract
:1. Introduction
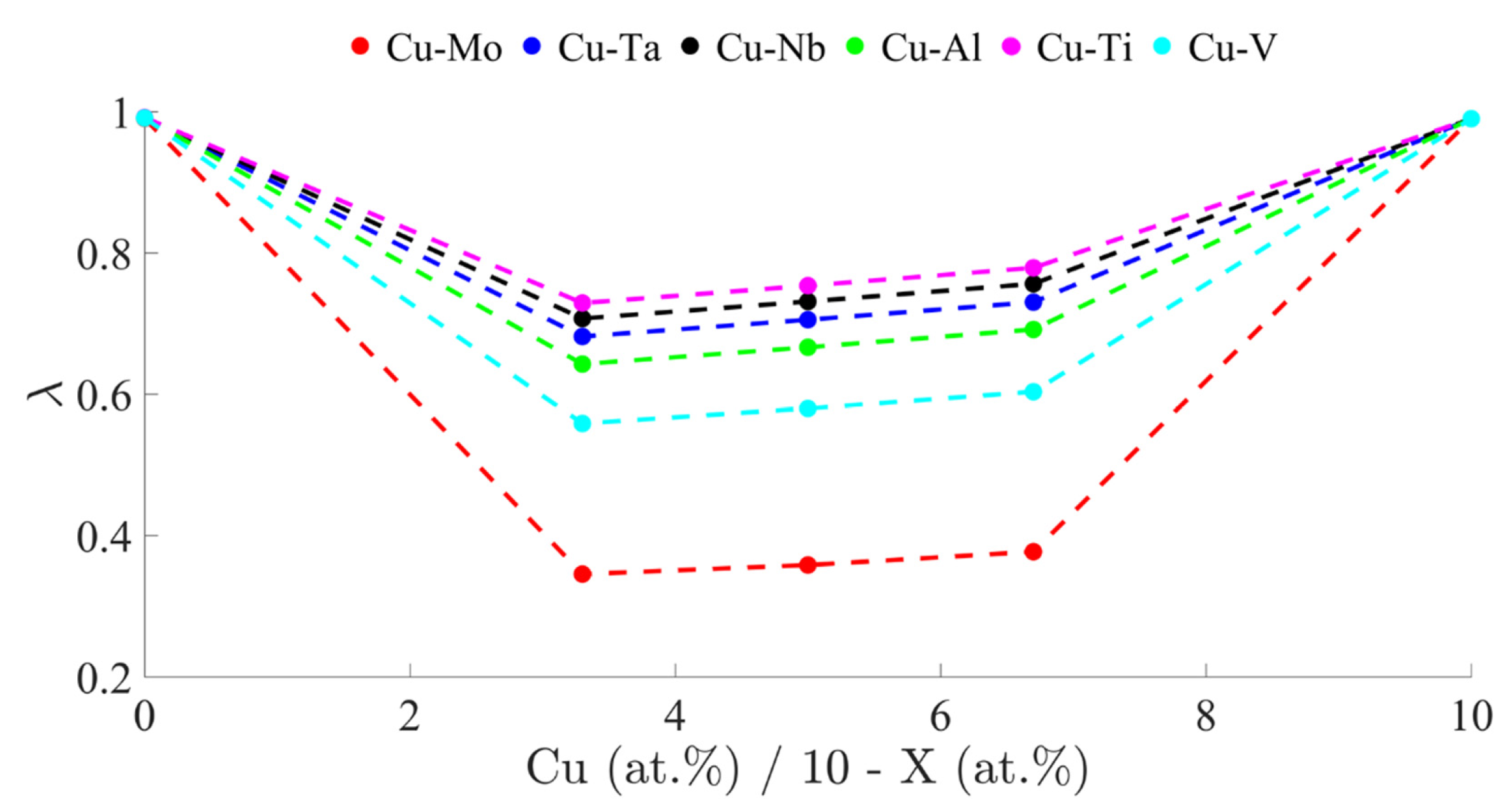
2. Alloy Design
3. Materials and Methods
4. Results
4.1. As-Cast Microstructure
4.2. Homogenization at 1200 °C and Forged Microstructures
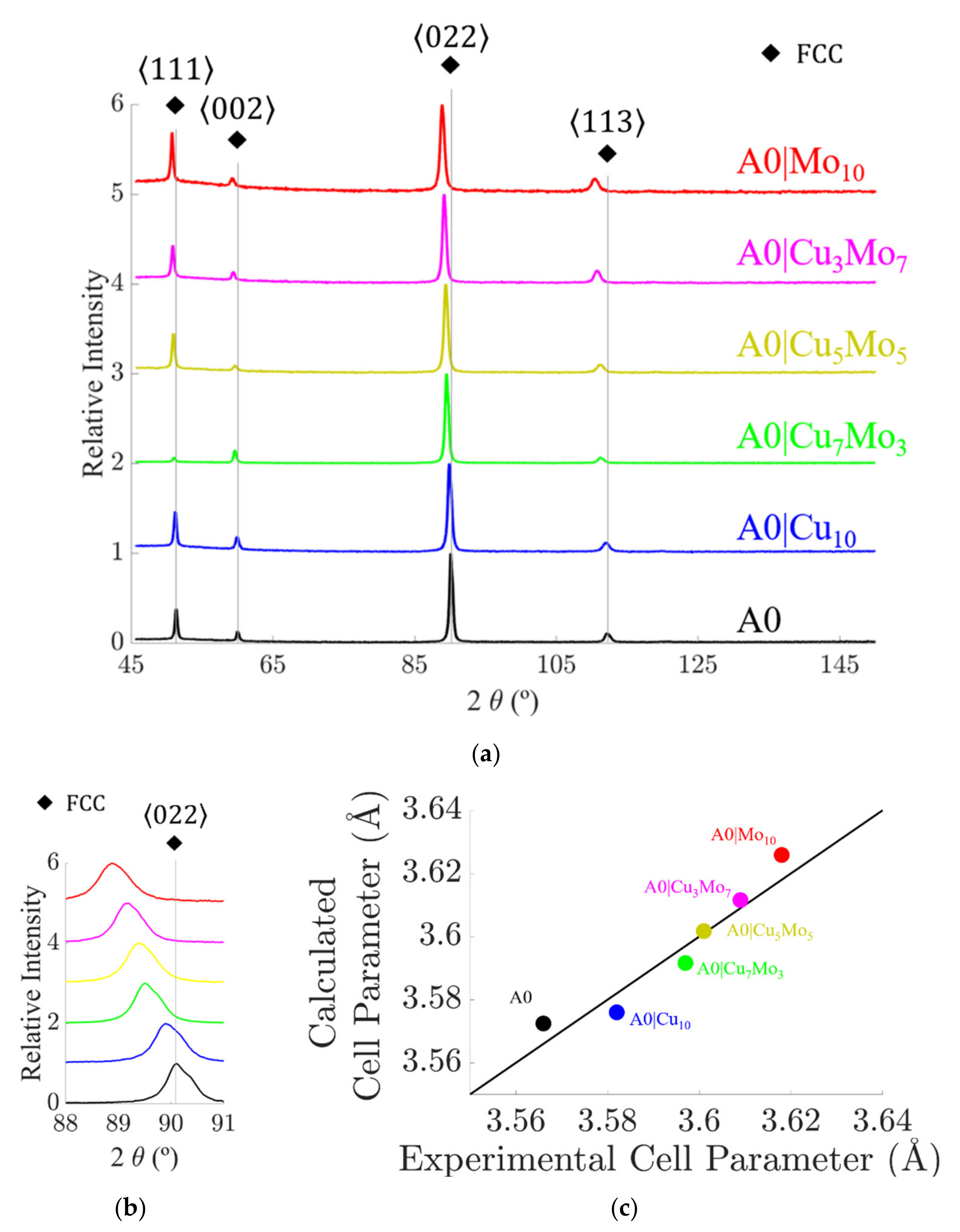
4.2.1. X-ray Diffraction
4.2.2. Saturation Magnetization Measurements
4.2.3. EPMA: Electron Probe Micro Analysis
4.3. Microstructure after Heat Treatments
4.3.1. Dilatometry
4.3.2. Heat Treatments at 500 °C, 700 °C and 900 °C
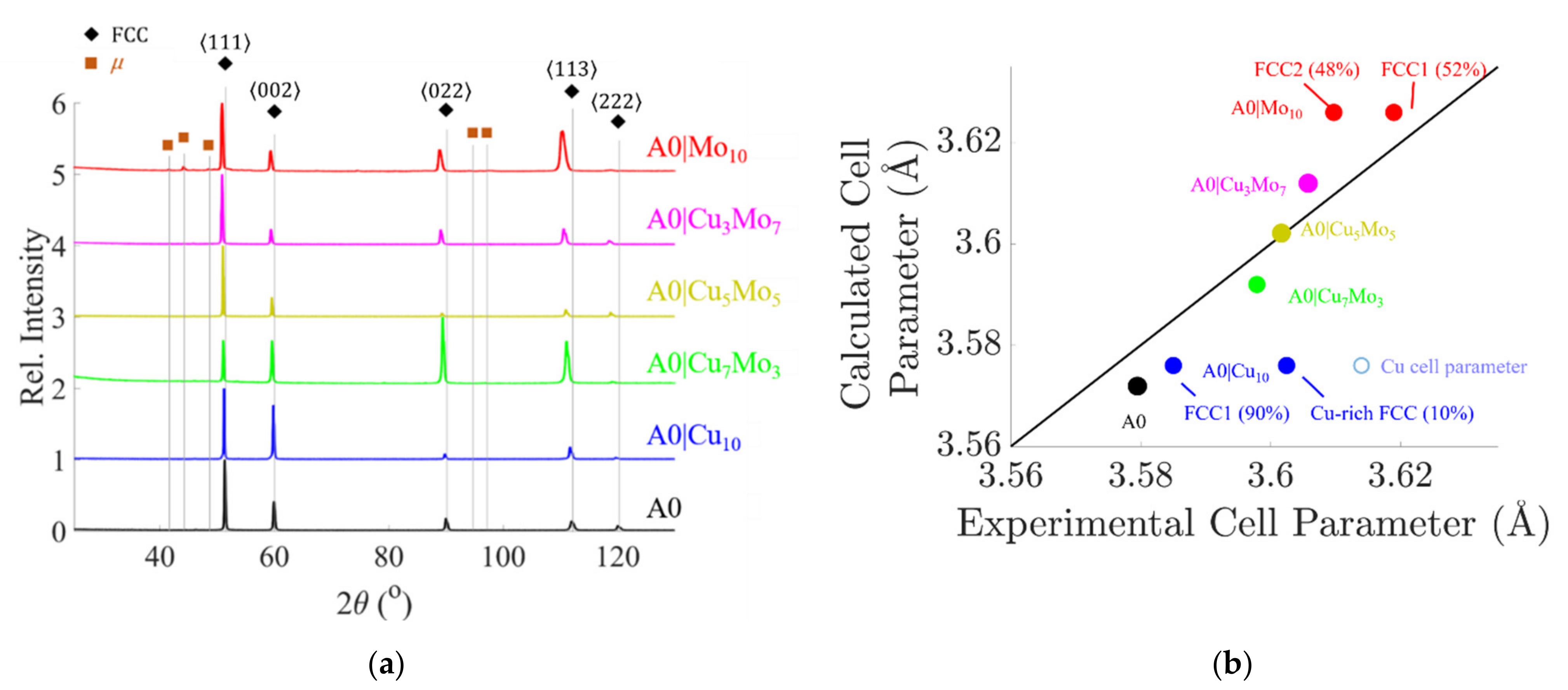
4.3.3. X-ray Analysis after Heat Treatment of 900 °C
5. Summary and Conclusions
- (a)
- Based on solid-solution formation rules, a segregation parameter (λ), and thermodynamic calculations, the CoCrFe2Ni2 system was developed by adding up to 10 at. % of Cu, Mo, or the combination of both elements. The alloys designed showed a single FCC phase once the microstructure was homogenized by a thermal treatment at 1200 °C, and therefore they constitute a new family of HEAs.
- (b)
- The EPMA compositional maps revealed that the interdendritic areas present an enrichment of Cu and Mo accompanied by a noticeable Fe depletion. This result is unexpected as thermodynamics predicts that these two elements should reject each other, in a similar way to Cu with Fe, and the Mo-enriched areas should be also enriched by Fe due to the positive enthalpy of the Fe-Cu interaction. This Fe depletion from the Mo-enriched zones highlights the complexity of the atomic interactions in HEAs and the difficulty of their prediction.
