Improvement of Erosion-Corrosion Behavior of AISI 420 Stainless Steel by Ion-Assisted Deposition ZrN Coatings
Abstract
:1. Introduction
2. Experiments
2.1. Materials
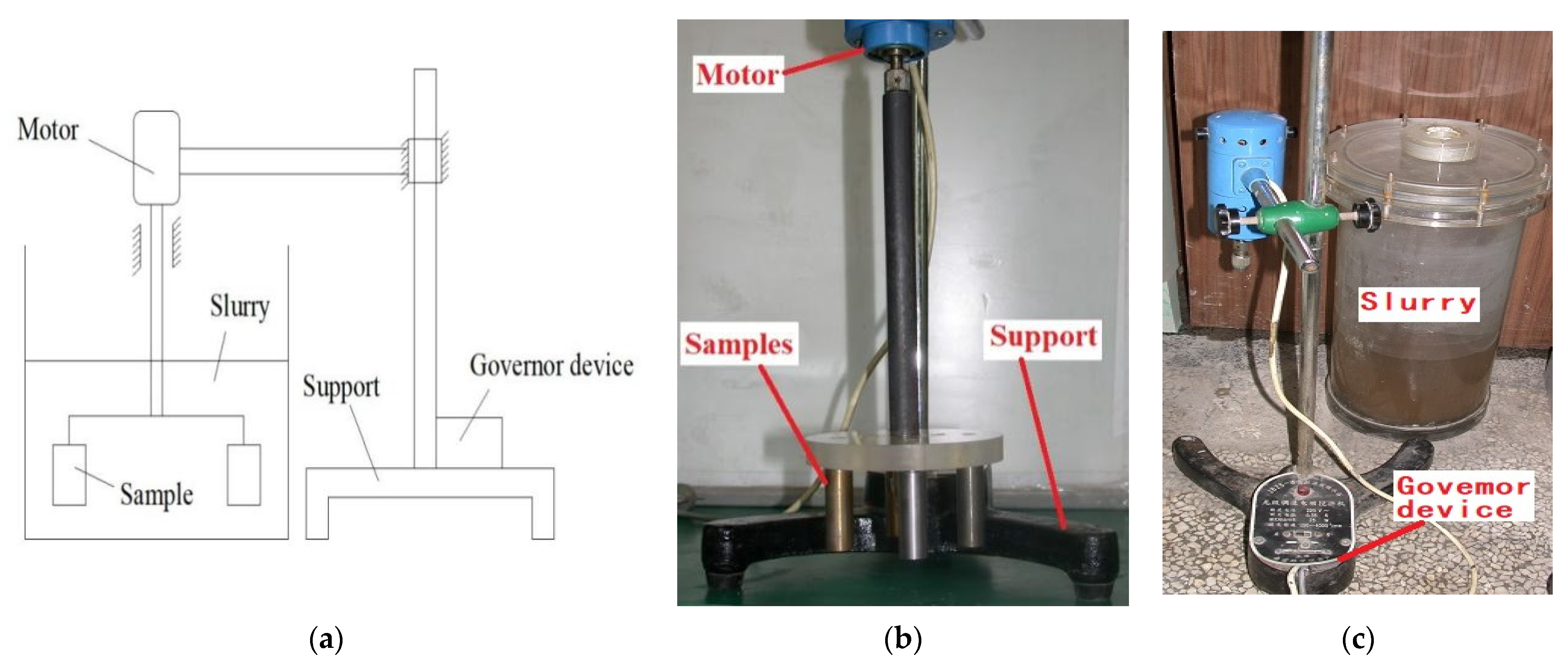
2.2. Experimental Setup
2.3. Experimental Procedures
2.3.1. Coating Deposition
2.3.2. Coating Characterization
2.3.3. Corrosion Tests
2.3.4. Slurry Erosion-Corrosion Tests
3. Results
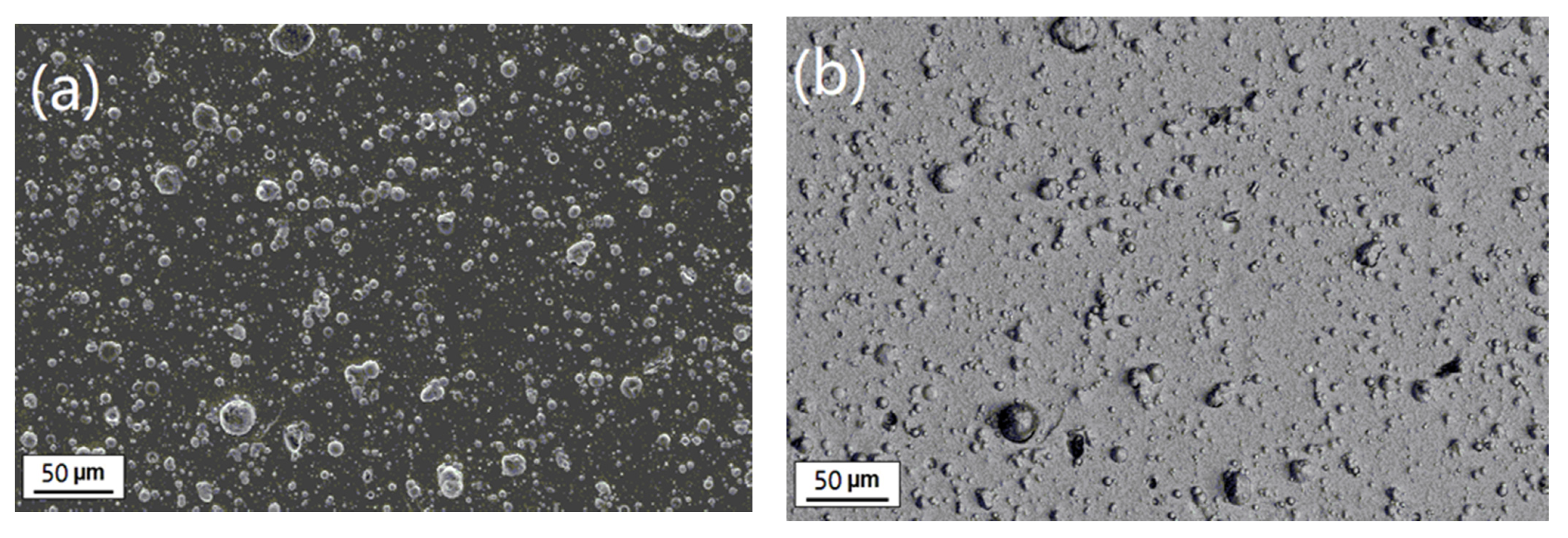
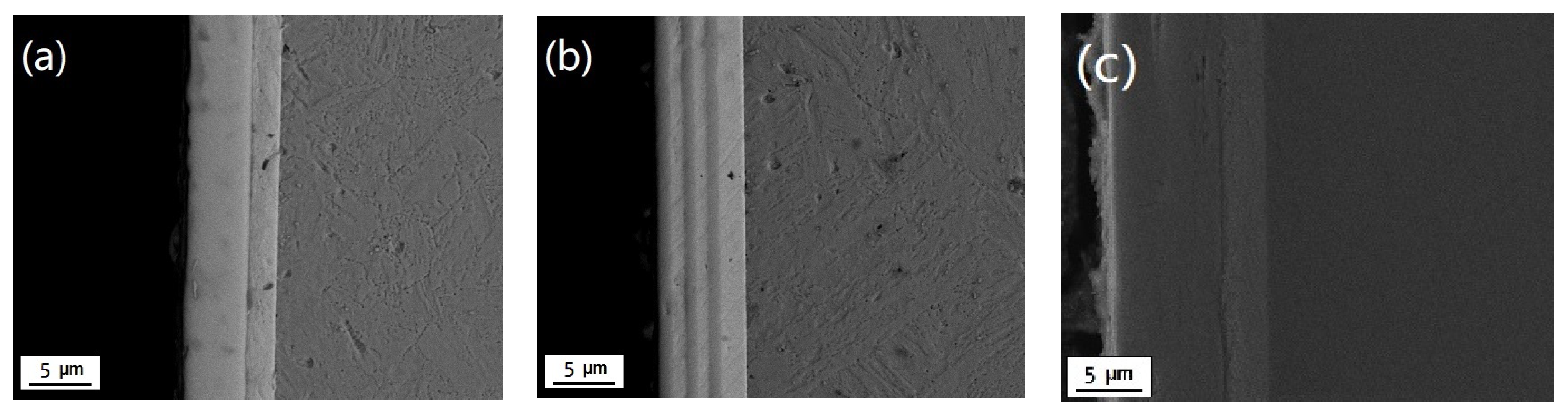
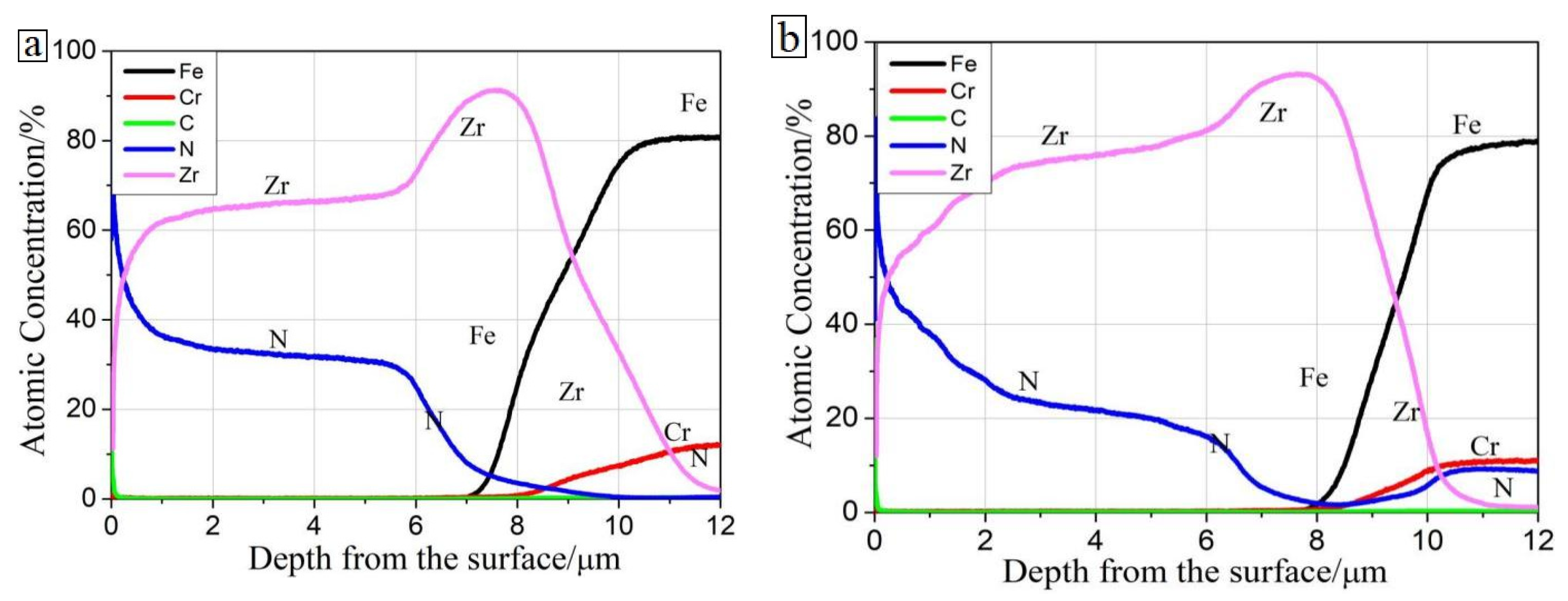
3.1. Surface and Cross-Sectional Morphology
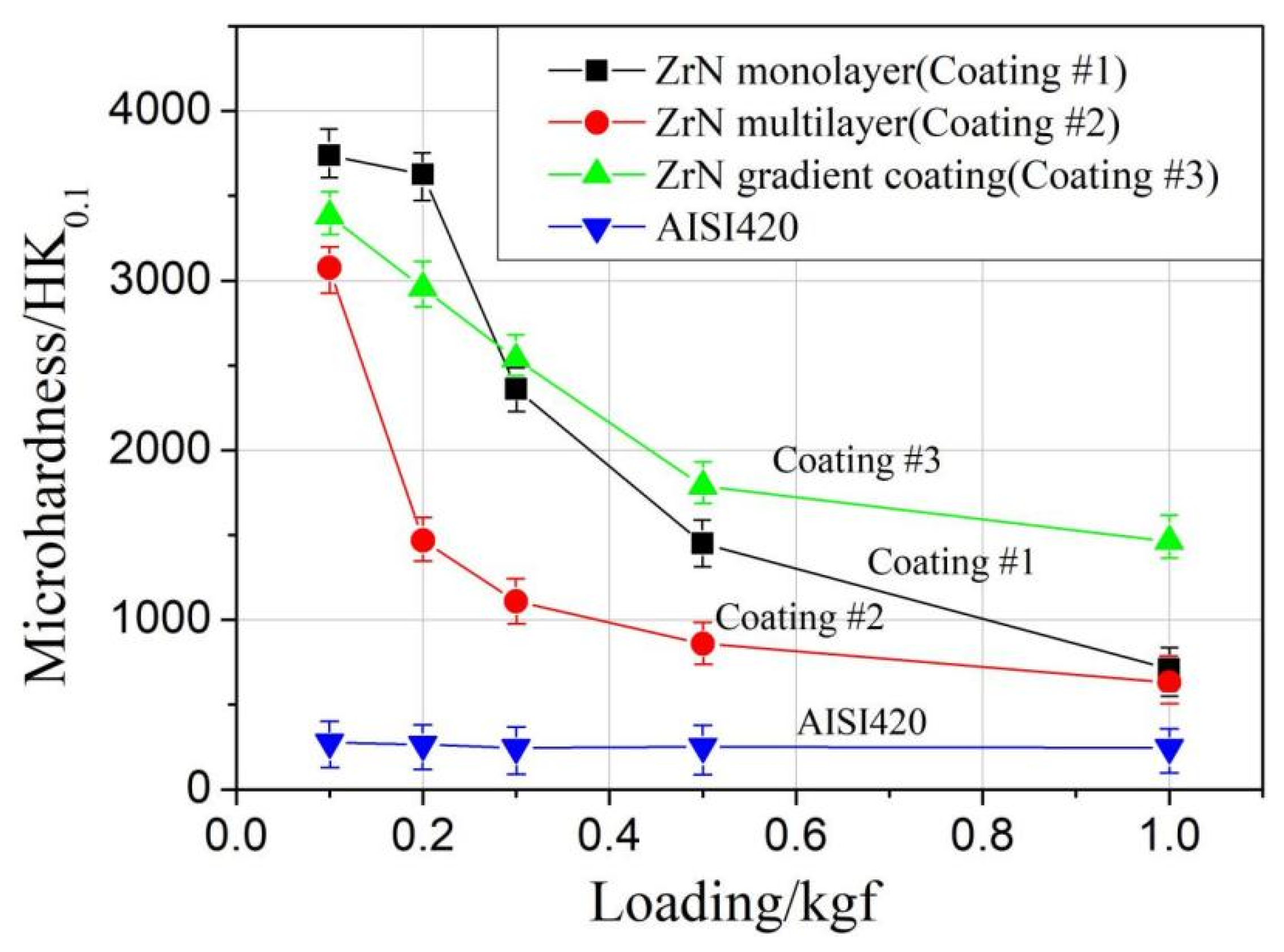
3.2. Microhardness
3.3. Corrosion Resistance
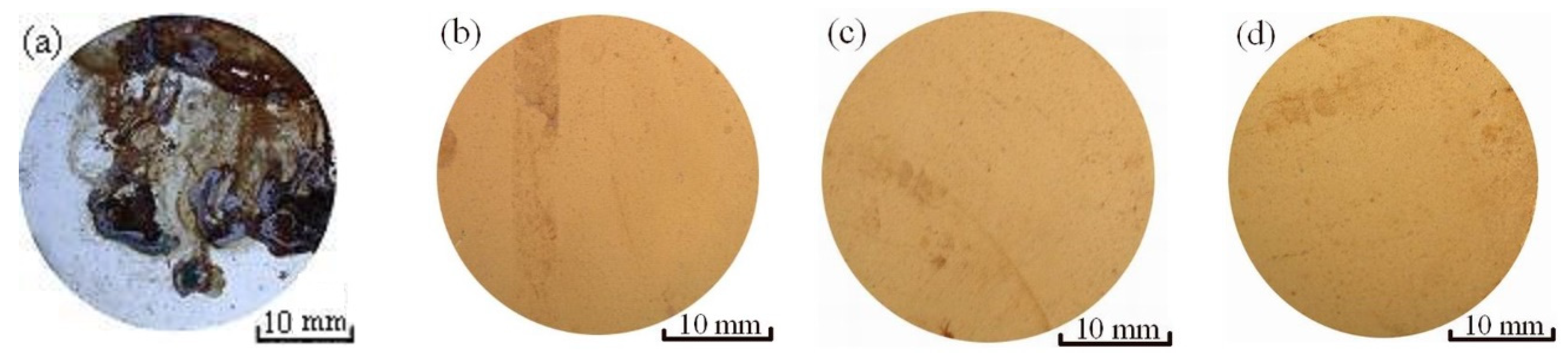
3.3.1. Salt Fog Spray Tests
3.3.2. Electrochemical Behavior Tests
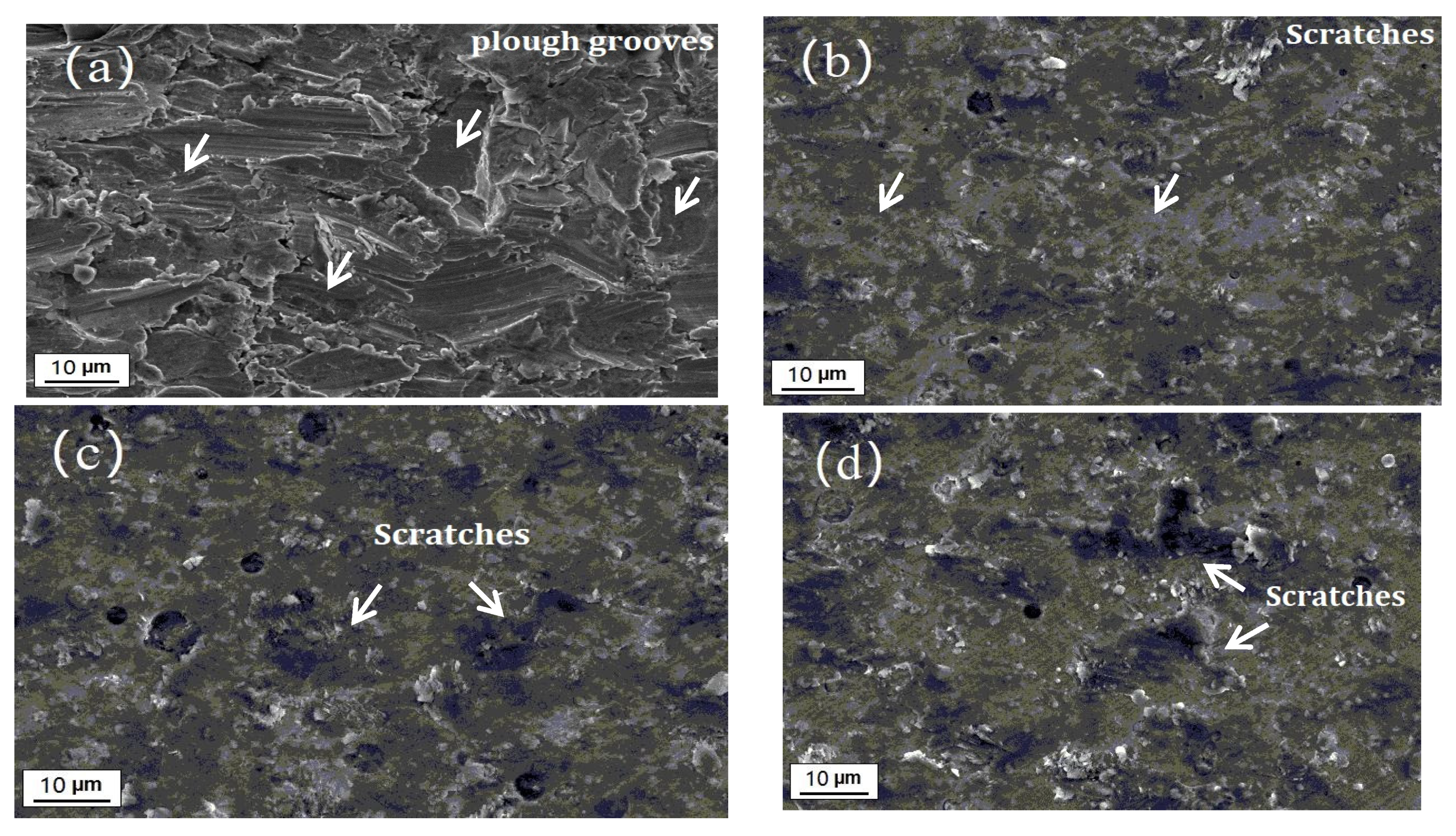
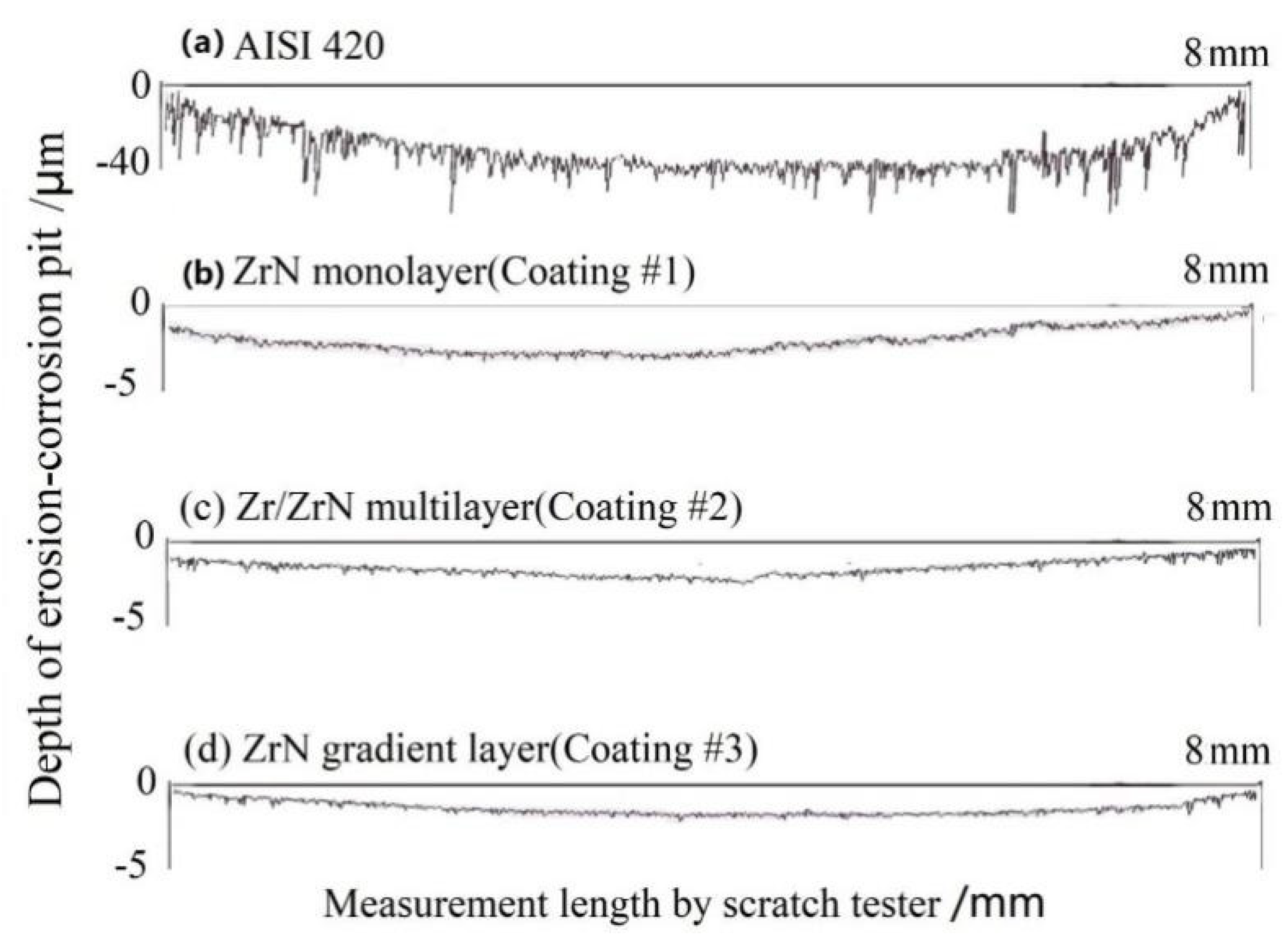
3.3.3. Erosion-Corrosion Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.; Schatz, M.; Casey, M.V. An empirical approach to predict droplet impact erosion in low-pressure stages of steam turbines. Wear 2018, 402–403, 57–63. [Google Scholar] [CrossRef]
- Xi, Y.T.; Liu, D.X.; Han, D. Improvement of corrosion and wear resistances of AISI 420 martensitic stainless steel using plasma nitriding at low temperature. Surf. Coat. Technol. 2008, 202, 2577–2583. [Google Scholar] [CrossRef]
- Ilieva, G.I. Erosion failure mechanisms in turbine stage with twisted rotor blade. Eng. Fail. Anal. 2016, 70, 90–104. [Google Scholar] [CrossRef]
