Plasmonic Gold Nanorod Size-Controlled: Optical, Morphological, and Electrical Properties of Efficiency Improved Tin Disulfide Vacuum-Free Hybrid Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Au Nanorods
2.2. Preparation of SnS2 Quantum Dots Nanoparticles
2.3. Preparation of (PEDOT:PSS + Au Nrods) Thin Film
2.4. Device Fabrication
2.5. Characterization
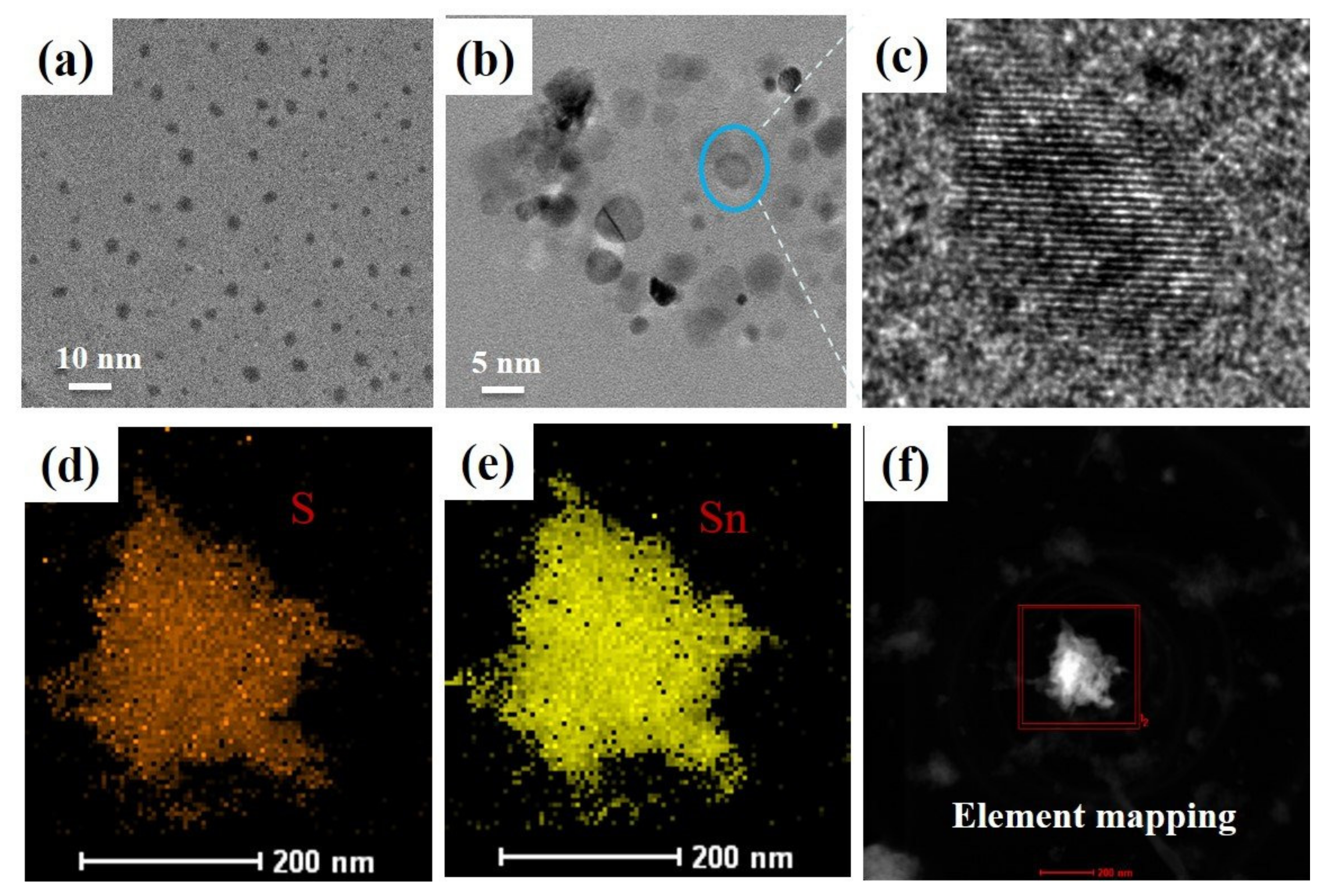
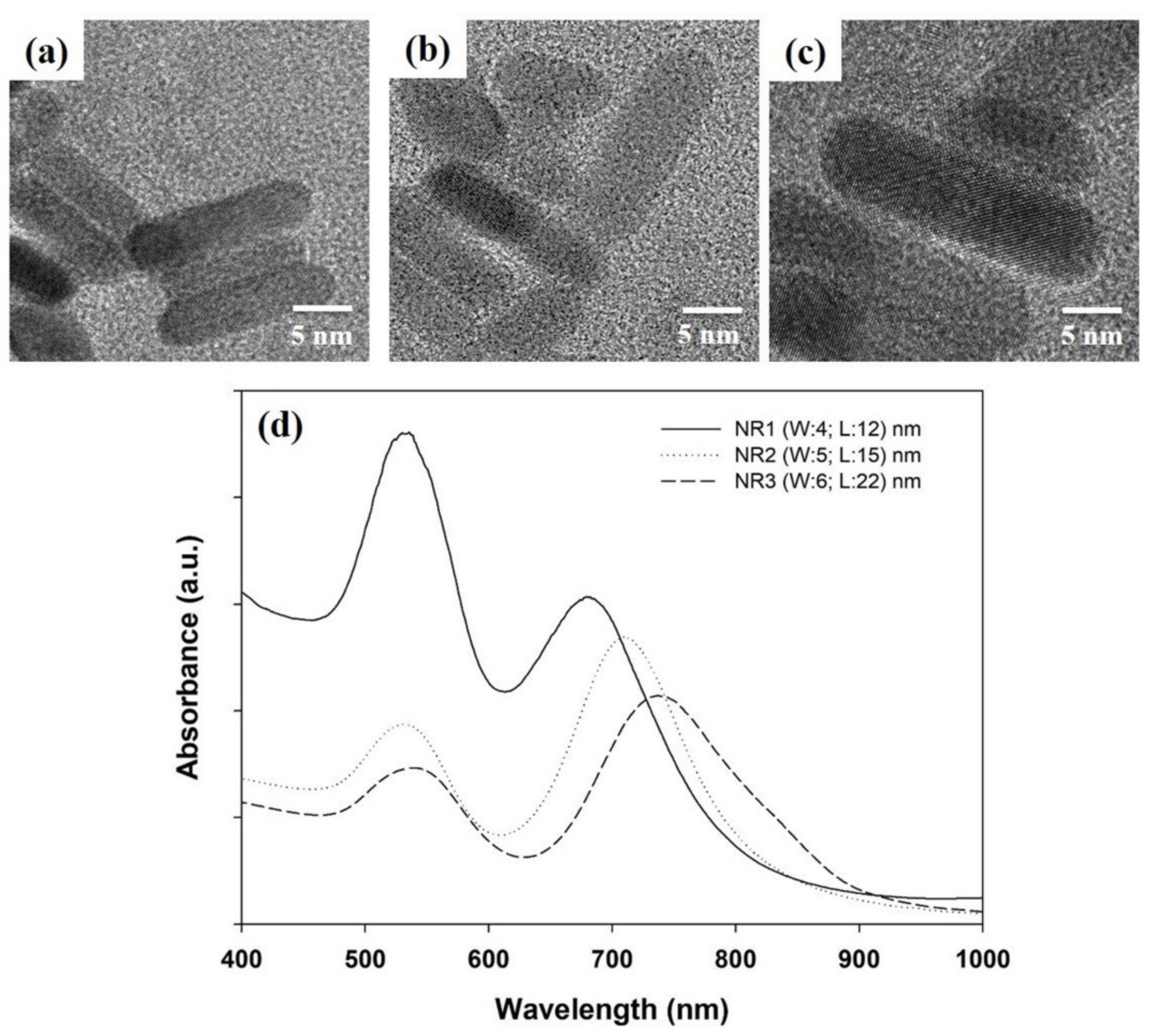
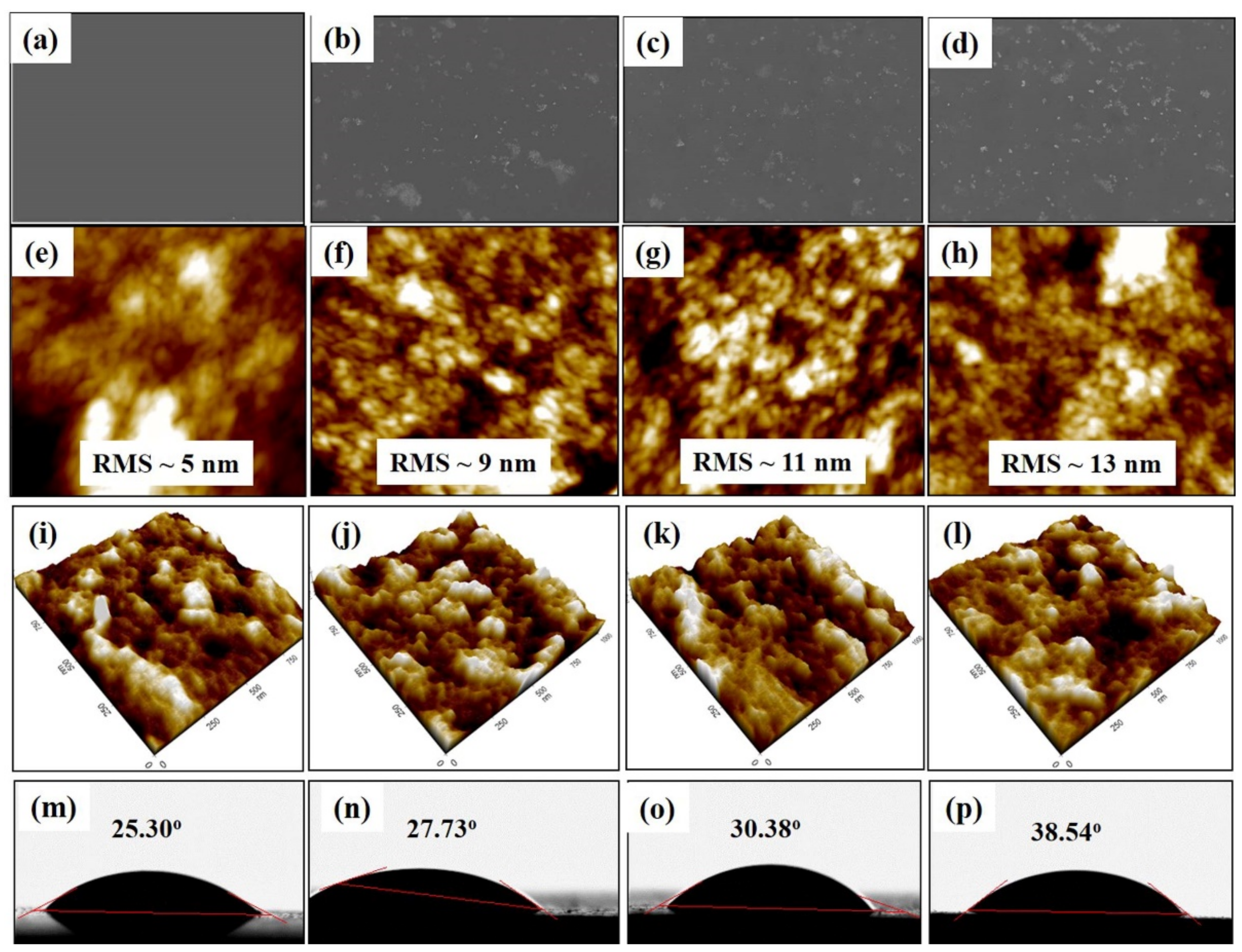
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watt, A.A.; Blake, D.; Warner, J.H.; Thomsen, E.A.; Tavenner, E.L.; Dunlop, H.R.; Meredith, P. Lead sulfide nanocrystal: Conducting polymer solar cells. J. Phys. D 2005, 38, 2006. [Google Scholar] [CrossRef] [Green Version]
- Zeng, T.W.; Lin, Y.Y.; Lo, H.H.; Chen, C.W.; Liu, S.C.; Huang, H.Y.; Su, W.F. A large interconnecting network within hybrid MEH-PPV/TiO2 nanorod photovoltaic devices. Nanotechnology 2006, 17, 5387. [Google Scholar] [CrossRef] [Green Version]
