Environmentally Benign Organic Dye Conversion under UV Light through TiO2-ZnO Nanocomposite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of TiO2, ZnO and TiO2-ZnO Composite
2.3. Characterization
2.4. Evaluation of Photocatalytic Performance
3. Results and Discussion
3.1. XRD Analysis
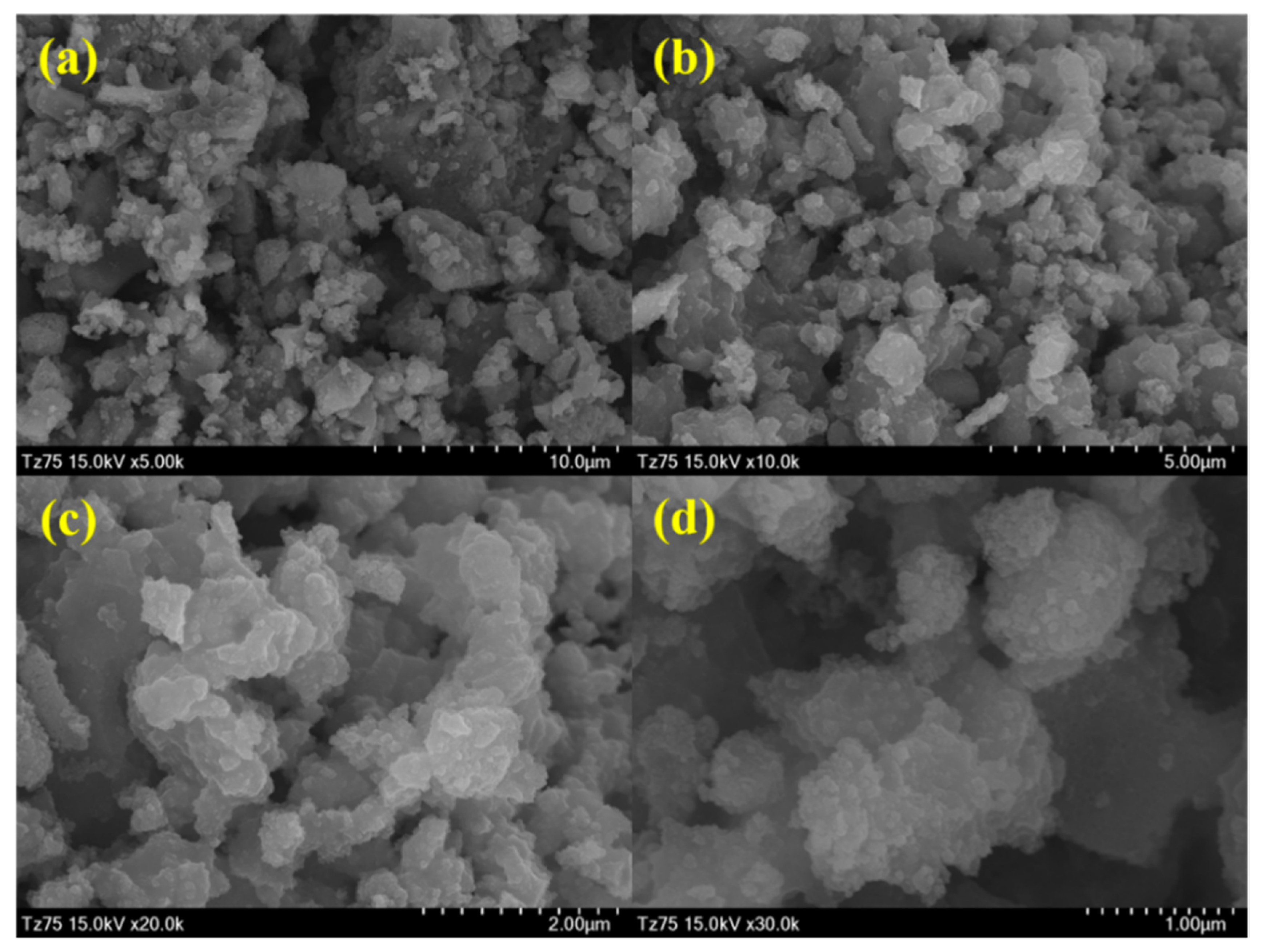
3.2. Structure and Morphological Analysis
3.3. Optical Properties
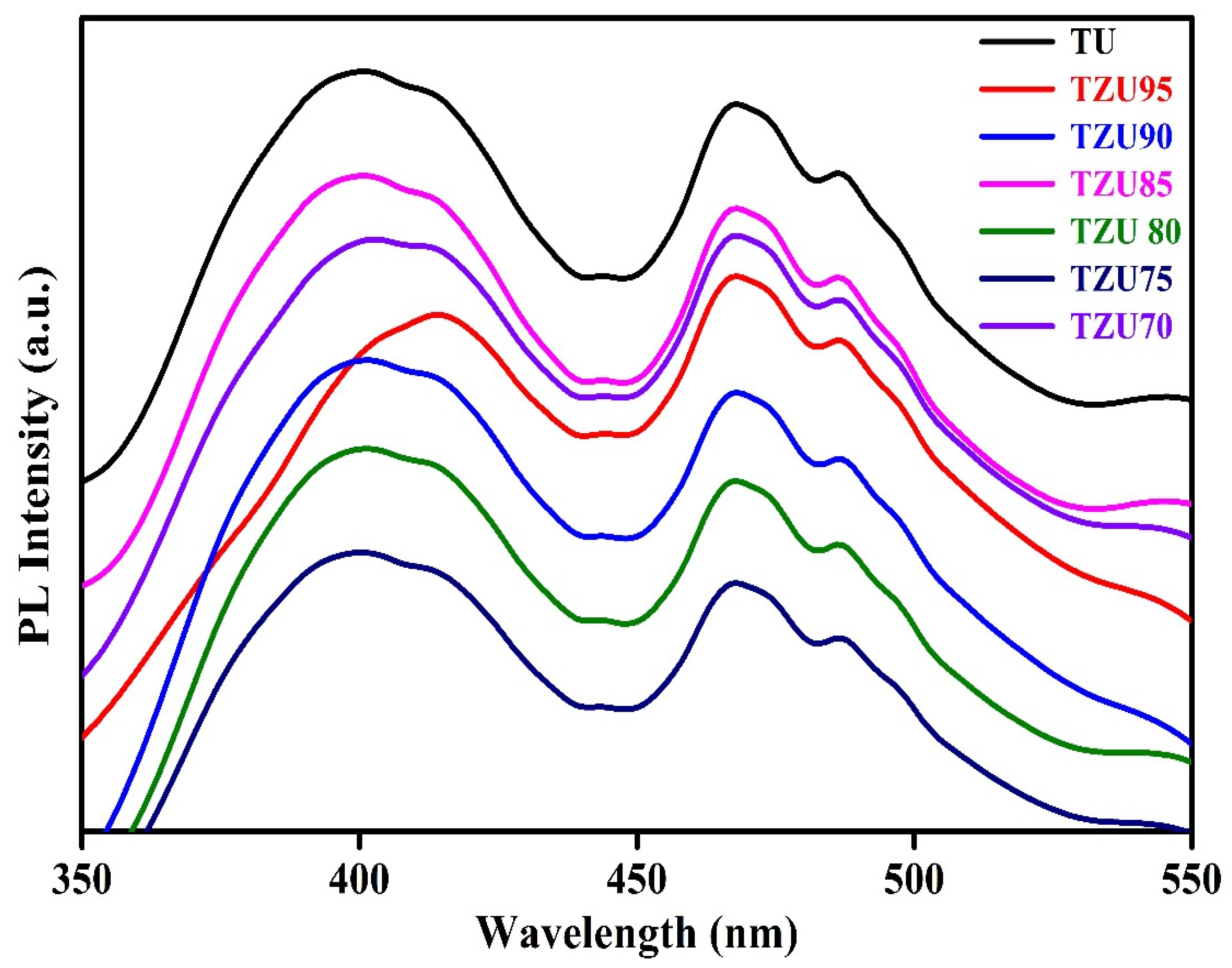
3.4. PL Spectra
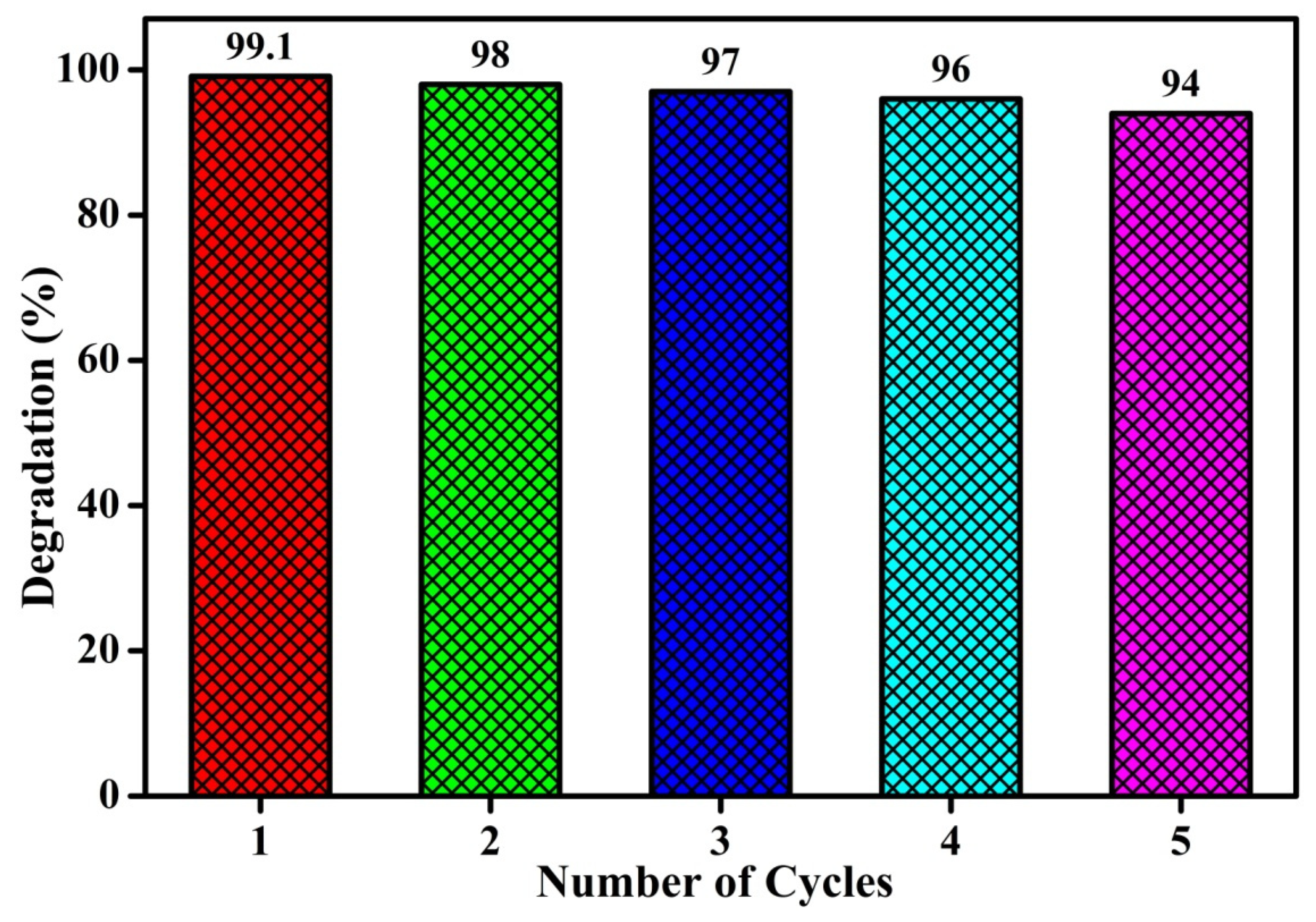
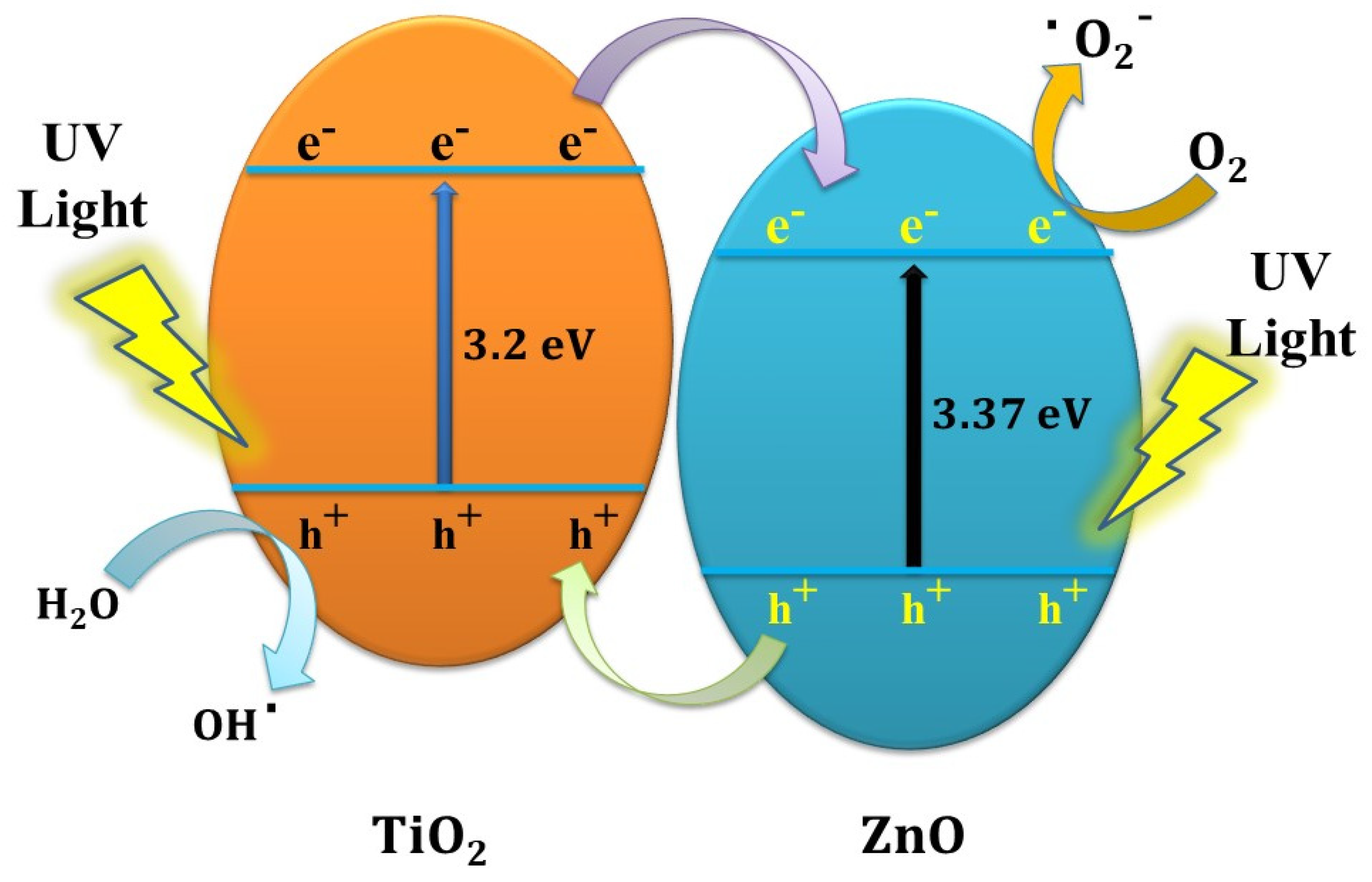
3.5. Photocatalytic Degradation of Methyl Orange Using UV–Visible Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Sci. Direct 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Long, L.L.; Liu, C.; Li, W.W.; Yu, H.Q. Chemical recycling of the waste anodic electrolyte from the TiO2 nanotube preparation process to synthesize facet-controlled TiO2 single crystals as an efficient photocatalyst. Green Chem. 2014, 16, 2745–2753. [Google Scholar] [CrossRef]
- Shaikh, A.F.; Arbuj, S.S.; Tamboli, M.S.; Naik, S.D.; Rane, S.B.; Kale, B.B. ZnSe/ZnO Nano-Heterostructures for Enhanced Solar Light Hydrogen Generation. Chem. Sel. 2017, 2, 9174–9180. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Yang, J.; Chen, Z.; Zhan, W.; Zhou, L.; Liu, S. Sonochemical synthesis of nanocrystallite Bi2O3 as a visible-light-driven photocatalyst. Appl. Catal. A 2006, 308, 105–110. [Google Scholar] [CrossRef]