- (c)
- The non-equimolar composition has shown an improvement in the system stability, delaying the appearance of secondary phases, especially for the Cu-containing alloys, in comparison with the behavior of some equimolar Cu-containing compositions described in the literature.
- (d)
- Cu-Mo interaction is responsible for inhibiting or delaying the precipitation of both Cu-rich and Mo-rich particles in the A0|Cu7Mo3, A0|Cu5Mo5, and A0|Cu3Mo7 alloys, and the maintenance of a single FCC crystal in the A0|Cu7Mo3 alloy during aging treatments. In A0|Cu5Mo5, A0|Cu3Mo7, and A0|Mo10 alloys, Mo-rich blocky precipitates of the µ phase decorate the grain boundaries after an aging heat treatment at high temperatures, where the diffusion of the elements is faster. In the case of the A0|Cu5Mo5 and A0|Cu3Mo7 alloys, the precipitation occurs only at 900 °C, while for the A0|Mo10 the precipitation had already started at 700 °C due to the absence of Cu that can inhibit or retard this phenomenon. For this alloy, aging at 900 °C also produces the formation of acicular precipitates, probably corresponding to the needle-shape µ phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Wang, W.R.; Wang, W.L.; Yeh, J.W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Zaddach, A.J.; Scattergood, R.O.; Koch, C.C. Tensile properties of low-stacking fault energy high-entropy alloys. Mater. Sci. Eng. A 2015, 636, 373–378. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Liu, W.H.; He, J.Y.; Huang, H.L.; Wang, H.; Lu, Z.P.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Toda-Caraballo, I.; Rivera-Díaz-Del-Castillo, P.E.J. Modelling solid solution hardening in high entropy alloys. Acta Mater. 2015, 85, 14–23. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Tasan, C.C.; Springer, H.; Gault, B.; Raabe, D. Interstitial atoms enable joint twinning and transformation induced plasticity in strong and ductile high-entropy alloys. Sci. Rep. 2017, 7, 40704. [Google Scholar] [CrossRef] [PubMed]
- Toda-Caraballo, I.; Wróbel, J.S.; Nguyen-Manh, D.; Pérez, P.; Rivera-Díaz-del-Castillo, P.E.J. Simulation and modeling in high entropy alloys. JOM 2017, 69, 2137–2149. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Xia, S.Q.; Yang, X.; Yang, T.F.; Liu, S.; Zhang, Y. Irradiation resistance in AlxCoCrFeNi high entropy alloys. JOM 2015, 67, 3–7. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Curado, T.M.; Zeng, Z.; Lopes, J.G.; Rossinyol, E.; Park, J.M.; Schell, N.; Braz Fernandes, F.M.; Kim, H.S. Gas tungsten arc welding of as-rolled CrMnFeCoNi high entropy alloy. Mater. Des. 2020, 189, 108505. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Shen, J.; Zeng, Z.; Park, J.M.; Choi, Y.T.; Schell, N.; Maawad, E.; Zhou, N.; Kim, H.S. Dissimilar laser welding of a CoCrFeMnNi high entropy alloy to 316 stainless steel. Scr. Mater. 2022, 206, 114219. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, K.R.; Won, J.W.; Na, Y.S.; Kim, H.S. Mechanical properties and deformation twinning behavior of as-cast CoCrFeMnNi high-entropy alloy at low and high temperatures. Mater. Sci. Eng. A 2018, 712, 108–113. [Google Scholar] [CrossRef]
- Sun, S.J.; Tian, Y.Z.; Lin, H.R.; Yang, H.J.; Dong, X.G.; Wang, Y.H.; Zhang, Z.F. Transition of twinning behavior in CoCrFeMnNi high entropy alloy with grain refinement. Mater. Sci. Eng. A 2018, 712, 603–607. [Google Scholar] [CrossRef]