- Dayal, S.; Kopidakis, N.; Olson, D.C.; Ginley, D.S.; Rumbles, G. Photovoltaic devices with a low band gap polymer and CdSe nanostructures exceeding 3% efficiency. Nano Lett. 2010, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Qu, S.; Wu, J.; Liu, K.; Zhou, S.; Wang, Z. Preparation of SnS2 colloidal quantum dots and their application in organic/inorganic hybrid solar cells. Nanoscale Res. Lett. 2011, 6, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, N.T.N.; Trinh, T.K.; Pham, V.T.H.; Smith, R.P.; Park, C. Overcoming the efficiency limitations of SnS2 nanoparticle-based bulk heterojunction solar cells. Jpn. J. Appl. Phys. 2018, 57, 045002. [Google Scholar] [CrossRef]
- Tan, F.; Qu, S.; Zeng, X.; Zhang, C.; Shi, M.; Wang, Z.; Jin, L.; Bi, Y.; Cao, J.; Wang, Z.; et al. Photovoltaic effect of tin disulfide with nanocrystalline/amorphous blended phases. Solid State Commun. 2010, 150, 58. [Google Scholar] [CrossRef]
- Han, L.; Qin, D.; Jiang, X.; Liu, Y.; Wang, L.; Chen, J.; Cao, Y. Synthesis of high quality zinc-blende CdSe nanocrystals and their application in hybrid solar cells. Nanotechnology 2006, 17, 4736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhong, H.; Hou, J.; Ding, Y.; Yang, C.; Li, Y. Hybrid nanocrystal/polymer solar cells based on tetrapod-shaped CdSexTe1−x nanocrystals. Nanotechnology 2006, 17, 4041. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, K.S.; Abdullah, S.M.; Sulaiman, K.; Zakaria, R. Effects of silver nanoparticles towards the efficiency of organic solar cells. Appl. Phys. A 2014, 115, 63–68. [Google Scholar] [CrossRef]