- Li, L.; Chu, Y.; Liu, Y.; Dong, L. Template-Free Synthesis and Photocatalytic Properties of Novel Fe2O3 Hollow Spheres. J. Phys. Chem. C 2007, 111, 2123–2127. [Google Scholar] [CrossRef]
- Deshpande, S.P.; Tamboli, M.S.; Arbuj, S.S.; Mulik, U.P.; Amalnerkar, D.P. Synthesis of Nanostructured Ta2O5 and Its Photocatalytic Performance Study. J. Nanoeng. Nanomanuf. 2014, 4, 215–220. [Google Scholar] [CrossRef]
- Smith, Y.R.; Kar, A.; Subramanian, V.R. Investigation of physicochemical parameters that influence photocatalytic degradation of methyl orange over TiO2 nanotubes. Ind. Eng. Chem. Res. 2009, 48, 10268–10276. [Google Scholar] [CrossRef]
- Jin, Z.; Duan, W.; Liu, B.; Chen, X.; Yang, F. Fabrication of efficient visible light activated Cu–P25–graphene ternary composite for photocatalytic degradation of methyl blue. J. Appl. Surf. Sci. 2015, 356, 707–718. [Google Scholar] [CrossRef]
- Margan, P.; Haghighi, M. Hydrothermal-assisted sol–gel synthesis of Cd-doped TiO2 nanophotocatalyst for removal of acid orange from wastewater. J. Sol-Gel Sci. Technol. 2017, 81, 556–718. [Google Scholar] [CrossRef]
- Wang, P.; Yi, X.; Lu, Y.; Yu, H.; Yu, J. In-situ synthesis of amorphous H2TiO3-modified TiO2 and its improved photocatalytic H2-evolution performance. J. Colloid Interface Sci. 2018, 532, 272–279. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.G.; Xiao, W.; Li, Q. Microwave-assisted hydrothermal synthesis of graphene based Au–TiO2 photocatalysts for efficient visible-light hydrogen production. J. Mater. Chem. A 2014, 2, 3847–3855. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, M.H.; Wu, J.Y.; Ma, Y.Y.; Ma, Z.Z. In situ synthesis of TiO2/SnOx–Au ternary heterostructures effectively promoting visible-light photocatalysis. Dalton Trans. 2015, 44, 11901–11910. [Google Scholar] [CrossRef]
- Muzakki, A.; Shabrany, H.; Saleh, R. Synthesis of ZnO/CuO and TiO2/CuO nanocomposites for light and ultrasound assisted degradation of a textile dye in aqueous solution. AIP Conf. Proc. 2016, 1725, 020051. [Google Scholar] [CrossRef]
- Vinoth, R.; Karthik, P.; Devan, K.; Neppolian, B.; Ashokkumar, M. TiO2–NiO p–n nanocomposite with enhanced sonophotocatalytic activity under diffused sunlight. Ultrason Sonochem. 2017, 35, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Lachom, V.; Poolcharuansin, P.; Laokul, P. Preparation, characterizations and photocatalytic activity of a ZnO/TiO2 nanocomposite. Mater. Res. Express. 2017, 4, 035006. [Google Scholar] [CrossRef]
- Arbuj, S.S.; Mulik, U.P.; Amalnerkar, D.P. Synthesis of Ta2O5/TiO2 Coupled Semiconductor Oxide Nanocomposites with High Photocatalytic Activity. Nanosci. Nanotechnol. Lett. 2013, 5, 968–973. [Google Scholar] [CrossRef]