- Chou, H.P.; Chang, Y.S.; Chen, S.K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 ≤ x ≤2) high-entropy alloys. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2009, 163, 184–189. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L. Equilibrium phase of high-entropy FeCoNiCrCu0.5 alloy at elevated temperature. J. Alloys Compd. 2010, 489, 30–35. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L.; Bor, H.Y. Effect of aging treatment on microstructure and properties of high-entropy Cu0.5CoCrFeNi alloy. Intermetallics 2010, 18, 1244–1250. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Martin, A.C.; Oliveira, J.P.; Fink, C. Elemental effects on weld cracking susceptibility in AlxCoCrCuyFeNi high-entropy alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 778–787. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yuan, H.; Cheng, G.; Xu, W.; Tsai, K.Y.; Tsai, C.W.; Jian, W.W.; Juan, C.C.; Shen, W.J.; Chuang, M.H.; et al. Morphology, structure and composition of precipitates in Al0.3CoCrCu0.5FeNi high-entropy alloy. Intermetallics 2013, 32, 329–336. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Mondal, S.; Van Smaalen, S.; Völkl, R.; Glatzel, U.; Wanderka, N. Investigation of phases in Al23Co15Cr23Cu8Fe15Ni16 and Al8Co17Cr17Cu8Fe17Ni33 high entropy alloys and comparison with equilibrium phases predicted by Thermo-Calc. J. Alloys Compd. 2013, 552, 430–436. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, Y.; Chen, G.L. Atomic packing efficiency and phase transition in a high entropy alloy. J. Alloys Compd. 2009, 478, 321–324. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B.S. Processing and properties of nanocrystalline CuNiCoZnAlTi high entropy alloys by mechanical alloying. Mater. Sci. Eng. A 2010, 527, 1027–1030. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Chen, H.Y.; Tsai, C.W.; Tung, C.C.; Yeh, J.W.; Shun, T.T.; Yang, C.C.; Chen, S.K. Effect of the substitution of Co by Mn in Al-Cr-Cu-Fe-Co-Ni high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 685–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Tsai, C.W.; Tsai, M.H.; Yeh, J.W.; Yang, C.C. Effect of temperature on mechanical properties of Al0.5CoCrCuFeNi wrought alloy. J. Alloys Compd. 2010, 490, 160–165. [Google Scholar] [CrossRef]
- Li, B.S.; Wang, Y.P.; Ren, M.X.; Yang, C.; Fu, H.Z. Effects of Mn, Ti and V on the microstructure and properties of AlCrFeCoNiCu high entropy alloy. Mater. Sci. Eng. A 2008, 498, 482–486. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Shaysultanov, D.G.; Stepanov, N.D.; Salishchev, G.A.; Senkov, O.N. Tensile properties of an AlCrCuNiFeCo high-entropy alloy in as-cast and wrought conditions. Mater. Sci. Eng. A 2012, 533, 107–118. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chuang, M.H. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, R.; Qin, G.; Li, X.; Su, Y.; Ding, H.; Guo, J.; Fu, H. Microstructure evolution, Cu segregation and tensile properties of CoCrFeNiCu high entropy alloy during directional solidification. J. Mater. Sci. Technol. 2020, 38, 19–27. [Google Scholar] [CrossRef]
- Xian, X.; Lin, L.; Zhong, Z.; Zhang, C.; Chen, C.; Song, K.; Cheng, J.; Wu, Y. Precipitation and its strengthening of Cu-rich phase in CrMnFeCoNiCux high-entropy alloys. Mater. Sci. Eng. A 2018, 713, 134–140. [Google Scholar] [CrossRef]
- Verma, A.; Tarate, P.; Abhyankar, A.C.; Mohape, M.R.; Gowtam, D.S.; Deshmukh, V.P.; Shanmugasundaram, T. High temperature wear in CoCrFeNiCux high entropy alloys: The role of Cu. Scr. Mater. 2019, 161, 28–31. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, F.J.; Chen, G.L. Phase transformation induced by lattice distortion in multiprincipal component CoCrFeNi Cux Al1-x solid-solution alloys. Appl. Phys. Lett. 2008, 92, 12–15. [Google Scholar] [CrossRef]
