Implementation of the Chicot–Lesage Composite Hardness Model in a Determination of Absolute Hardness of Copper Coatings Obtained by the Electrodeposition Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of the Cu Coatings by Electrodeposition
- (a)
- the basic electrolyte: 240 g L−1 CuSO4·5 H2O in 60 g L−1 H2SO4 (electrolyte I), and
- (b)
- the electrolyte with additives: 240 g L−1 CuSO4∙5H2O, 60 g L−1 H2SO4, 0.124 g L−1 NaCl, 1 g L−1 PEG 6000 (polyethylene glycol), 0.0015 g L−1 MPSA (3–Mercapto–1–propanesulfonic acid) (electrolyte II).
2.2. Morphological and Structural Analysis of the Electrolytically Produced Cu Coatings
- (a)
- Scanning electron microscope (SEM): morphology of the electrolytically obtained Cu coatings was examined using JEOL JSM-6610LV (JEOL Ltd., Tokyo, Japan).
- (b)
- Atomic force microscope (AFM): topography of the coatings was monitored in the contact mode using Auto Probe CP Research; TM Microscopes—Veeco Instruments, Santa Barbara, CA, USA. The software SPMLab (SPMLab NT Ver. 6.0.2., Veeco Instruments, Santa Barbara, CA, USA) was used for an estimation of roughness of obtained coatings by the determination of the arithmetic average of the absolute (Ra) roughness parameters, and for generation of histogram of topography.
- (c)
- X-ray diffractometer (XRD): The crystal structure of obtained Cu coatings electrodeposited on Si(111) cathode was analyzed using RIGAKU Ultima IV diffractometer (Rigaku Co. Ltd., Tokyo, Japan) in Bragg–Brentano geometry with CuKα radiation in a 2θ range from 30° to 95°. For an estimation of the preferred orientation of Cu coatings, methodology based on the determination of the “Texture Coefficient“, TC(hkl) and the “Relative Texture Coefficient“, RTC(hkl) was applied. The detailed description of a procedure for the determination of these coefficients is presented in [21,25,26].