- Arbuj, S.S.; Hawaldar, R.R.; Mulik, U.P.; Amalnerkar, D.P. Preparation, Characterisation and Photocatalytic Activity of Nb2O5/TiO2 Coupled Semiconductor Oxides. J. Nanoeng. Nanomanuf. 2013, 3, 79–83. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Q.; Xie, Y.; Ling, Y.; Hou, Y. Designed synthesis of anatase–TiO2 (B) biphase nanowire/ZnO nanoparticle heterojunction for enhanced photocatalysis. J. Mater. Chem. A 2018, 6, 8289–8298. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wei, X.; Liu, G.; Li, J. ZnO nanoparticles implanted in TiO2 macrochannels as an effective direct Z-scheme heterojunction photocatalyst for degradation of RhB. Appl. Surf. Sci. 2018, 456, 666–675. [Google Scholar] [CrossRef]
- Liao, M.-H.; Hsu, C.-H.; Chen, D.-H. Preparation and properties of amorphous titania-coated zinc oxide nanoparticles. J. Solid State Chem. 2006, 179, 2020–2026. [Google Scholar] [CrossRef]
- Chakma, S.; Moholkar, V.S. Synthesis of bi-metallic oxides nanotubes for fast removal of dye using adsorption and sonocatalysis process. J. Ind. Eng. Chem. 2016, 37, 84–89. [Google Scholar] [CrossRef]
- King, D.M.; Liang, X.; Zhou, Y.; Carney, C.S.; Hakim, L.F.; Li, P.; Weimer, A.W. Atomic layer deposition of TiO2 films on particles in a fluidized bed reactor. Powder Technol. 2008, 183, 356–363. [Google Scholar] [CrossRef]
- Bahadur, N.M.; Furusawa, T.; Sato, M.; Kurayama, F.; Suzuki, N. Rapid synthesis, characterization and optical properties of TiO2 coated ZnO nanocomposite particles by a novel microwave irradiation method. Mater. Res. Bull. 2010, 45, 1383–1388. [Google Scholar] [CrossRef]