- Aliabadi, M.A.F.; Lakzian, E.; Jahangiri, A.; Khazaei, I. Numerical investigation of effects polydispersed droplets on the erosion rate and condensation loss in the wet steam flow in the turbine blade cascade. Appl. Therm. Eng. 2020, 164, 114478. [Google Scholar] [CrossRef]
- Silva, H.R.; Ferraresi, V.A. Effect of cobalt alloy addition in erosive wear and cavitation of coatings welds. Wear 2019, 426–427, 302–313. [Google Scholar] [CrossRef]
- Lavigne, S.; Pougoum, F.; Savoie, S.; Martinu, L.; Schulz, R. Cavitation erosion behavior of HVOF CaviTec coatings. Wear 2017, 386–387, 90–98. [Google Scholar] [CrossRef]
- Nagentrau, M.; Mohd Tobi, A.L.; Sambu, M.; Jamian, S. The influence of welding condition on the microstructure of WC hardfacing coating on carbon steel substrate. Int. J. Refract. Met. Hard Mater. 2019, 82, 43–57. [Google Scholar] [CrossRef]
- Kuznetsov, M.; Turichin, G.; Silevich, V.; Ochkasov, V.; Sorokin, A. Research of technological possibility of increasing erosion resistance rotor blade using laser cladding. Procedia Manuf. 2019, 36, 163–175. [Google Scholar] [CrossRef]
- Ding, J.C.; Zhang, T.F.; Mane, R.S.; Kim, K.H.; Kang, M.C.; Zou, C.W.; Wang, Q.M. Low-temperature deposition of nanocrystalline Al2O3 films by ion source-assisted magnetron sputtering. Vacuum 2018, 149, 284–290. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, L.; Wang, Q.M.; Li, M.X. A superhard CrAlSiN superlattice coating deposited by multi-arc ion plating: I. Microstructure and mechanical properties. Surf. Coat. Technol. 2013, 214, 160–167. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Shugurov, V.V.; Koval, N.N.; Shulepov, I.A.; Surmenev, R.A. Physico-mechanical properties of Ti-Zr coatings fabricated via ion-assisted arc-plasma deposition. Vacuum 2018, 149, 129–133. [Google Scholar] [CrossRef]
- Shang, H.F.; Li, J.; Shao, T.M. Mechanical properties and thermal stability of TiAlN/Ta multilayer film deposited by ion beam assisted deposition. Appl. Surf. Sci. 2014, 310, 317–320. [Google Scholar] [CrossRef]
- Choi, J.; Hayashi, N.; Kato, T.; Kawaguchi, M. Mechanical properties and thermal stability of SiBCN films prepared by ion beam assisted sputter deposition. Diam. Relat. Mater. 2013, 34, 95–99. [Google Scholar] [CrossRef]
- Liu, D.X.; Xi, Y.T.; Han, D.; Zhang, X. Study on solid particle erosion behaviors of ZrN gradient coatings prepared by ion assisting arc deposition. J. Aeronaut. Mater. 2010, 30, 31–37. [Google Scholar]
- Xi, Y.T.; Liu, D.X.; Han, D. Influence of ZrN monolayer, multilayer, gradient film and duplex treatment on solid particle erosion behavior of stainless steel. Tribology 2008, 28, 293–298. [Google Scholar]