- Kim, S.S.; Na, S.I.; Jo, J.; Kim, D.Y.; Nah, Y.C. Plasmon enhanced performance of organic solar cells using electrodeposited Ag nanoparticles. Appl. Phys. Lett. 2008, 93, 305. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, D.; Zuo, L.; Ye, Y.; Qian, J.; Chen, H.; He, S. Localized surface plasmon resonance enhanced organic solar cell with gold nanospheres. Appl. Energy 2011, 88, 848–852. [Google Scholar] [CrossRef]
- Xie, F.X.; Choy, W.C.; Wang, C.C.; Sha, W.E.; Fung, D.D. Improving the efficiency of polymer solar cells by incorporating gold nanoparticles into all polymer layers. Appl. Phys. Lett. 2011, 99, 219. [Google Scholar] [CrossRef] [Green Version]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 24, 20–22. [Google Scholar] [CrossRef]
- Guillermo, G.R.; Vished, K.; Pablo, L.; Pablo, D.N.; Eva, B.; Thomas, A.; Sara, B.; Ovidio, P.R.; Eva, G.N.; Luis, G.M.; et al. Disconnecting symmetry breaking from seeded growth for the reproducible synthesis of high quality gold nanorods. ACS Nano 2019, 13, 4424. [Google Scholar]