- Troparevsky, M.C.; Morris, J.R.; Daene, M.; Wang, Y.; Lupini, A.R.; Stocks, G.M. Beyond atomic sizes and hume-rothery rules: Understanding and predicting high-entropy alloys. JOM 2015, 67, 2350–2363. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shim, S.H.; Lee, B.J.; Hong, S.I. Correlation between mechanical properties and thermodynamic parameters of dual-fcc-phase CoCrFeCuxNi (x = 1, 1.71) and CoCu1.71FeMnNi. Mater. Lett. 2020, 272, 127866. [Google Scholar] [CrossRef]
- Shim, S.H.; Pouraliakbar, H.; Hong, S.I. High strength dual fcc phase CoCuFeMnNi high-entropy alloy wires with dislocation wall boundaries stabilized by phase boundaries. Mater. Sci. Eng. A 2021, 825, 141875. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Zhao, Y.L.; He, Q.F.; Lu, J.; Yang, Y. Elemental segregation in solid-solution high-entropy alloys: Experiments and modeling. J. Alloys Compd. 2016, 681, 167–174. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Toda-Caraballo, I.; Rivera-Díaz-del-Castillo, P.E.J. A criterion for the formation of high entropy alloys based on lattice distortion. Intermetallics 2016, 71, 76–87. [Google Scholar] [CrossRef]
- Poletti, M.G.; Battezzati, L. Electronic and thermodynamic criteria for the occurrence of high entropy alloys in metallic systems. Acta Mater. 2014, 75, 297–306. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K.; Pearson, W.B. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds; Release 20.; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Wang, L.; Mercier, D.; Zanna, S.; Seyeux, A.; Laurent-Brocq, M.; Perrière, L.; Guillot, I.; Marcus, P. Study of the surface oxides and corrosion behaviour of an equiatomic CoCrFeMnNi high entropy alloy by XPS and ToF-SIMS. Corros. Sci. 2020, 167, 108507. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Zajusz, M.; Jawańska, M.; Gil, A.; Jedliński, J.; Mroczka, K.; Matsuda, K.; Kulik, T.; et al. Oxidation behavior of Alx(CoCrFeNi)100−x high-entropy alloys under thermal-cycling conditions. Oxid. Met. 2021, 96, 307–321. [Google Scholar] [CrossRef]
- Holcomb, G.R.; Tylczak, J.; Carney, C. Oxidation of CoCrFeMnNi high entropy alloys. JOM 2015, 67, 2326–2339. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Chen, C.; Chang, I.; Zhou, P.; Wang, X. Compositional dependence of phase selection in CoCrCu0.1FeMoNi-based high-entropy alloys. Materials 2018, 11, 1290. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Zhao, J.C. Determination of the Fe-Cr-Mo phase diagram at intermediate temperatures using dual-anneal diffusion multiples. J. Phase Equilibria Diffus. 2016, 37, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Toda-Caraballo, I.; Wróbel, J.S.; Dudarev, S.L.L.; Nguyen-Manh, D.; Rivera-Díaz-Del-Castillo, P.E.J. Interatomic spacing distribution in multicomponent alloys. Acta Mater. 2015, 97, 156–169. [Google Scholar] [CrossRef]
- Buerger, M.J. A handbook of lattice spacings and structures of metals and alloys. Zeitschrift für Krist. 1961, 115, 319–320. [Google Scholar] [CrossRef]
- Wróbel, J.S.; Nguyen-Manh, D.; Lavrentiev, M.Y.; Muzyk, M.; Dudarev, S.L. Phase stability of ternary fcc and bcc Fe-Cr-Ni alloys. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 91, 024108. [Google Scholar] [CrossRef] [Green Version]
- Leong, Z.; Dudarev, S.L.; Goodall, R.; Todd, I.; Nguyen-Manh, D. The effect of electronic structure on the phases present in high entropy alloys. Sci. Rep. 2017, 7, 39803. [Google Scholar] [CrossRef]