- Samim, P.M.; Fattah-alhosseini, A.; Elmkhah, H.; Imantalab, O.; Nouri, M. A study on comparing surface characterization and electrochemical properties of single-layer CrN coating with nanostructured multilayer ZrN/CrN coating in 3.5 wt.% NaCl solution. Surf. Interfaces 2020, 21, 100721. [Google Scholar] [CrossRef]
- Samim, P.M.; Fattah-alhosseini, A.; Elmkhah, H.; Imantalab, O. Structure and corrosion behavior of ZrN/CrN nano-multilayer coating deposited on AISI 304 stainless steel by CAE-PVD technique. J. Asian Ceram. Soc. 2020, 8, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Samim, P.M.; Fattah-alhosseini, A.; Elmkhah, H.; Imantalab, O. A study on the corrosion resistance of ZrN/CrN multilayer nanostructured coating applied on AISI 304 stainless steel using Arc-PVD method in 3.5 wt% NaCl solution. Mater. Res. Express 2019, 6, 126426. [Google Scholar] [CrossRef]
- Maksakova, O.; Simoes, S.; Pogrebnjak, A.; Bondar, O.; Kravchenko, Y.; Beresnev, V.; Erdybaeva, N. The influence of deposition conditions and bilayer thickness on physical-mechanical properties of CA-PVD multilayer ZrN/CrN coatings. Mater. Charact. 2018, 140, 189–196. [Google Scholar] [CrossRef]
- Aperador, W.; Caicedo, J.C.; España, C.; Cabrera, G.; Amaya, C. Bilayer period effect on corrosion–erosion resistance for [TiN/AlTiN]n multilayered growth on AISI 1045 steel. J. Phys. Chem. Solids 2010, 71, 1754–1759. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Cabrera, G.; Aperador, W.; Caicedo, H.H.; Mejia, A. Determination of the best behavior among AISI D3 steel, 304 stainless steel and CrN/AlN coatings under erosive-corrosive effect. Vacuum 2012, 86, 1886–1894. [Google Scholar] [CrossRef]
