Dual Cluster Model for Medium-Range Order in Metallic Glasses
Abstract
:1. Introduction
2. Methods
2.1. Interatomic Potentials
2.2. Simulation Procedure
2.3. Voronoi Tessellation and Frank–Kasper Clusters
3. Results
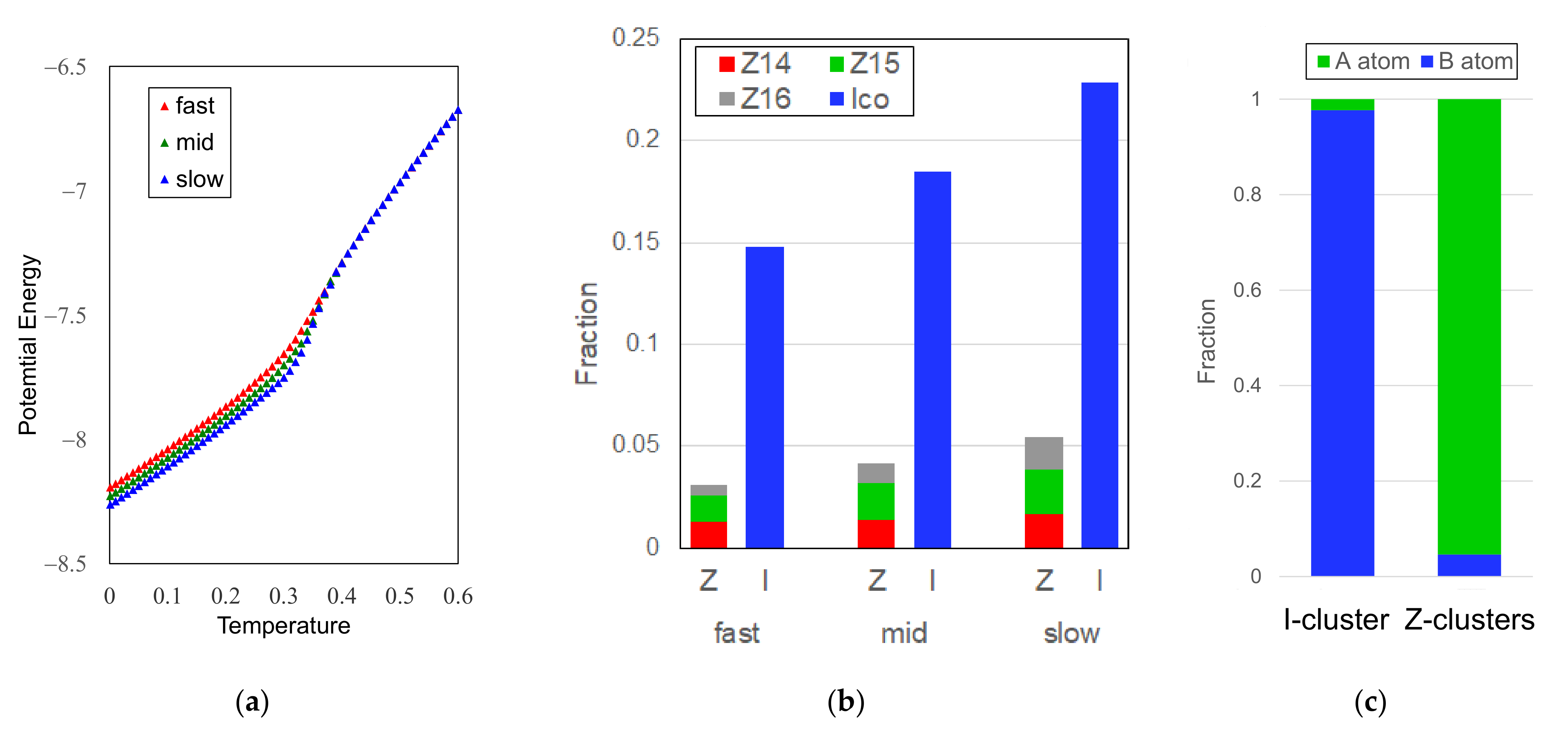
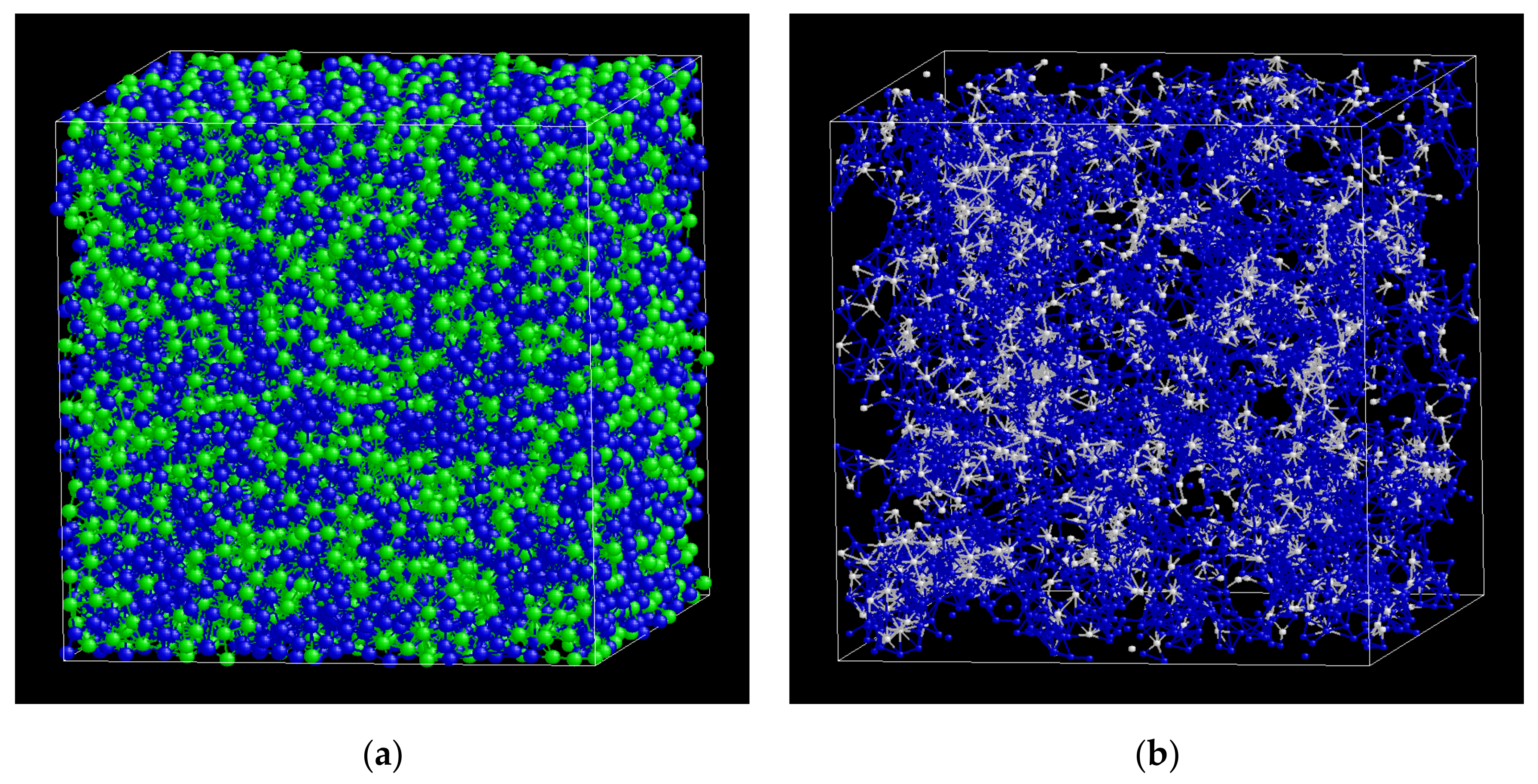
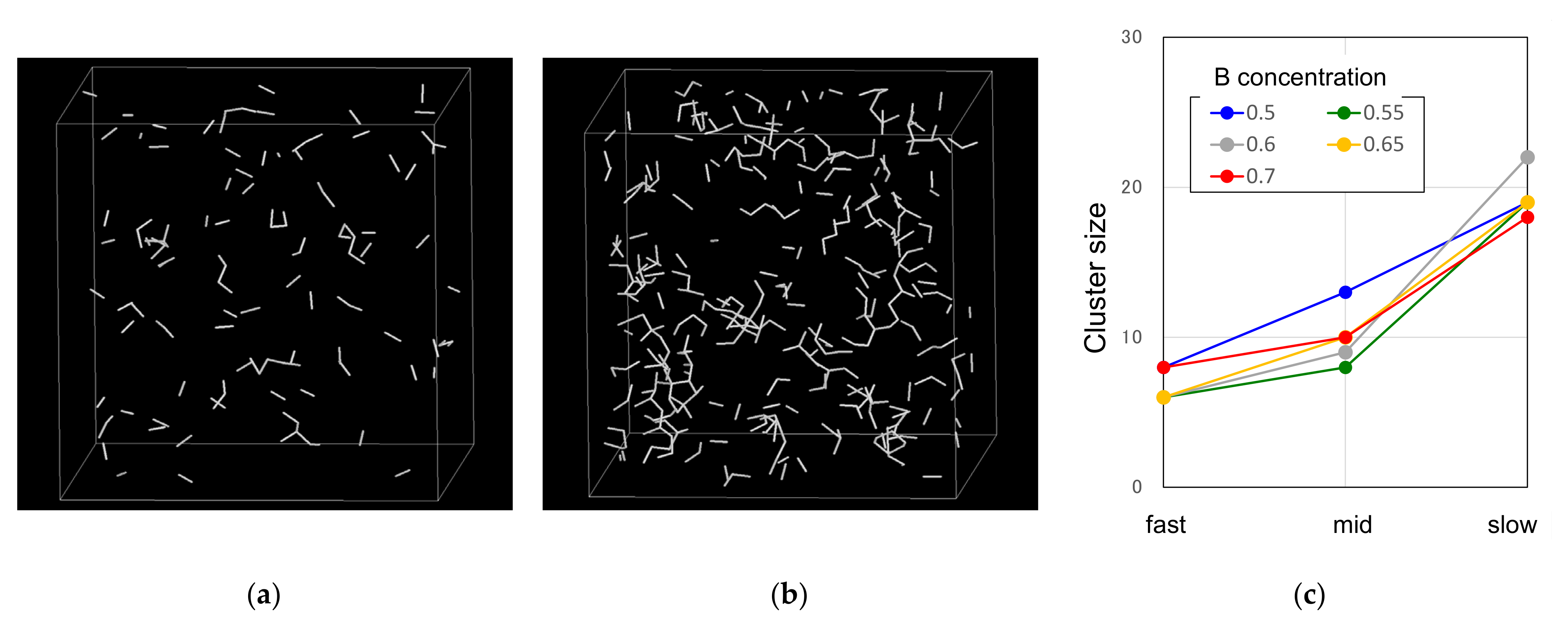
3.1. Glass Formation by Liquid Quenching
3.2. Icosahedral Order in Glassy Phases
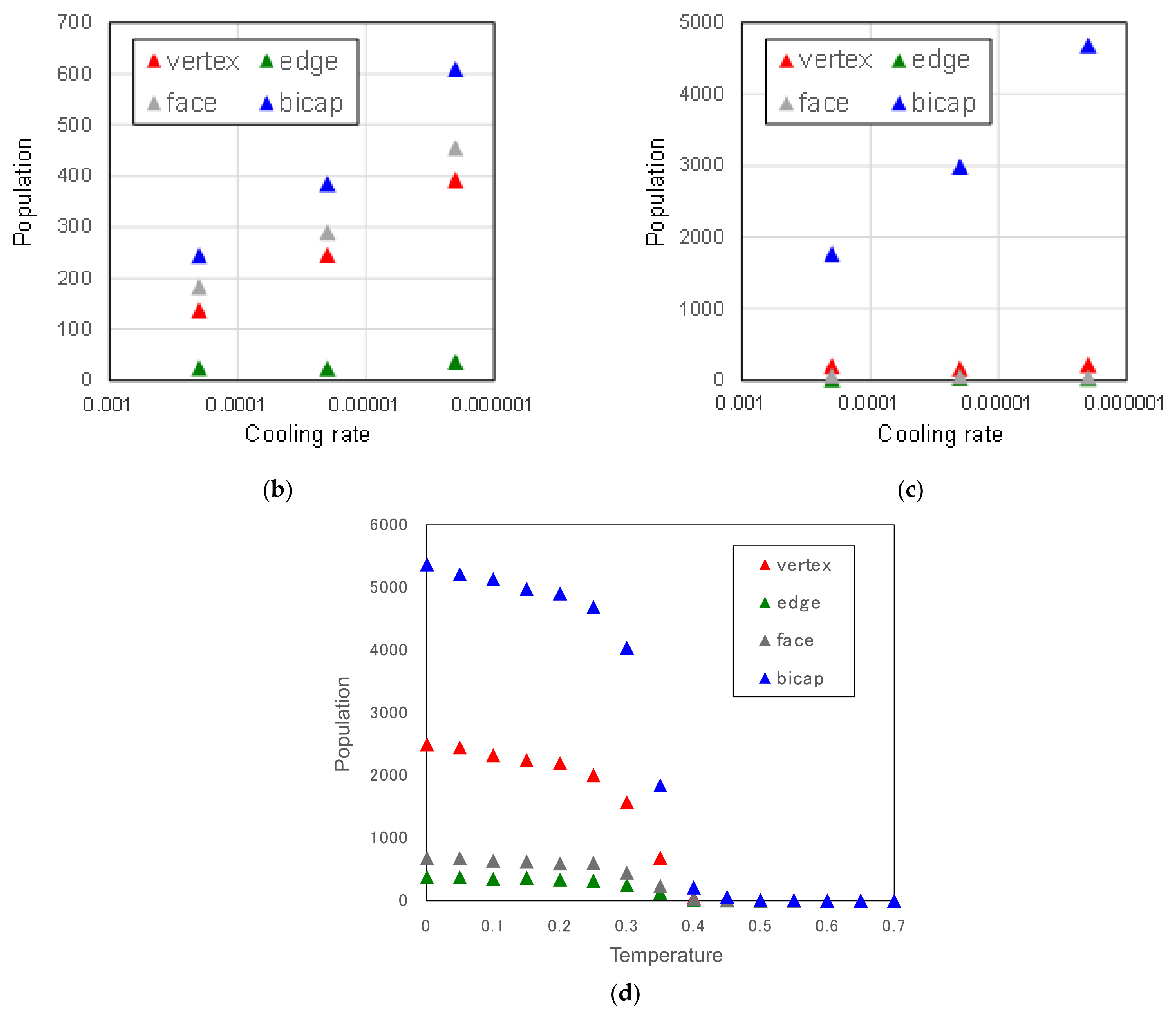
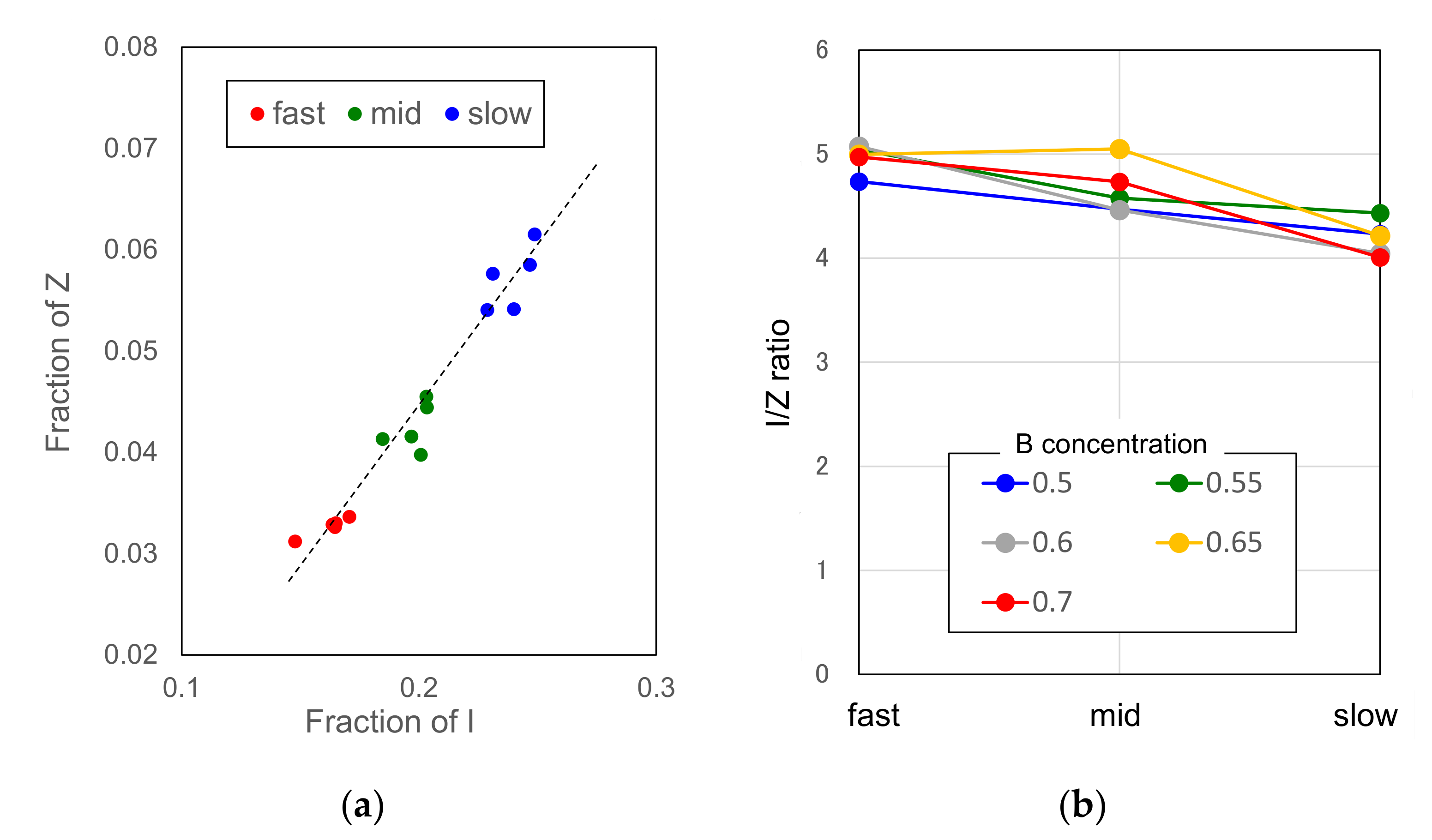
3.2.1. Cooling Rate Dependence of Icosahedral Order
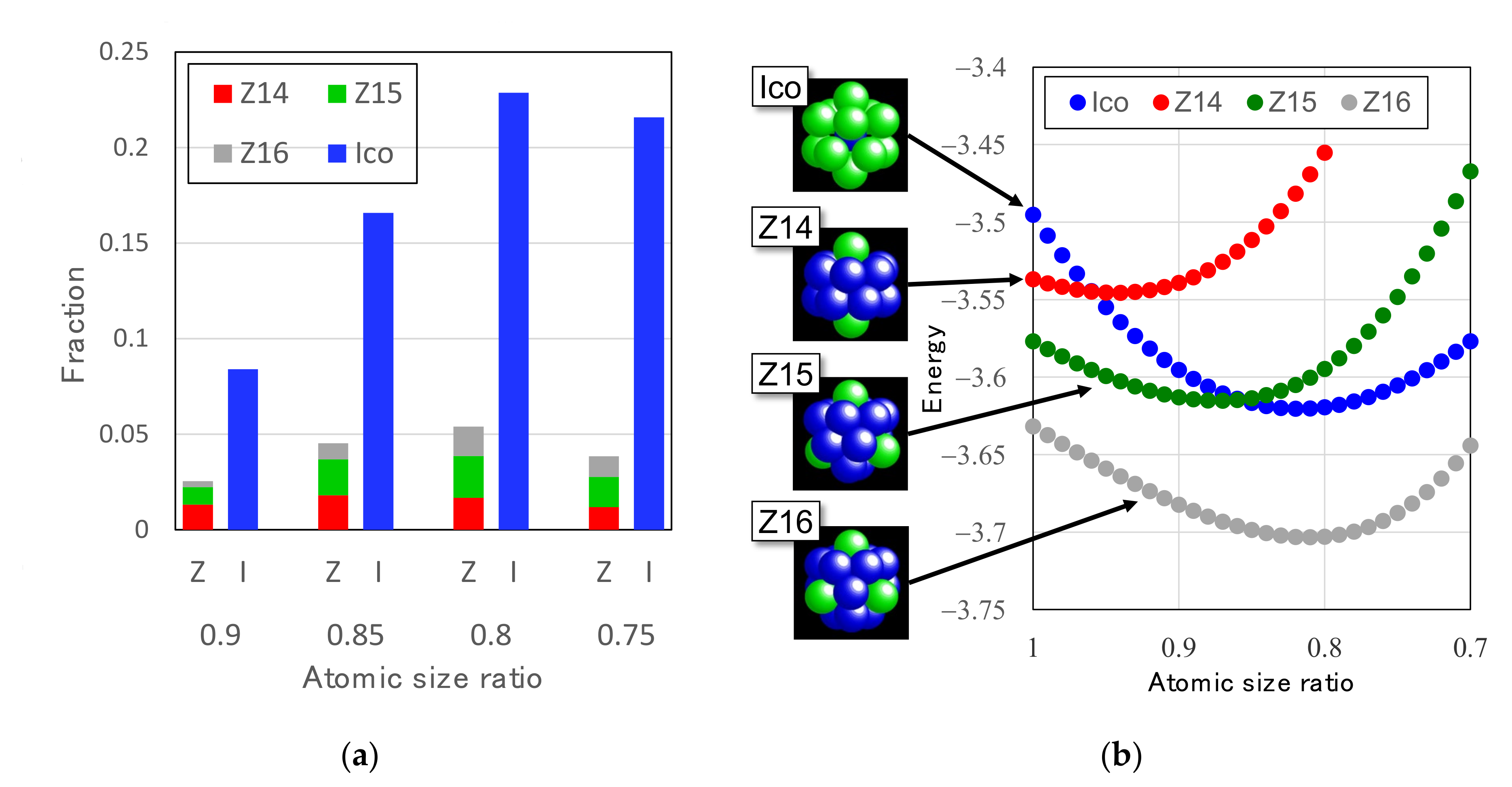
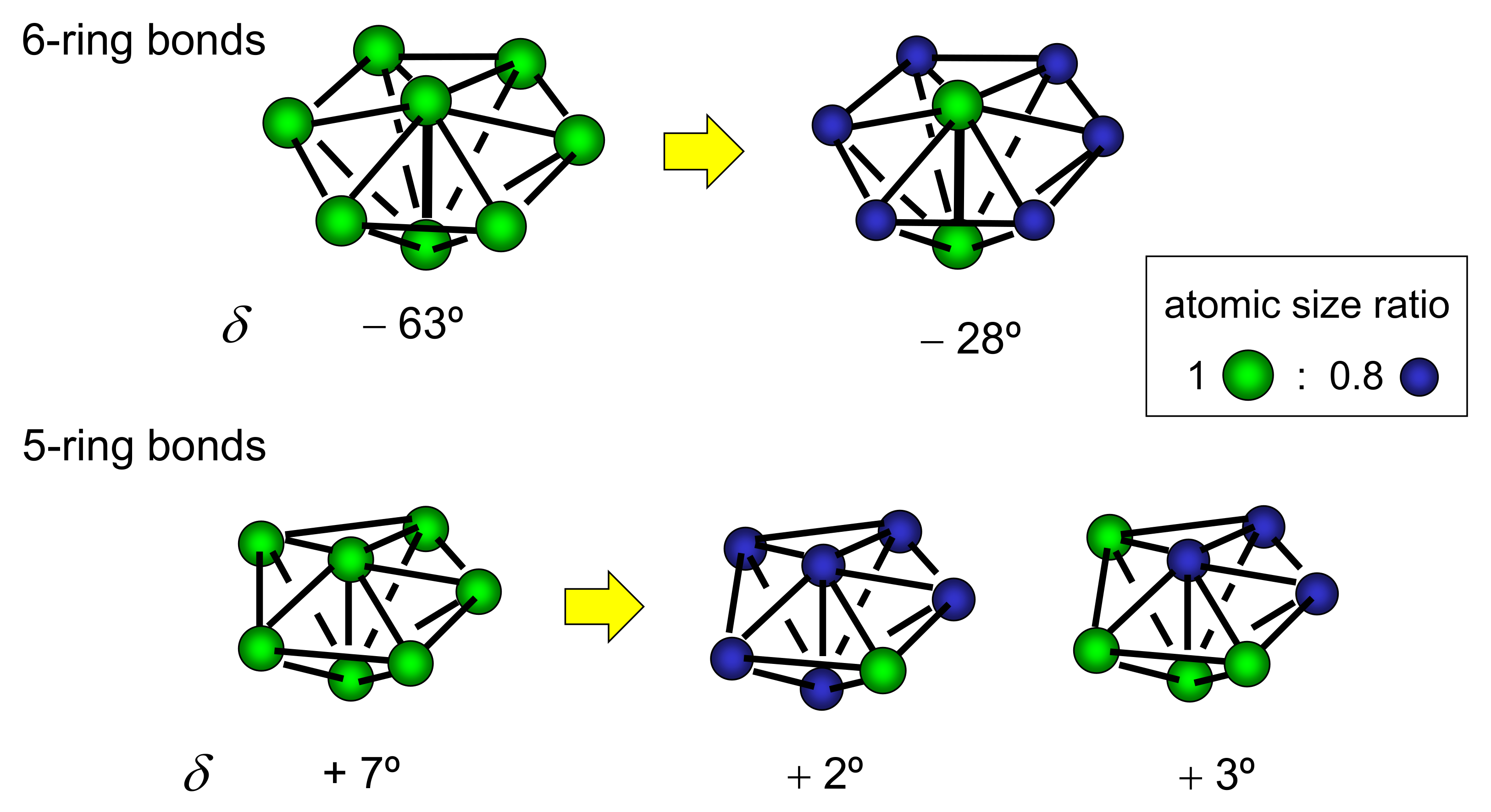
3.2.2. Atomic Size Effect on Icosahedral Order
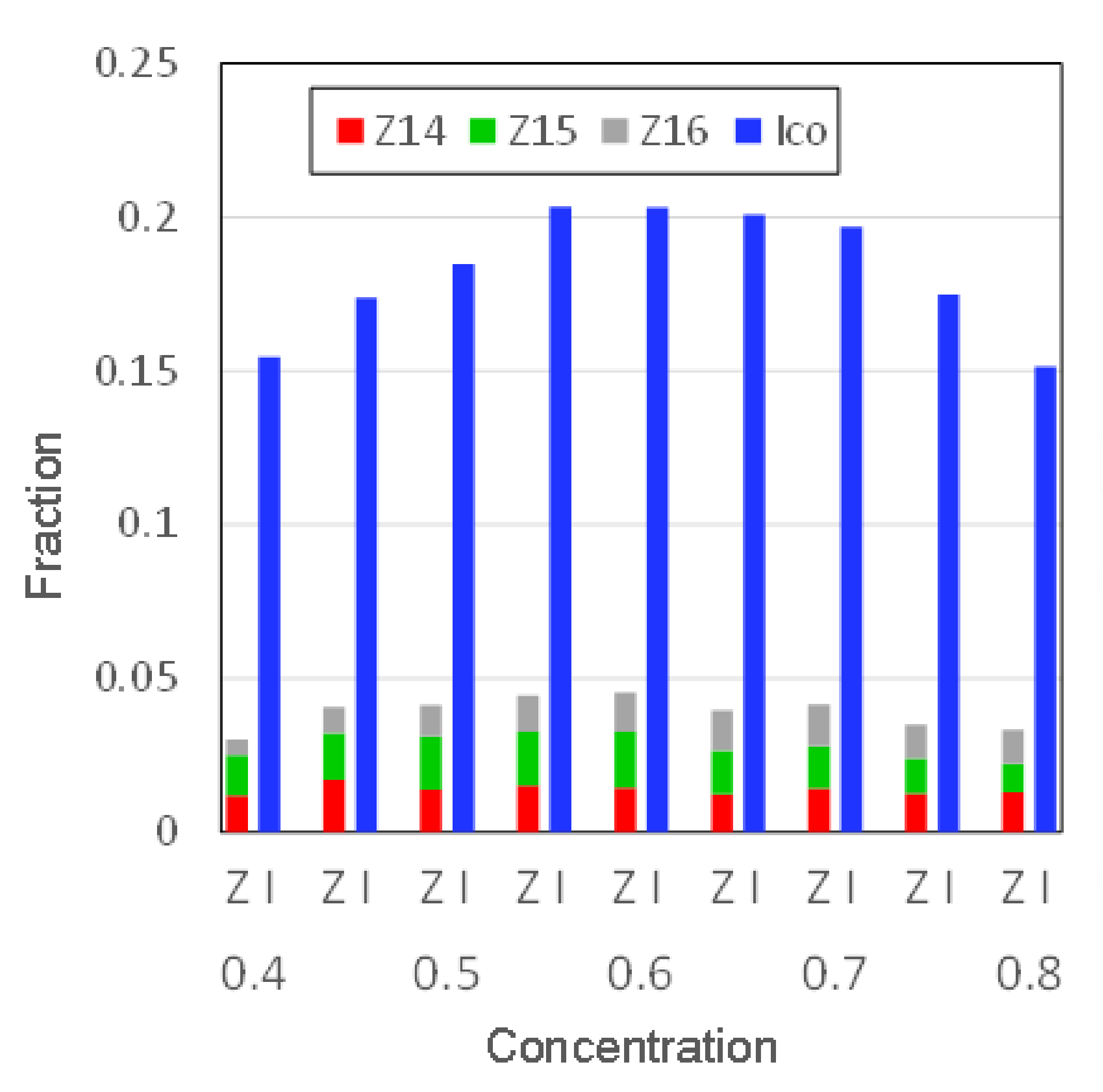
3.2.3. Concentration Dependence of Icosahedral Order
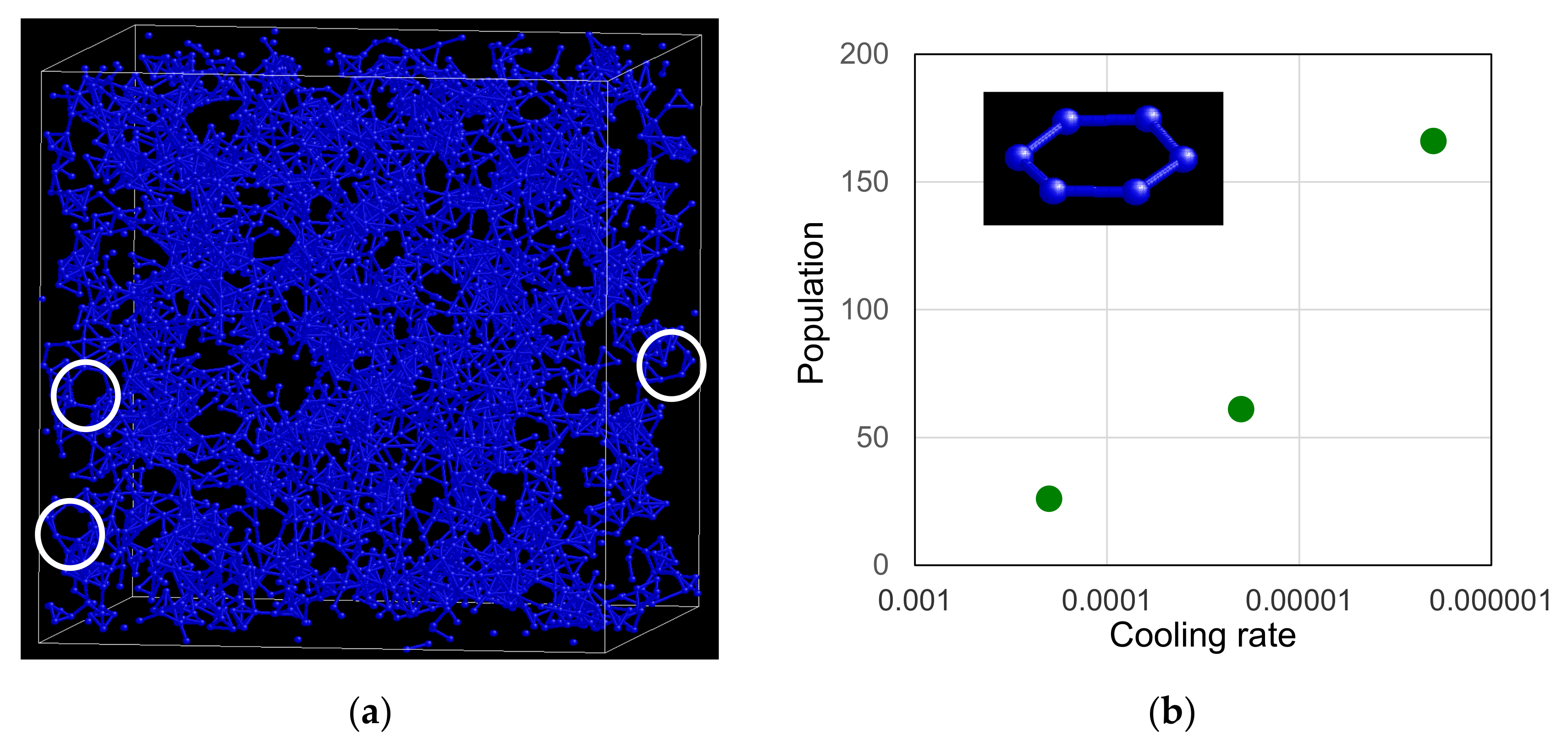
3.3. Topological Feature of Icosahedral Medium-Range Order
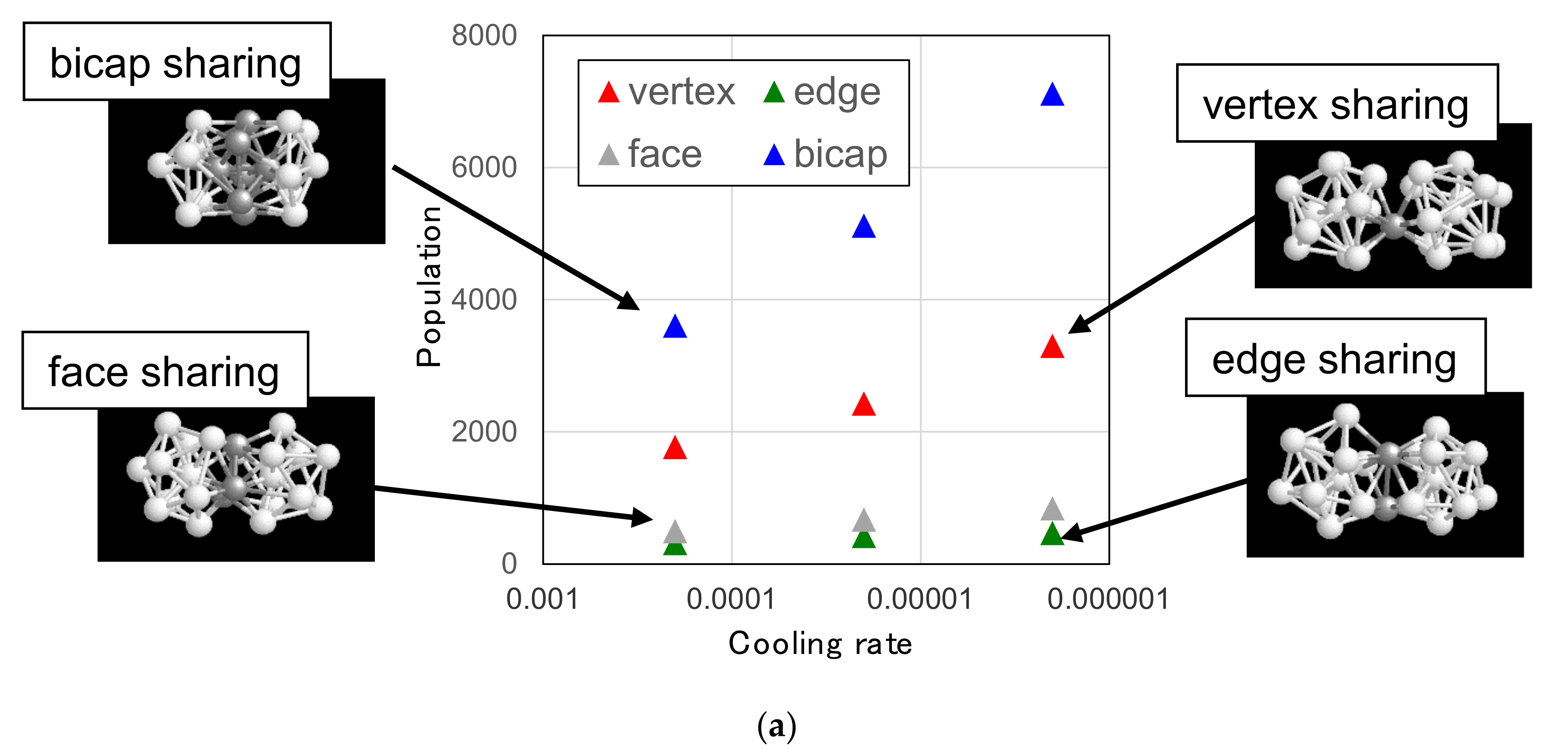
3.3.1. Linking Patterns between F-K Clusters
3.3.2. Typical Structural Unit in the Network
4. Discussion