- Deshmukh, S.M.; Tamboli, M.S.; Shaikh, H.; Babar, S.B.; Hiwarale, D.P.; Thate, A.G.; Shaikh, A.F.; Alam, M.A.; Khetre, S.M.; Bamane, S.R. A Facile Urea-Assisted Thermal Decomposition Process of TiO2 Nanoparticles and Their Photocatalytic Activity. Coatings 2021, 11, 165. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Houskova, V.; Stengl, V.; Bakardjieva, S.; Murafa, N. Photoactive materials prepared by homogeneous hydrolysis with thioacetamide: Part 2—TiO2/ZnO nanocomposites. J. Phys. Chem. Solids 2008, 69, 1623–1631. [Google Scholar] [CrossRef] [Green Version]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction Reading; Addison-Wesley: London, UK, 1978; Available online: https://www.worldcat.org/title/elements-of-x-ray-diffraction/oclc/3672627 (accessed on 10 September 2021).
- Xiao, F.-X. Construction of Highly Ordered ZnO–TiO2 Nanotube Arrays (ZnO/TNTs) Heterostructure for Photocatalytic Application. ACS Appl. Mater. Interfaces 2012, 4, 7055–7063. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.S.; Lee, W.J.; Pathan, H.M.; Han, S.H. Nanocrystalline TiO2/ZnO Thin Films: Fabrication and Application to Dye-Sensitized Solar Cells. J. Phys. Chem. B 2005, 109, 24254–24259. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Nikfarjam, A.; Hajghassem, H.; Salehifar, N. Hierarchical Dense Array of ZnO Nanowires Spatially Grown on ZnO/TiO2 Nanofibers and Their Ultraviolet Activated Gas Sensing Properties. J. Phys. Chem. C 2020, 124, 322–335. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, S.M.; Arbuj, S.S.; Babar, S.B.; Shaikh, S.F.; Tamboli, A.M.; Nguyen Truong, N.T.; Kim, C.-D.; Khetre, S.M.; Tamboli, M.S.; Bamane, S.R. Environmentally Benign Organic Dye Conversion under UV Light through TiO2-ZnO Nanocomposite. Metals 2021, 11, 1787. https://doi.org/10.3390/met11111787
Deshmukh SM, Arbuj SS, Babar SB, Shaikh SF, Tamboli AM, Nguyen Truong NT, Kim C-D, Khetre SM, Tamboli MS, Bamane SR. Environmentally Benign Organic Dye Conversion under UV Light through TiO2-ZnO Nanocomposite. Metals. 2021; 11(11):1787. https://doi.org/10.3390/met11111787
Chicago/Turabian StyleDeshmukh, Sandip M., Sudhir S. Arbuj, Santosh B. Babar, Shoyebmohamad F. Shaikh, Asiya M. Tamboli, Nguyen Tam Nguyen Truong, Chang-Duk Kim, Sanjay M. Khetre, Mohaseen S. Tamboli, and Sambhaji R. Bamane. 2021. "Environmentally Benign Organic Dye Conversion under UV Light through TiO2-ZnO Nanocomposite" Metals 11, no. 11: 1787. https://doi.org/10.3390/met11111787
APA StyleDeshmukh, S. M., Arbuj, S. S., Babar, S. B., Shaikh, S. F., Tamboli, A. M., Nguyen Truong, N. T., Kim, C.-D., Khetre, S. M., Tamboli, M. S., & Bamane, S. R. (2021). Environmentally Benign Organic Dye Conversion under UV Light through TiO2-ZnO Nanocomposite. Metals, 11(11), 1787. https://doi.org/10.3390/met11111787