- Souza, V.A.D.; Neville, A. Corrosion and synergy in a WCCoCr HVOF thermal spray coating-understanding their role in erosion–corrosion degradation. Wear 2005, 259, 171–180. [Google Scholar] [CrossRef]
- He, Q.; Paiva, J.M.; Kohlscheen, J.; Beake, B.D.; Veldhuis, S.C. An integrative approach to coating/carbide substrate design of CVD and PVD coated cutting tools during the machining of austenitic stainless steel. Ceram. Int. 2020, 46, 5149–5158. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Guo, L.; Miao, W.H.; Guo, H.B.; Gong, S.K. Degradation of EB-PVD thermal barrier coatings caused by CMAS deposits. Prog. Nat. Sci. Mater. Int. 2012, 22, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, H.; Restrepo, E.; Devia, A. Effect of the substrate temperature in ZrN coatings grown by the pulsed arc technique studied by XRD. Surf. Coat. Technol. 2006, 201, 1594–1601. [Google Scholar] [CrossRef]
- Tian, C.X.; Han, B.; Zou, C.W.; Xie, X.; Li, S.Q.; Liang, F.; Tang, X.S.; Wang, Z.S.; Pelenovich, V.O.; Zeng, X.M.; et al. Synthesis of monolayer MoNx and nanomultilayer CrN/Mo2N coatings using arc ion plating. Surf. Coat. Technol. 2019, 370, 125–129. [Google Scholar] [CrossRef]