- Truong, N.T.N.; Park, C. Synthesis and characterization of tin disulfide nanocrystals for hybrid bulk hetero-junction solar cell applications. Electron. Mater. Lett. 2016, 12, 308. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 1–11. [Google Scholar]
- Chang, S.H. Modeling and design of Ag, Au, and Cu nanoplasmonic structures for enhancing the absorption of P3HT: PCBM-based photovoltaics. IEEE Photonics J. 2013, 5, 4800509. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Sau, T.K.; Murphy, C.J. Shape-Dependent Plasmon-Resonant Gold Nanoparticles. Small 2006, 2, 636–639. [Google Scholar] [CrossRef] [PubMed]
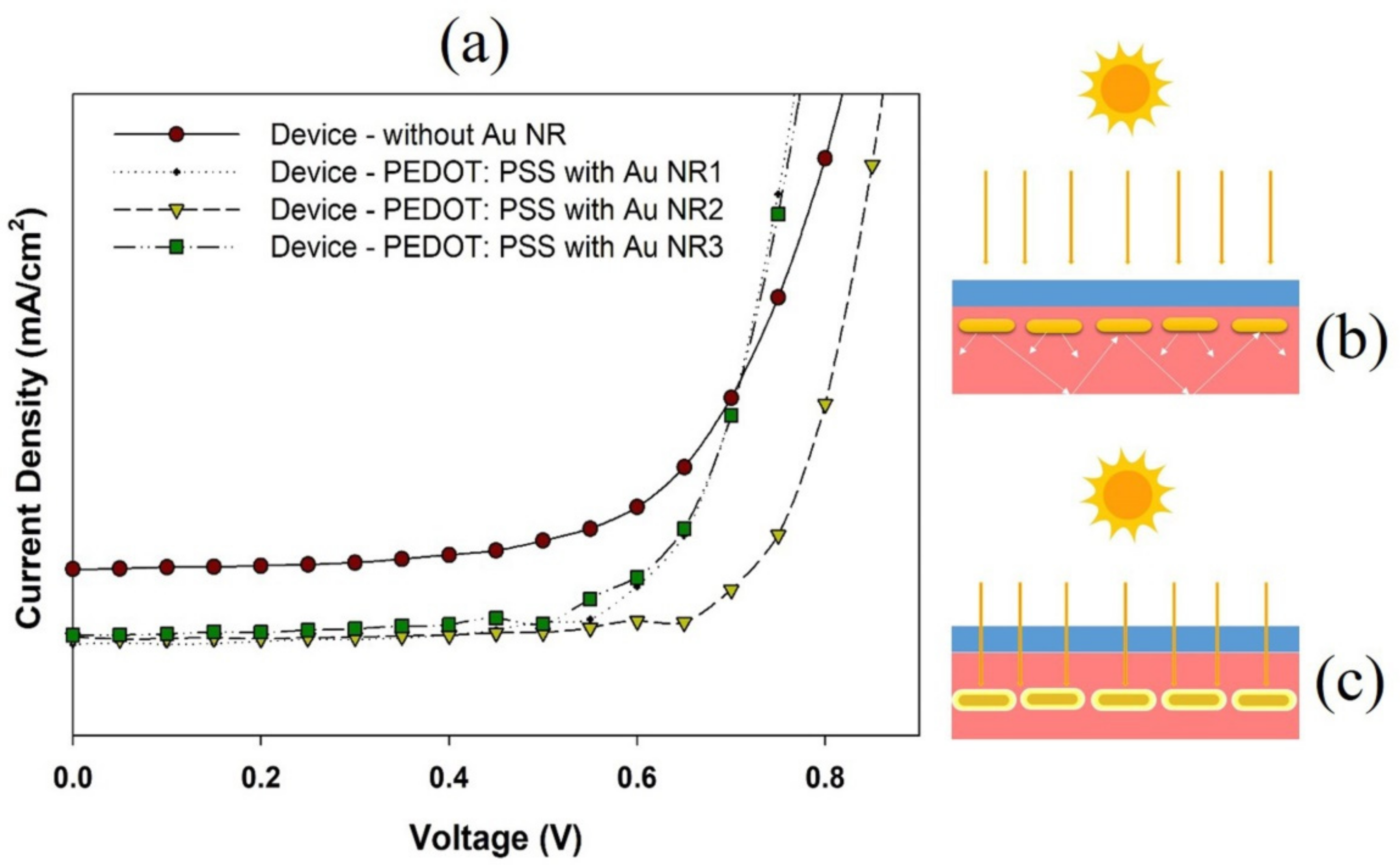

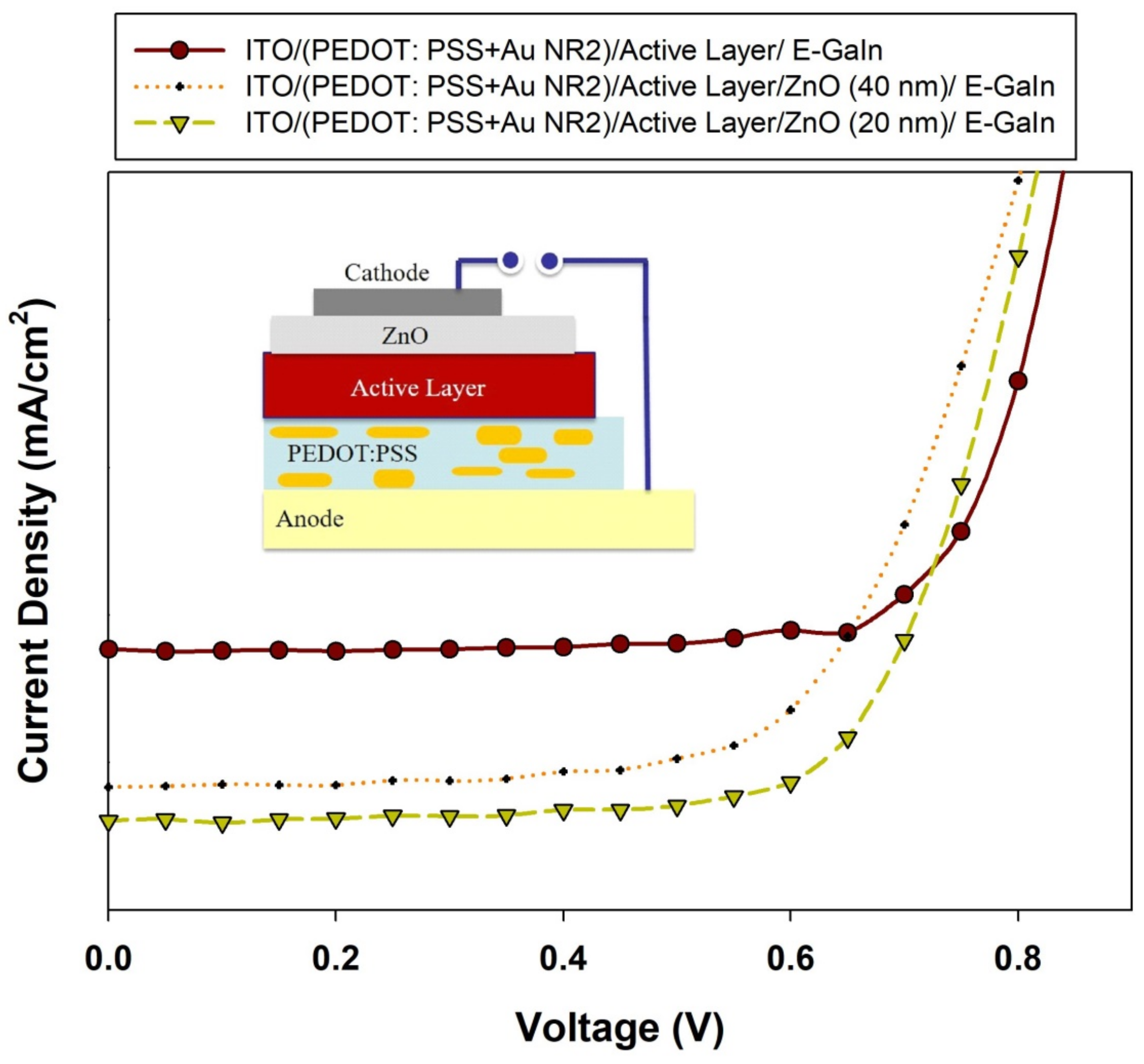
| Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | |
|---|---|---|---|---|
| Cell without Au NR | 0.72 ± 0.02 | 3.42 ± 0.01 | 61 ± 2 | 1.5 ± 0.04 |