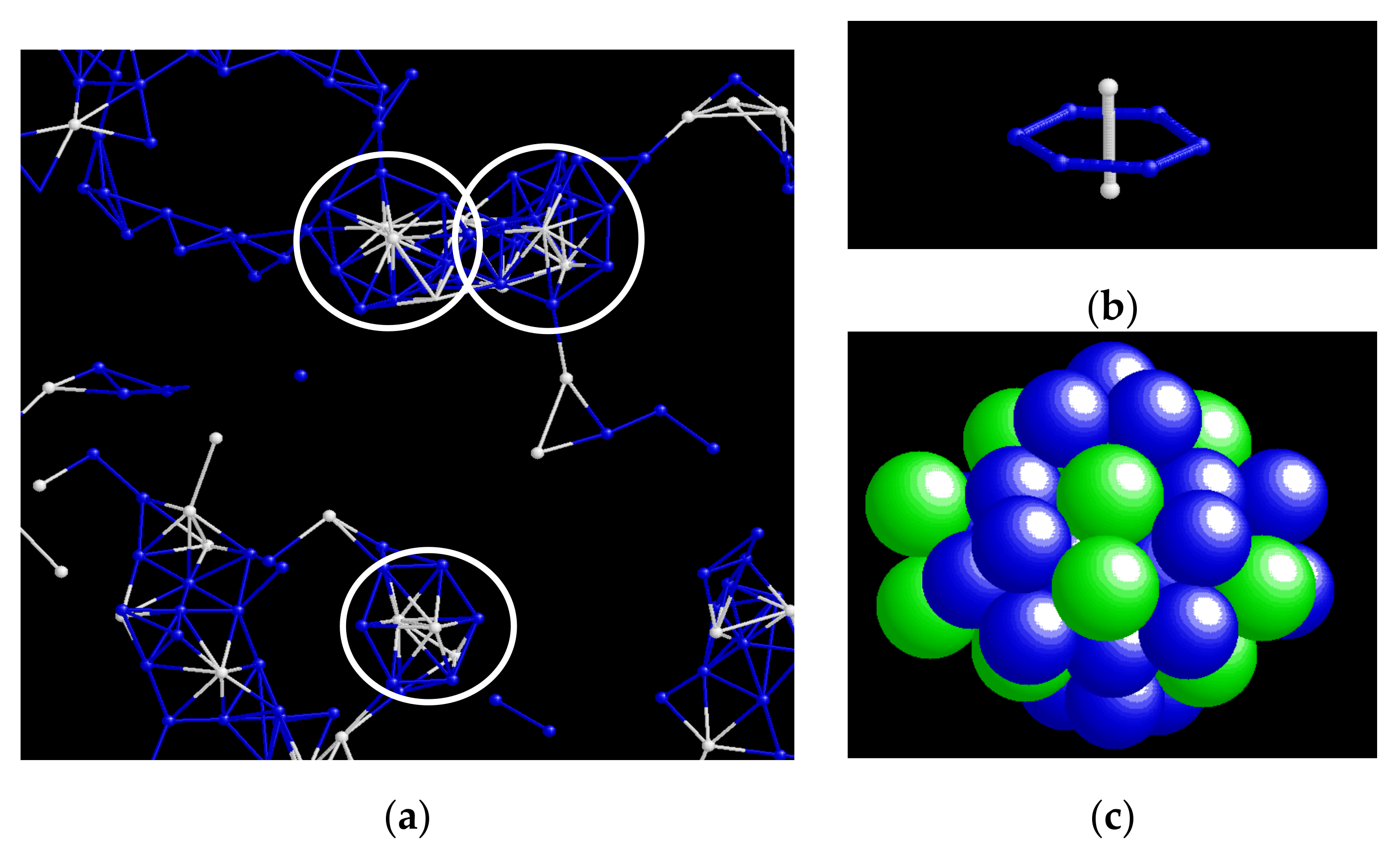
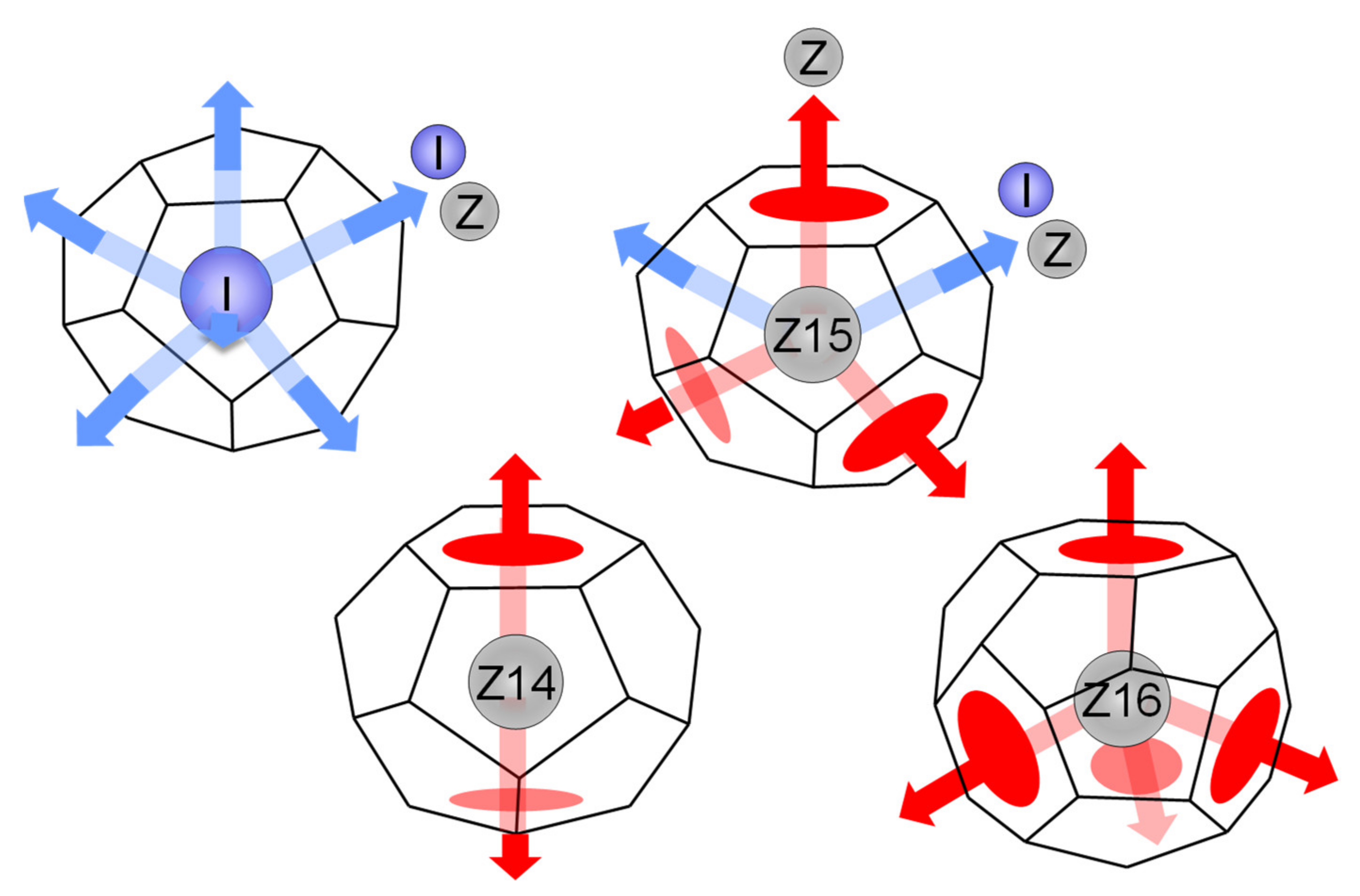
4.1. Geometrical Features of Connection between I- and Z-Clusters
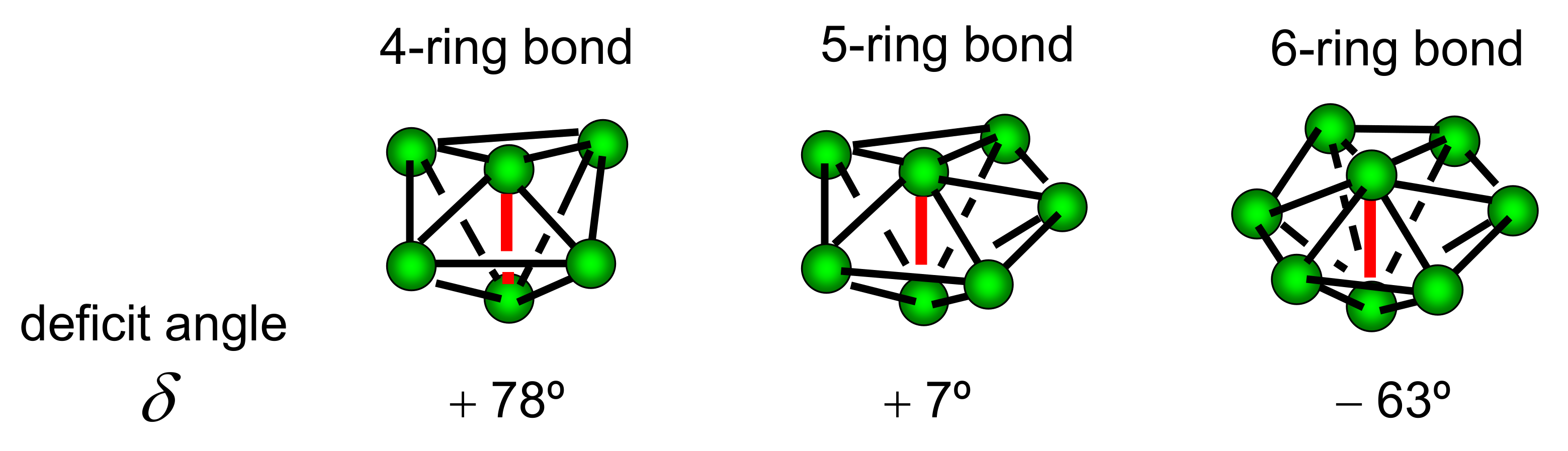
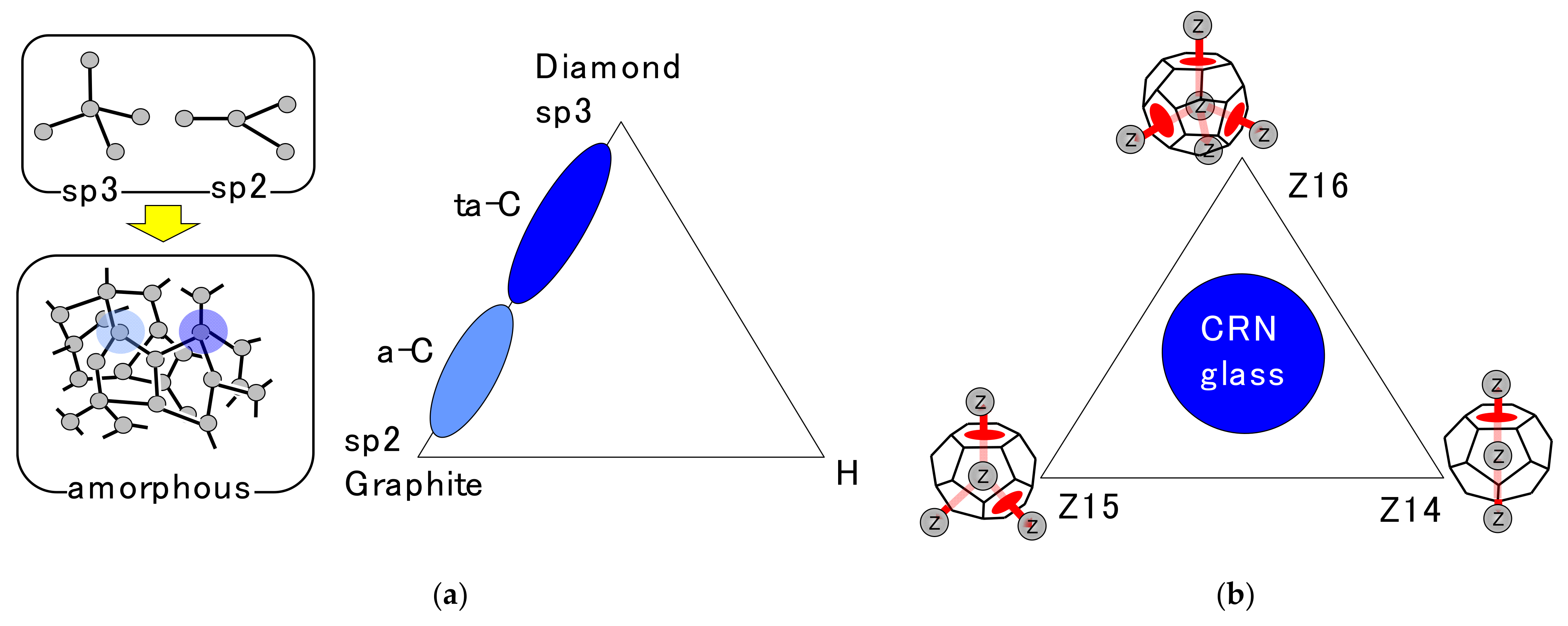
4.2. DRP Model and Regge Calculus
4.3. Disclination Theory
4.4. Disclination Lines in Z-Clusters’ Network
4.5. Role of I-Clusters in Z-Clusters’ Network
4.6. CRN Model for Z-Disclination Network
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernal, J.D. A Geometrical Approach to the Structure of Liquids. Nature 1959, 183, 141–147. [Google Scholar] [CrossRef]
- Cargill, G.S., III. Dense random packing of hard spheres as a structural model for noncrystalline metallic solids. J. Appl. Phys. 1970, 41, 2248–2250. [Google Scholar] [CrossRef]
- Finney, J.L. Modelling the structures of amorphous metals and alloys. Nature 1977, 266, 309–314. [Google Scholar] [CrossRef]