- Selivanov, K.S.; Smyslov, A.M.; Dyblenko, Y.M.; Semenova, I.P. Erosive wear behavior of Ti/Ti(V,Zr)N multilayered PVD coatings for Ti-6Al-4V alloy. Wear 2019, 418–419, 160–166. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Żukowska, L.W.; Mikuła, J.; Gołombek, K.; Pakuła, D.; Pancielejko, M. Structure and mechanical properties of gradient PVD coatings. J. Mater. Process. Technol. 2008, 201, 310–314. [Google Scholar] [CrossRef]
- Raaif, M. Investigating the structure and tribo-mechanical performance of PVD TiN on bearing TiN substrate constructed by RF plasma. Mater. Chem. Phys. 2019, 22415, 117–123. [Google Scholar] [CrossRef]
- Dalmau, A.; Richard, C.; Muñoz, A.I. Degradation mechanisms in martensitic stainless steels: Wear, corrosion and tribocorrosion appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Fischer, D.A.; Vargas, I.T.; Pizarro, G.E.; Armijo, F.; Walczak, M. The effect of scan rate on the precision of determining corrosion current by Tafel extrapolation: A numerical study on the example of pure Cu in chloride containing medium. Electrochim. Acta 2019, 3131, 457–467. [Google Scholar] [CrossRef]
- Poorqasemi, E.; Abootalebi, O.; Peikari, M.; Haqdar, F. Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corros. Sci. 2009, 51, 1043–1054. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Jamison, L.; Seidman, D.N.; Mohamed, W.; Bei, Y.; Pellin, M.J.; Yacout, A.M. Nanocrystalline ZrN thin film development via atomic layer deposition for U-Mo powder. J. Nucl. Mater. 2019, 5261, 151770. [Google Scholar] [CrossRef]