- Lucas, M.S.; Belyea, D.; Bauer, C.; Bryant, N.; Michel, E.; Turgut, Z.; Leontsev, S.O.; Horwath, J.; Semiatin, S.L.; McHenry, M.E.; et al. Thermomagnetic analysis of FeCoCrxNi alloys: Magnetic entropy of high-entropy alloys. J. Appl. Phys. 2013, 113, 17A923. [Google Scholar] [CrossRef] [Green Version]
- Zuo, T.; Gao, M.C.; Ouyang, L.; Yang, X.; Cheng, Y.; Feng, R.; Chen, S.; Liaw, P.K.; Hawk, J.A.; Zhang, Y. Tailoring magnetic behavior of CoFeMnNiX (X = Al, Cr, Ga, and Sn) high entropy alloys by metal doping. Acta Mater. 2017, 130, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Crangle, J.; Hallam, G.C. The magnetization of face-centred cubic and body-centred cubic iron + nickel alloys. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1963, 272, 119–132. [Google Scholar] [CrossRef]
- Celada, C.; Toda-Caraballo, I.; Kim, B.; San Martín, D. Chemical banding revealed by chemical etching in a cold-rolled metastable stainless steel. Mater. Charact. 2013, 84, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Basso, A.; Toda-caraballo, I.; San-martín, D.; Caballero, F.G. Influence of cast part size on macro- and microsegregation patterns in a high carbon high silicon steel. J. Mater. Res. Technol. 2020, 9, 3013–3025. [Google Scholar] [CrossRef]
- Gómez-Acebo, T.; Navarcorena, B.; Castro, F. Interdiffusion in multiphase, Al-Co-Cr-Ni-Ti diffusion couples. J. Phase Equilibria Diffus. 2004, 25, 237–251. [Google Scholar] [CrossRef]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.P.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef] [Green Version]
- García De Andrés, C.; Caballero, F.G.; Capdevila, C.; Álvarez, L.F. Application of dilatometric analysis to the study of solid-solid phase transformations in steels. Mater. Charact. 2002, 48, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Hunkel, M.; Surm, H.; Steinbacher, M. Dilatometry. Handb. Therm. Anal. Calorim. 2018, 6, 103–129. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W.; Li, D. Quantification of phase transformation kinetics of 18 wt.% Ni C250 maraging steel. Mater. Sci. Eng. A 2004, 373, 10–20. [Google Scholar] [CrossRef]
- Goldberg, A.; O’Connor, D.G. Influence of heating rate on transformations in an 18 per cent nickel maraging steel. Nature 1967, 213, 170–171. [Google Scholar] [CrossRef]
- Primig, S.; Leitner, H. Transformation from continuous-to-isothermal aging applied on a maraging steel. Mater. Sci. Eng. A 2010, 527, 4399–4405. [Google Scholar] [CrossRef]
- Rowolt, C.; Milkereit, B.; Andreazza, P.; Kessler, O. Quantitative high temperature calorimetry on precipitation in steel and nickel alloys. Thermochim. Acta 2019, 677, 169–179. [Google Scholar] [CrossRef]
- Huang, D.; Yan, J.; Zuo, X. Co-precipitation kinetics, microstructural evolution and interfacial segregation in multicomponent nano-precipitated steels. Mater. Charact. 2019, 155, 109786. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Dey, G.K.; Asundi, M.K. Precipitation hardening in 350 grade maraging steel. Metall. Trans. A 1993, 24, 2429–2442. [Google Scholar] [CrossRef]
- Koohdar, H.; Nili-Ahmadabadi, M.; Habibi-Parsa, M.; Jafarian, H.R.; Bhattacharjee, T.; Tsuji, N. On the Stability of Reversely Formed Austenite and Related Mechanism of Transformation in an Fe-Ni-Mn Martensitic Steel Aided by Electron Backscattering Diffraction and Atom Probe Tomography. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 5244–5257. [Google Scholar] [CrossRef]