- Yamamoto, R.; Doyama, M. The polyhedron and cavity analyses of a structural model of amorphous iron. J. Phys. F Metal Phys. 1979, 9, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Zhang, T.; Masumoto, T. Zr-Al-Ni amorphous alloys with high glass transition temperature and significant supercooled liquid region. Mater. Trans. JIM 1990, 31, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.; Johnson, W.L. A highly processable metallic glass: Zr41.2Ti13.8Cu12.5Ni10Be22.5. Appl. Phys. Lett. 1993, 63, 2342–2344. [Google Scholar]
- Saida, J.; Sanada, T.; Sato, S.; Imafuku, M.; Matsubara, E.; Inoue, A. Local structure study in Zr-based metallic glasses. Mater. Trans. 2007, 48, 1703–1707. [Google Scholar] [CrossRef] [Green Version]
- Hui, X.; Gao, R.; Chen, G.L.; Shang, S.L.; Wang, Y.; Liu, Z.K. Short-to-medium-range order in Mg65Cu25Y10 metallic glass. Phys. Lett. A 2008, 372, 3078–3084. [Google Scholar] [CrossRef]
- Shen, Y.T.; Kim, T.H.; Gangopadhy, A.K.; Kelton, K.F. Icosahedral order, frustration, and the glass transition: Evidence from time-dependent nucleation and supercooled liquid structure studies. Phys. Rev. Lett. 2009, 102, 057801. [Google Scholar] [CrossRef]