- Alishahi, M.; Mahboubi, F.; Khoie, S.M.M.; Aparicio, M.; Gago, R. Electrochemical behavior of nanocrystalline Ta/TaN multilayer on 316L stainless steel: Novel bipolar plates for proton exchange membrane fuel-cells. J. Power Sources 2016, 3221, 1–9. [Google Scholar] [CrossRef]
- Luo, Q.; Qin, Z.; Wu, Z.; Shen, B.; Liu, L.; Hu, W. The corrosion behavior of Ni-Cu gradient layer on the nickel aluminum-bronze (NAB) alloy. Corros. Sci. 2018, 1381, 8–19. [Google Scholar] [CrossRef]
- Barik, R.C.; Wharton, J.A.; Wood, R.J.K.; Stokes, K.R.; Jones, R.L. Corrosion, erosion and erosion–corrosion performance of plasma electrolytic oxidation (PEO) deposited Al2O3 coatings. Surf. Coat. Technol. 2005, 199, 158–167. [Google Scholar] [CrossRef]
- Ali, B.; Imar, S.; Laffir, F.; Kailas, L.; Maccato, C.; McCormac, T. Electrochemical, surface and electrocatalytic properties of layer-by-layer multilayer assemblies composed of silver nanoparticles and a Ni(II)-crown type polyoxometalate. J. Electroanal. Chem. 2018, 824, 75–82. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, L.R.; Patnaik, P. Erosion performance, corrosion characteristics and hydrophobicity of nanolayered and multilayered metal nitride coating. Surf. Coat. Technol. 2019, 37515, 763–772. [Google Scholar] [CrossRef]



| C | Cr | Mn | Si | Ni | Cu | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.190 | 12.650 | 0.200 | 0.280 | 0.120 | 0.110 | 0.028 | 0.007 | balance |