- Kapoor, R.; Batra, I.S. On the α to γ transformation in maraging (grade 350), PH 13-8 Mo and 17-4 PH steels. Mater. Sci. Eng. A 2004, 371, 324–334. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Houserová, J.; Vřešťál, J.; Šob, M. Phase diagram calculations in the Co-Mo and Fe-Mo systems using first-principles results for the sigma phase. Calphad Comput. Coupling Phase Diagrams Thermochem. 2005, 29, 133–139. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Divinski, S.V.; Pokoev, A.V.; Esakkiraja, N.; Paul, A. A Mystery of “sluggish diffusion” in high-entropy alloys: The truth or a myth? Diffus. Found. 2018, 17, 69–104. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Sohn, Y. Investigation of sluggish diffusion in FCC Al0.25CoCrFeNi high-entropy alloy. Mater. Res. Lett. 2021, 9, 239–246. [Google Scholar] [CrossRef]
- Qin, X.Z.; Guo, J.T.; Yuan, C.; Yang, G.X.; Zhou, L.Z.; Ye, H.Q. μ-Phase behavior in a cast Ni-base superalloy. J. Mater. Sci. 2009, 44, 4840–4847. [Google Scholar] [CrossRef]







| Alloys | Notation | Composition at. % | ||||||
|---|---|---|---|---|---|---|---|---|
| Co | Cr | Cu | Fe | Mo | Ni | |||
| CoCrFe2Ni2 | A0 | Nominal | 16.67 | 16.67 | - | 33.33 | - | 33.33 |
| Experimental | 16.8 | 17.0 | - | 33.4 | - | 32.8 | ||
| (A0)0.9(Cu)0.1 | A0|Cu10 | Nominal | 15 | 15 | 10 | 30 | - | 30 |
| Experimental | 16.0 | 16.0 | 9.9 | 30.2 | - | 29.5 | ||
| (A0)0.9(Cu2Mo)0.1 | A0|Cu7Mo3 | Nominal | 15 | 15 | 6.67 | 30 | 3.33 | 30 |
| Experimental | 14.6 | 14.6 | 6.5 | 31.6 | 3.2 | 29.0 | ||
| (A0)0.9(CuMo)0.1 | A0|Cu5Mo5 | Nominal | 15 | 15 | 5 | 30 | 5 | 30 |
| Experimental | 14.5 | 14.5 | 4.9 | 62.3 | 4.8 | 28.8 | ||
| (A0)0.9(CuMo2)0.1 | A0|Cu3Mo7 | Nominal | 15 | 15 | 3.33 | 30 | 6.67 | 30 |
| Experimental | 14.3 | 14.3 | 3.1 | 33.1 | 6.7 | 28.6 | ||
| (A0)0.9(Mo)0.1 | A0|Mo10 | Nominal | 15 | 15 | - | 30 | 10 | 30 |
| Experimental | 14.0 | 14.0 | - | 33.5 | 9.3 | 28.2 | ||
| Composition at. % | ||||||
|---|---|---|---|---|---|---|
| Co | Cr | Cu | Fe | Mo | Ni | |
| Cu-rich precipitates | 2.5–3.0 | 1.5–2.5 | 80–82 | 5–6 | - | 7–8 |
| Mo-rich precipitates | 9–12 | 15–20 | - | 18–23 | 32–38 | 12–17 |
| Treatment | A0 | A0|Cu10 | A0|Cu7Mo3 | A0|Cu5Mo5 | A0|Cu3Mo7 | A0|Mo10 |
|---|---|---|---|---|---|---|
| 500 °C—8 h | - | - | - | - | - | - |
| 700 °C—8 h | - | - | - | - | - | Blocky μ (GB) |
| 900 °—8 h | - | FCC Cu-rich (GB) | - | - Traces of blocky μ (GB) | - Blocky μ (GB) | FCC1 + FCC2 + Blocky μ (GB) + Needle-shape μ or R phase |
| 1200 °C—5 h (homogenization treatment)- | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toda-Caraballo, I.; Jiménez, J.A.; Milenkovic, S.; Jimenez-Aguirre, J.; San-Martín, D. Microstructural Stability of the CoCrFe2Ni2 High Entropy Alloys with Additions of Cu and Mo. Metals 2021, 11, 1994. https://doi.org/10.3390/met11121994
Toda-Caraballo I, Jiménez JA, Milenkovic S, Jimenez-Aguirre J, San-Martín D. Microstructural Stability of the CoCrFe2Ni2 High Entropy Alloys with Additions of Cu and Mo. Metals. 2021; 11(12):1994. https://doi.org/10.3390/met11121994
Chicago/Turabian StyleToda-Caraballo, Isaac, Jose Antonio Jiménez, Srdjan Milenkovic, Jorge Jimenez-Aguirre, and David San-Martín. 2021. "Microstructural Stability of the CoCrFe2Ni2 High Entropy Alloys with Additions of Cu and Mo" Metals 11, no. 12: 1994. https://doi.org/10.3390/met11121994
APA StyleToda-Caraballo, I., Jiménez, J. A., Milenkovic, S., Jimenez-Aguirre, J., & San-Martín, D. (2021). Microstructural Stability of the CoCrFe2Ni2 High Entropy Alloys with Additions of Cu and Mo. Metals, 11(12), 1994. https://doi.org/10.3390/met11121994