- Hirata, A.; Guan, P.-F.; Fujita, T.; Hirotsu, Y.; Inoue, A.; Yavari, A.R.; Sakurai, T.; Chen, M.-W. Direct observation of local atomic order in a metallic glass. Nat. Mater. 2011, 10, 28–32. [Google Scholar] [CrossRef]
- Hwang, J.; Melgarejo, Z.H.; Kalay, Y.E.; Kalay, I.; Kramer, M.J.; Stone, D.S.; Voyles, P.M. Nanoscale Structure and Structural Relaxation in Zr50Cu45Al5 Bulk Metallic Glass. Phys. Rev. Lett. 2012, 108, 195505. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.C.Y.; Neish, M.J.; Stokol, G.; Buckley, G.A.; Smillie, L.A.; de Jonge, M.D.; Ott, R.T.; Kramer, M.J.; Bourgeois, L.J. Systematic Mapping of Icosahedral Short-Range Order in a Melt-Spun Zr36Cu64 Metallic Glass. Phys. Rev. Lett. 2013, 110, 205505. [Google Scholar] [CrossRef]
- Miracle, D.B. A structural model for metallic glasses. Nat. Mater. 2004, 3, 97–702. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.W.; Luo, W.K.; Alamgir, F.M.; Bai, J.M.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Shimono, M.; Onodera, H. Icosahedral order in supercooled liquids and glassy alloys. Mat. Sci. Forum 2007, 539–543, 2031–2035. [Google Scholar] [CrossRef] [Green Version]