| Test Medium | Sample | Volume Loss (mm3) | Erosion-Corrosion Volume Damage Ratio (%) |
|---|---|---|---|
| Acid slurry (pH: 3 ± 0.2) | AISI 420 | 21.5 | - |
| ZrN monolayer(Coating #1) | 1.34 | 6.2 | |
| Zr/ZrN multilayer(Coating #2) | 1.40 | 6.5 | |
| ZrN gradient layer(Coating #3) | 1.02 | 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Y.; Wan, L.; Hou, J.; Wang, Z.; Wang, L.; Yang, D.; Liu, D.; Lei, S. Improvement of Erosion-Corrosion Behavior of AISI 420 Stainless Steel by Ion-Assisted Deposition ZrN Coatings. Metals 2021, 11, 1811. https://doi.org/10.3390/met11111811
Xi Y, Wan L, Hou J, Wang Z, Wang L, Yang D, Liu D, Lei S. Improvement of Erosion-Corrosion Behavior of AISI 420 Stainless Steel by Ion-Assisted Deposition ZrN Coatings. Metals. 2021; 11(11):1811. https://doi.org/10.3390/met11111811
Chicago/Turabian StyleXi, Yuntao, Lin Wan, Jungang Hou, Zhiyong Wang, Lei Wang, Daoyong Yang, Daoxin Liu, and Shubin Lei. 2021. "Improvement of Erosion-Corrosion Behavior of AISI 420 Stainless Steel by Ion-Assisted Deposition ZrN Coatings" Metals 11, no. 11: 1811. https://doi.org/10.3390/met11111811
APA StyleXi, Y., Wan, L., Hou, J., Wang, Z., Wang, L., Yang, D., Liu, D., & Lei, S. (2021). Improvement of Erosion-Corrosion Behavior of AISI 420 Stainless Steel by Ion-Assisted Deposition ZrN Coatings. Metals, 11(11), 1811. https://doi.org/10.3390/met11111811