| Cell with Au NR1 | 0.72 ± 0.04 | 4.47 ± 0.03 | 70 ± 4 | 2.3 ± 0.05 |
| Cell with Au NR2 | 0.77 ± 0.03 | 4.47 ± 0.02 | 76 ± 2 | 2.8 ± 0.05 |
| Cell with Au NR3 | 0.70 ± 0.04 | 4.33 ± 0.03 | 69 ± 2 | 2.1 ± 0.01 |
| Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | |
|---|---|---|---|---|
| Cell-without ZnO | 0.77 ± 0.03 | 4.47 ± 0.02 | 76 ± 2 | 2.8 ± 0.05 |
| Cell-with ZnO (T ~ 40 nm) | 0.75 ± 0.00 | 6.30 ± 0.03 | 65 ± 3 | 3.1 ± 0.07 |
| Cell-with ZnO (T ~ 20 nm) | 0.77 ± 0.03 | 6.80 ± 0.02 | 70 ± 2 | 3.7 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Truong, N.T.N.; Lam, N.H.; Le, N.; Tamboli, A.M.; Tamboli, M.S.; Jung, J.H. Plasmonic Gold Nanorod Size-Controlled: Optical, Morphological, and Electrical Properties of Efficiency Improved Tin Disulfide Vacuum-Free Hybrid Solar Cells. Metals 2021, 11, 1911. https://doi.org/10.3390/met11121911