- Wakeda, M.; Shibutani, Y. Icosahedral clustering with medium-range order and local elastic properties of amorphous metals. Acta Mater. 2010, 58, 3963–3969. [Google Scholar] [CrossRef]
- Xie, Z.-C.; Gao, T.-H.; Guo, X.-T.; Qin, X.-M.; Xie, Q. Growth of icosahedral mediumrange order in liquid TiAl alloy during rapid solidification. J. Non-Cryst. Solids 2014, 394–395, 16–21. [Google Scholar] [CrossRef]
- Li, M.Z.; Wang, C.Z.; Hao, S.G.; Kramer, M.J.; Ho, K.M. Structural heterogeneity and medium-range order in ZrxCu100-x metallic glasses. Phys. Rev. B 2009, 80, 184201. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H.-K.; Lee, J.-C. Icosahedral medium-range orders and backbone formation in an amorphous alloy. Met. Mater. Int. 2010, 16, 877–881. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E.; Sheng, H.W. Atomic level structure in multicomponent bulk metallic glass. Phys. Rev. Lett. 2009, 102, 245501. [Google Scholar] [CrossRef] [PubMed]
- Lekka, C.E.; Evangelakis, G.A. Bonding characteristics and strengthening of CuZr fundamental clusters upon small Al additions from density functional theory calculations. Scr. Mater. 2009, 61, 974–977. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Frank, F.C.; Kasper, J.S. Complex alloy structures regarded as sphere packings. I. Definitions and basic principles. Acta Cryst. 1958, 11, 184–190. [Google Scholar] [CrossRef]
- Nelson, D.R. Order, frustration, and defects in liquids and glasses. Phys. Rev. B 1983, 28, 5515–5535. [Google Scholar] [CrossRef]
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates. Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.M.; Barefoot, J.R.; Jarrett, R.N.; Tien, J.K. Modeling of phase equilibrium in the Nickel-Aluminum system. Acta Metall. 1984, 32, 1519–1525. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Cheng, Y.-Q.; Ma, E. Full icosahedra dominate local order in Cu64Zr34 metallic glass and supercooled liquid. Acta Mater. 2014, 69, 343–354. [Google Scholar] [CrossRef]