2.3. Characterization of the Mechanical Properties of Cu Coatings
3. Results
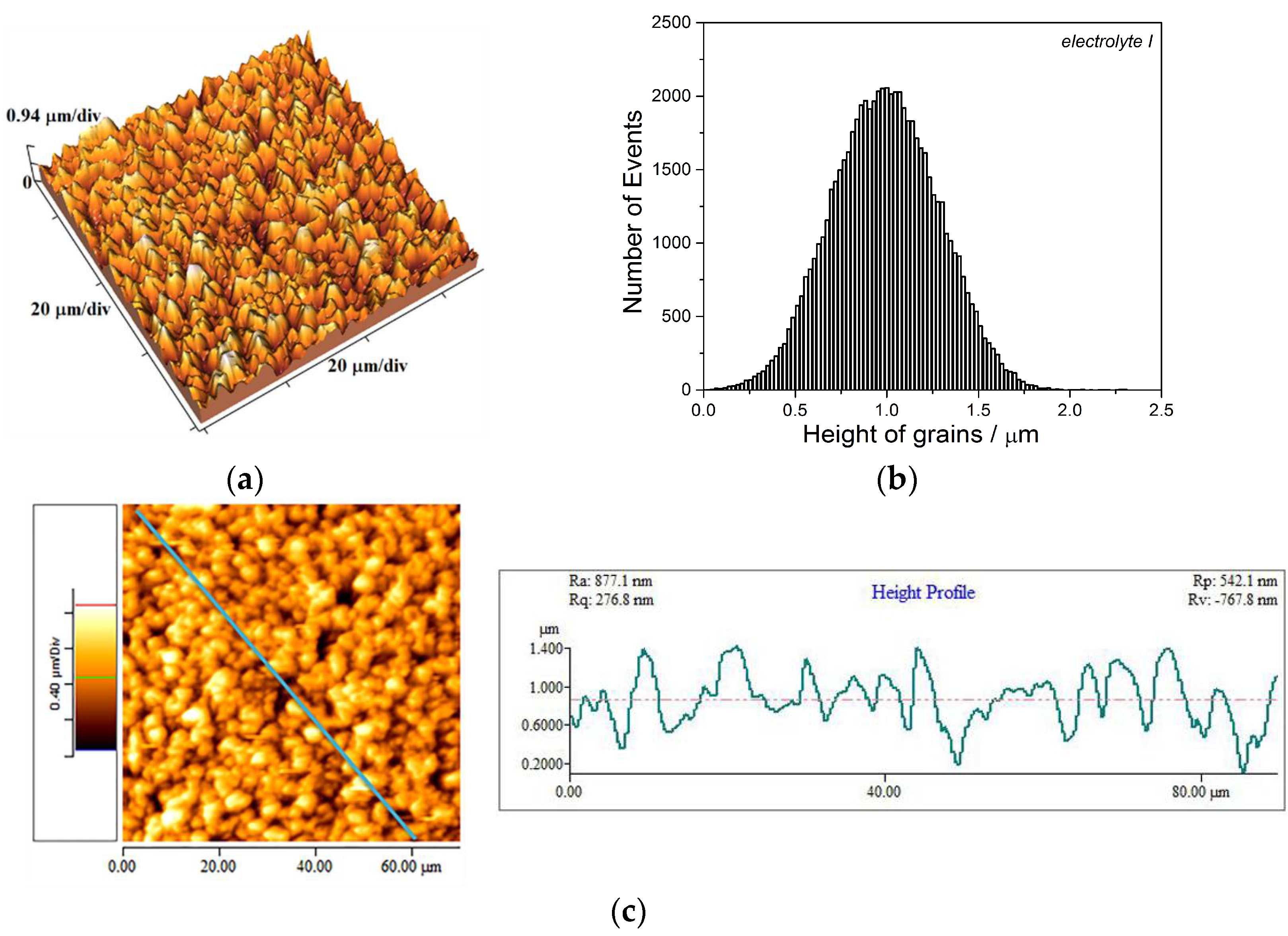
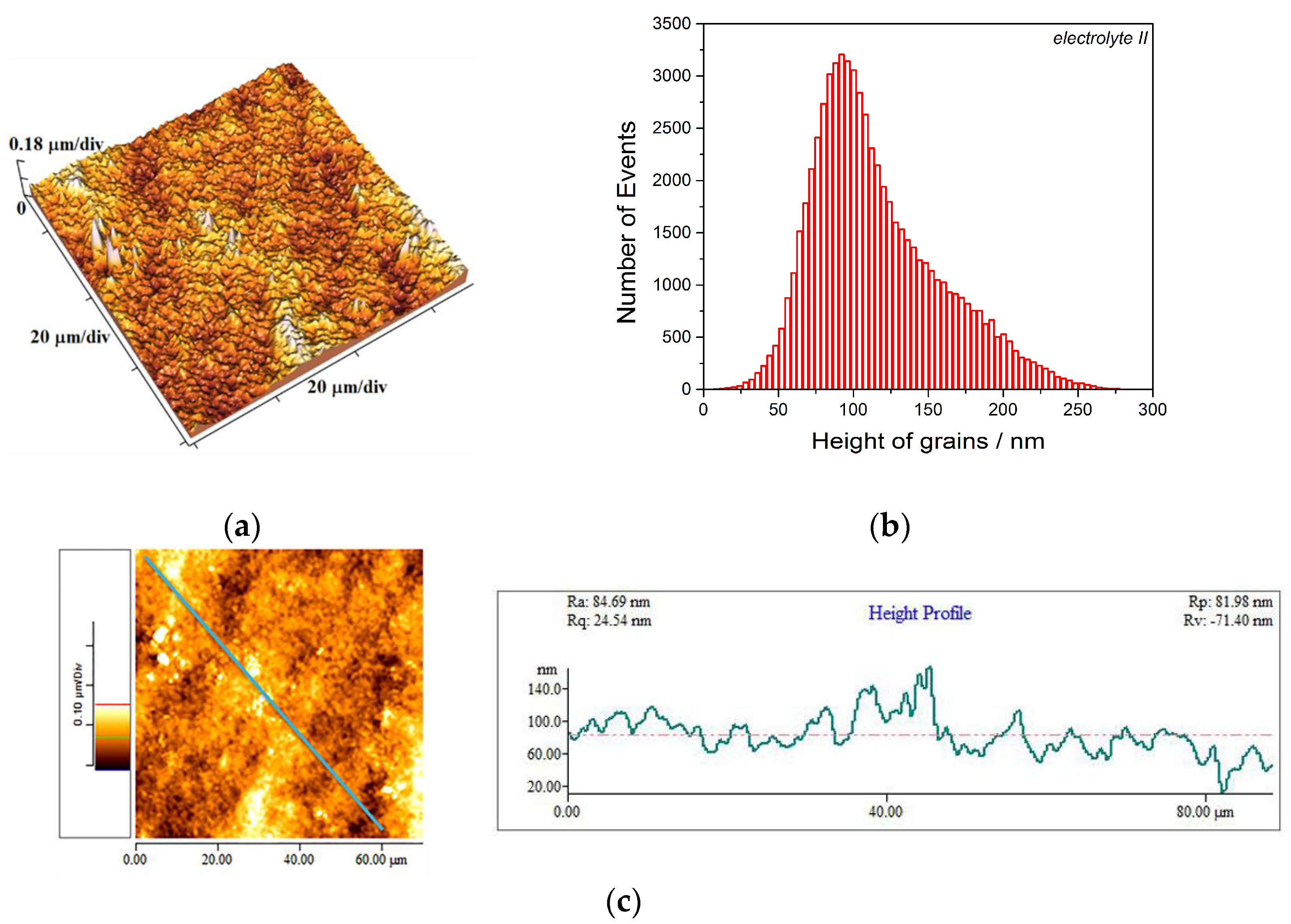
3.1. Analysis of the Copper Coatings Electrodeposited on the Brass Cathode
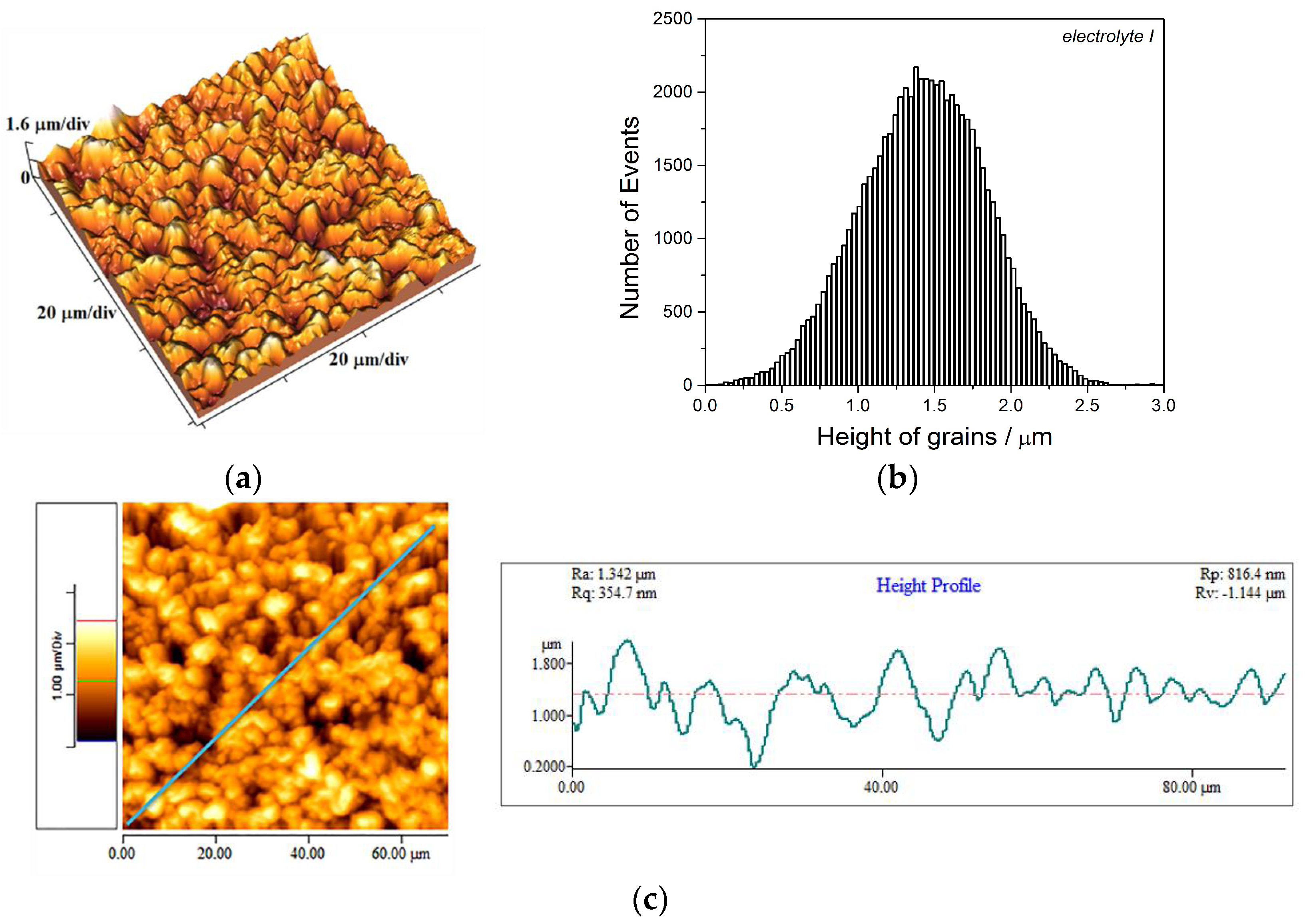
3.2. Analysis of the Copper Coatings Electrodeposited on Si(111) Cathode
3.3. Analysis of the Copper Coatings Electrodeposited on the Si(111) Cathode—Influence of Coating Thickness
4. Discussion
5. Conclusions
- (a)
- The Cu coatings obtained from the basic (sulfate) electrolyte were fine-grained with mat appearance. These coatings showed strong (220) preferred orientation. The smooth mirror bright Cu coatings of strong (200) preferred orientation were obtained from the electrolyte with additives for leveling and brightening. The roughness of the fine-grained coatings was considerably larger than the roughness of smooth coatings.
- (b)
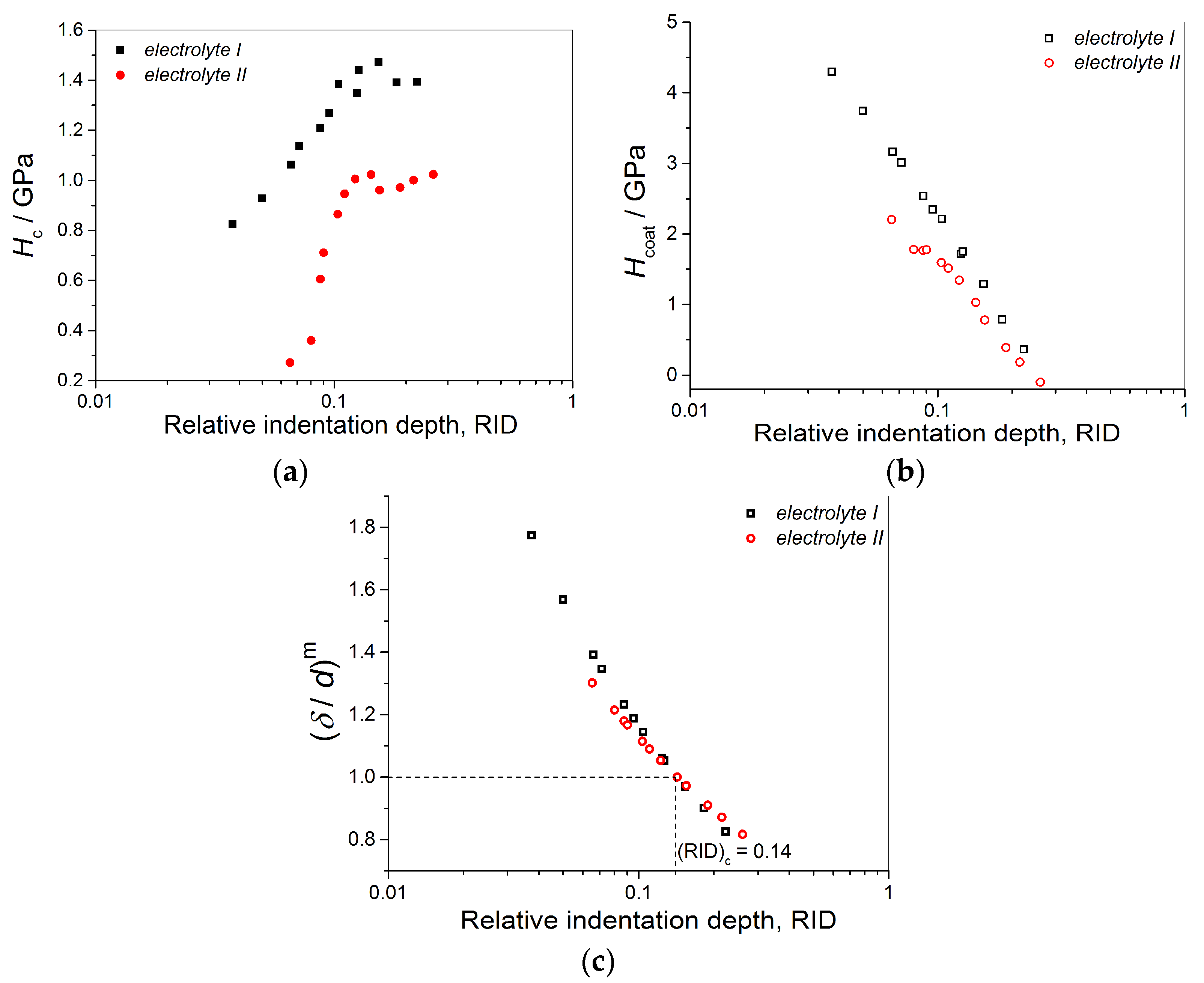
- Hardness of the mat Cu coatings was larger than that obtained for mirror bright Cu coatings. This difference can be attributed to numerous boundaries among grains in the fine-grained mat coatings.
- (c)
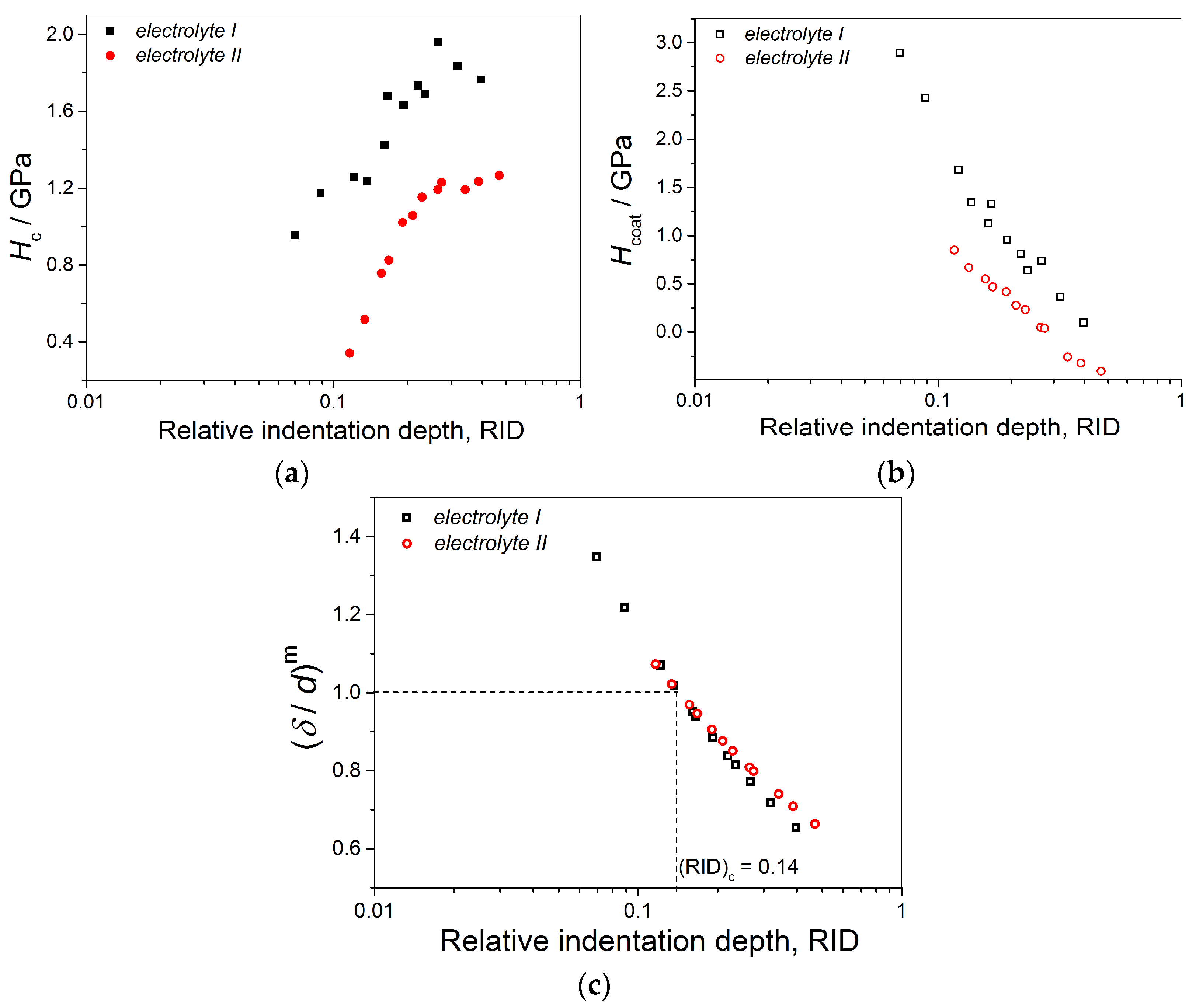
- The shapes of the dependencies of the coating hardness calculated by the C–L model on the RID differ mutually for the Cu coatings obtained on the brass and the Si(111) cathodes. This indicated the strong effect of cathode hardness on coating hardness.