Kim M, Truong NTN, Lam NH, Le N, Tamboli AM, Tamboli MS, Jung JH. Plasmonic Gold Nanorod Size-Controlled: Optical, Morphological, and Electrical Properties of Efficiency Improved Tin Disulfide Vacuum-Free Hybrid Solar Cells. Metals. 2021; 11(12):1911. https://doi.org/10.3390/met11121911
Chicago/Turabian StyleKim, Minsu, Nguyen Tam Nguyen Truong, Nguyen Hoang Lam, Nam Le, Asiya M. Tamboli, Mohaseen S. Tamboli, and Jae Hak Jung. 2021. "Plasmonic Gold Nanorod Size-Controlled: Optical, Morphological, and Electrical Properties of Efficiency Improved Tin Disulfide Vacuum-Free Hybrid Solar Cells" Metals 11, no. 12: 1911. https://doi.org/10.3390/met11121911
APA StyleKim, M., Truong, N. T. N., Lam, N. H., Le, N., Tamboli, A. M., Tamboli, M. S., & Jung, J. H. (2021). Plasmonic Gold Nanorod Size-Controlled: Optical, Morphological, and Electrical Properties of Efficiency Improved Tin Disulfide Vacuum-Free Hybrid Solar Cells. Metals, 11(12), 1911. https://doi.org/10.3390/met11121911