- Shimono, M.; Onodera, H. Geometrical and chemical factors in the glass-forming ability. Scr. Mater. 2001, 44, 1595–1598. [Google Scholar] [CrossRef]
- Shimono, M.; Onodera, H. Structural Relaxation in Supercooled Liquids. Mater. Trans. 2005, 46, 2830–2837. [Google Scholar] [CrossRef] [Green Version]
- Shimono, M.; Onodera, H. Dynamics and Geometry of Icosahedral Order in Liquid and Glassy Phases of Metallic Glasses. Metals 2015, 5, 1163–1187. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.P.; Qin, J.Y.; Wang, W.M.; Gu, T.K. Origin of splitting of the second peak in the pair-distribution function for metallic glasses. Phys. Rev. B 2011, 84, 092201. [Google Scholar] [CrossRef]
- Xie, Z.-C.; Gao, T.-H.; Guo, X.-T.; Qin, X.-M.; Xie, Q. Glass formation and icosahedral medium-range order in liquid Ti–Al alloys. Comput. Mater. Sci. 2014, 95, 502–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Wang, C.Z.; Mendelev, M.I.; Kramer, M.J.; Ho, K.M. Cooling rates dependence of medium-range order development in Cu64.5Zr35.5 metallic glass. Phys. Rev. B 2015, 91, 064105. [Google Scholar] [CrossRef] [Green Version]
- Regge, T. General relativity without coordinates. Nuovo Cim. 1961, 19, 558–571. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The atomic arrangement in glass. J. Am. Chem. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimono, M.; Onodera, H. Dual Cluster Model for Medium-Range Order in Metallic Glasses. Metals 2021, 11, 1840. https://doi.org/10.3390/met11111840
Shimono M, Onodera H. Dual Cluster Model for Medium-Range Order in Metallic Glasses. Metals. 2021; 11(11):1840. https://doi.org/10.3390/met11111840
Chicago/Turabian StyleShimono, Masato, and Hidehiro Onodera. 2021. "Dual Cluster Model for Medium-Range Order in Metallic Glasses" Metals 11, no. 11: 1840. https://doi.org/10.3390/met11111840
APA StyleShimono, M., & Onodera, H. (2021). Dual Cluster Model for Medium-Range Order in Metallic Glasses. Metals, 11(11), 1840. https://doi.org/10.3390/met11111840