- (d)
- Irrespective of conditions of electrolysis, the critical or limiting relative indentation depth (RID) of 0.14 was established for all types of the coatings. This value separates the zone in which the composite hardness can be equaled with the coating hardness (negligible effect of the cathode hardness on the composite hardness) and the zone of a necessary application of the C–L model for a determination of the absolute hardness of the Cu coatings (the strong effect of the cathode hardness on the composite hardness).
- (e)
- The established RID value of 0.14 obtained by implementation of the C–L model represents novel criterion for an estimation of the absolute hardness of electrolytically obtained Cu coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copper Plating Service. Available online: https://www.sharrettsplating.com/coatings/copper (accessed on 27 September 2021).
- Bharadishettar, N.; Bhat, U.K.; Panemangalore, D.B. Coating technologies for copper based antimicrobial active surfaces: A perspective review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Rohan, J.F.; Thompson, D. Frontiers of Cu Electrodeposition and Electroless plating for On-chip Interconnects. In Copper Electrodeposition for Nanofabrication of Electronics Devices; Kondo, K., Alkolkar, R.N., Barkey, D.P., Yokoi, M., Eds.; Springer: New York, NY, USA, 2014; Volume 171, pp. 99–101. [Google Scholar] [CrossRef]
- Wei, C.; Wu, G.; Yang, S.; Liu, Q. Electrochemical deposition of layered copper thin films based on the diffusion limited aggregation. Sci. Rep. 2016, 6, 34779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, K.I.; Djokić, S.S.; Nikolić, N.D.; Jović, V.D. Morphology of Electrochemically and Chemically Deposited Metals; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Chandrasekar, M.S.; Pushpavanam, M. Pulse and pulse reverse plating—Conceptual, advantages and applications. Electrochim. Acta 2008, 53, 3313–3322. [Google Scholar] [CrossRef]
- Lesage, J.; Pertuz, A.; Chicot, D. A New Method to Determine the Hardness of Thin Films. Matéria 2004, 9, 13–22. [Google Scholar]
- Lesage, J.; Pertuz, A.; Puchi-Cabrera, E.S.; Chicot, D. A model to determine the surface hardness of thin films from standard micro-indentation tests. Thin Solid Films 2006, 497, 232–238. [Google Scholar] [CrossRef]
- Lesage, J.; Chicot, D. A model for hardness determination of thin coatings from standard microindentation test. Surf. Coat. Technol. 2005, 200, 886–889. [Google Scholar] [CrossRef]
- Chicot, D.; Lesage, J. Absolute hardness of films and coatings. Thin Solid Films 1995, 254, 123–130. [Google Scholar] [CrossRef]
- Korsunsky, A.M.; McGurk, M.R.; Bull, S.J.; Page, T.F. On the hardness of coated systems. Surf. Coat. Technol. 1998, 99, 171–183. [Google Scholar] [CrossRef]
- Tuck, J.R.; Korunsky, A.M.; Bull, S.J.; Davidson, R.I. On the application of the work-of-indentation approach to depth-sensing indentation experiments in coated systems. Surf. Coat. Technol. 2001, 137, 217–224. [Google Scholar] [CrossRef]
- Lamovec, J.; Jovic, V.; Randjelovic, D.; Aleksic, R.; Radojevic, V. Analysis of the composite and film hardness of electrodeposited nickel coatings on different substrates. Thin Solid Films 2008, 516, 8646–8654. [Google Scholar] [CrossRef]
- Ma, Z.S.; Zhou, Y.C.; Long, S.G.; Lu, C. On the intrinsic hardness of a metallic film/substrate system: Indentation size and substrate effects. Int. J. Plast. 2012, 34, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Gao, J. The adhesion of copper films coated on silicon and glass substrates. Mod. Phys. Lett. B 2000, 14, 103–108. [Google Scholar] [CrossRef]
- He, J.L.; Li, W.Z.; Li, H.D. Hardness measurement of thin films: Separation from composite hardness. Appl. Phys. Lett. 1996, 69, 1402. [Google Scholar] [CrossRef]
- Hou, Q.; Gao, J.; Li, S. Adhesion and its influence on micro-hardness of DLC and SiC films. Eur. Phys. J. B 1999, 8, 493–496. [Google Scholar] [CrossRef]
- Magagnin, L.; Maboudian, R.; Carraro, C. Adhesion evaluation of immersion plating copper films on silicon by microindentation measurements. Thin Solid Films 2003, 434, 100–105. [Google Scholar] [CrossRef]
- Burnett, P.J.; Rickerby, D.S. The mechanical properties of wear-resistant coatings. II: Experimental studies and interpretation of hardness. Thin Solid Films 1987, 148, 51–65. [Google Scholar] [CrossRef]
- Bull, S.J.; Rickerby, D.S. New developments in the modeling of the hardness and scratch adhesion of thin films. Surf. Coat. Technol. 1990, 42, 149–164. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Lamovec, J.S.; Vasiljević Radović, D.G.; Vasilić, R.; Radojević, V.J.; Nikolić, N.D. Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111). Metals 2020, 10, 488. [Google Scholar] [CrossRef] [Green Version]
- Mladenović, I.O.; Nikolić, N.D.; Lamovec, J.S.; Vasiljević Radović, D.G.; Radojević, V.J. Application of the Composite Hardness Models in the Analysis of Mechanical Characteristics of Electrolytically Deposited Copper Coatings: The Effect of the Type of Substrate. Metals 2021, 11, 111. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Rakočević, Z.; Popov, K.I. Structural Characteristics of Bright Copper Surfaces. J. Electroanal. Chem. 2001, 514, 56–66. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Rakočević, Z.; Popov, K.I. Reflection and structural analyses of mirror bright metal coatings. J. Solid State Electrochem. 2004, 8, 526–531. [Google Scholar] [CrossRef]
- Berube, L.P.; Esperance, G.L. A Quantitative Method of Determining of the Degree of Texture of Zinc Electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Maksimović, V.M.; Avramović, L. Correlation of Morphology and Crystal Structure of Metal Powders Produced by Electrolysis Processes. Metals 2021, 11, 859. [Google Scholar] [CrossRef]
- Li, H.; Bradt, R.C. Knoop microhardness anisotropy of single-crystal LaB6. Mater. Sci. Eng. A 1991, 142, 51–61. [Google Scholar] [CrossRef]
- Buckle, H. The Science of Hardness Testing and Its Research Applications; Westbrook, J.W., Conrad, H., Eds.; American Society for Metals: Metals Park, OH, USA, 1973; p. 453. [Google Scholar]
- Mladenović, I.O.; Lamovec, J.S.; Jović, V.B.; Obradov, M.; Vasiljević Radović, D.G.; Nikolić, N.D.; Radojević, V.J. Mechanical characterization of copper coatings electrodeposited onto different substrates with and without ultrasound assistance. J. Serb. Chem. Soc. 2019, 84, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Mladenović, I.O.; Lamovec, J.S.; Vasiljević-Radović, D.G.; Radojević, V.J.; Nikolić, N.D. Mechanical features of copper coatings electrodeposited by the pulsating current (PC) regime on Si(111) substrate. Int. J. Electrochem. Sci. 2020, 15, 12173–12191. [Google Scholar] [CrossRef]
- Avramović, L.; Maksimović, V.M.; Baščarević, Z.; Ignjatović, N.; Bugarin, M.; Marković, R.; Nikolić, N.D. Influence of the Shape of Copper Powder Particles on the Crystal Structure and Some Decisive Characteristics of the Metal Powders. Metals 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Avramović, L.; Pavlović, M.M.; Maksimović, V.M.; Vuković, M.; Stevanović, J.S.; Bugarin, M.; Nikolić, N.D. Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles. Metals 2017, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Plieth, W. Additives in the electrocrystallization process. Electrochim. Acta 1992, 37, 2115–2121. [Google Scholar] [CrossRef]
- Muresan, L.M.; Varvara, S.C. Leveling and Brightening Mechanisms in Metal Electrodeposition. In Metal Electrodeposition; Nunez, M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2005; pp. 1–45. [Google Scholar]
- Kim, J.J.; Kim, S.-K.; Kim, Y.S. Catalytic behavior of 3-mercapto-1-propane sulfonic acid on Cu electrodeposition and its effect on Cu film properties for CMOS device metallization. J. Electroanal. Chem. 2003, 542, 61–66. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotech. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Varea, A.; Pellicer, E.; Pané, S.; Nelson, B.J.; Suriñach, S.; Baró, M.D.; Sort, J. Mechanical Properties and Corrosion Behaviour of Nanostructured Cu-rich CuNi Electrodeposited Films. Int. J. Electrochem. Sci. 2012, 7, 1288–1302. [Google Scholar]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Mishra, R.; Basu, B.; Balasubramaniam, R. Effect of grain size on the tribological behavior of nanocrystalline nickel. Mater. Sci. Eng. A 2004, 373, 370–373. [Google Scholar] [CrossRef]
- Manika, I.; Maniks, J. Effect of substrate hardness and film structure on indentation depth criteria for film hardness testing. J. Phys. D Appl. Phys. 2008, 41, 074010. [Google Scholar] [CrossRef]
- Manika, I.; Maniks, J. Characteristics of deformation localization and limits to the microhardness testing of amorphous and polycrystalline coatings. Thin Solid Films 1992, 208, 223–227. [Google Scholar] [CrossRef]
- Lebouvier, D.; Gilormini, P.; Felder, E. A kinematic model for plastic indentation of a bilayer. Thin Solid Films 1989, 172, 227–239. [Google Scholar] [CrossRef]
- Bull, S.J. Nanoindentation of coatings. J. Phys. D Appl. Phys. 2005, 38, R393. [Google Scholar] [CrossRef]
- Kramer, D.E.; Volinsky, A.A.; Moody, N.R.; Gerberich, W.W. Substrate effects on indentation plastic zone development in thin soft films. J. Mater. Res. 2001, 16, 3150–3157. [Google Scholar] [CrossRef] [Green Version]
- Beegan, D.; Chowdhury, S.; Laugier, M.T. The nanoindentation behaviour of hard and soft films on silicon substrates. Thin Solid Films 2004, 466, 167–174. [Google Scholar] [CrossRef]
- Wu, T.W.; Moshref, M.; Alexopoulos, P.S. The effect of the interfacial strength on the mechanical properties of aluminum films. Thin Solid Films 1990, 187, 295–307. [Google Scholar] [CrossRef]






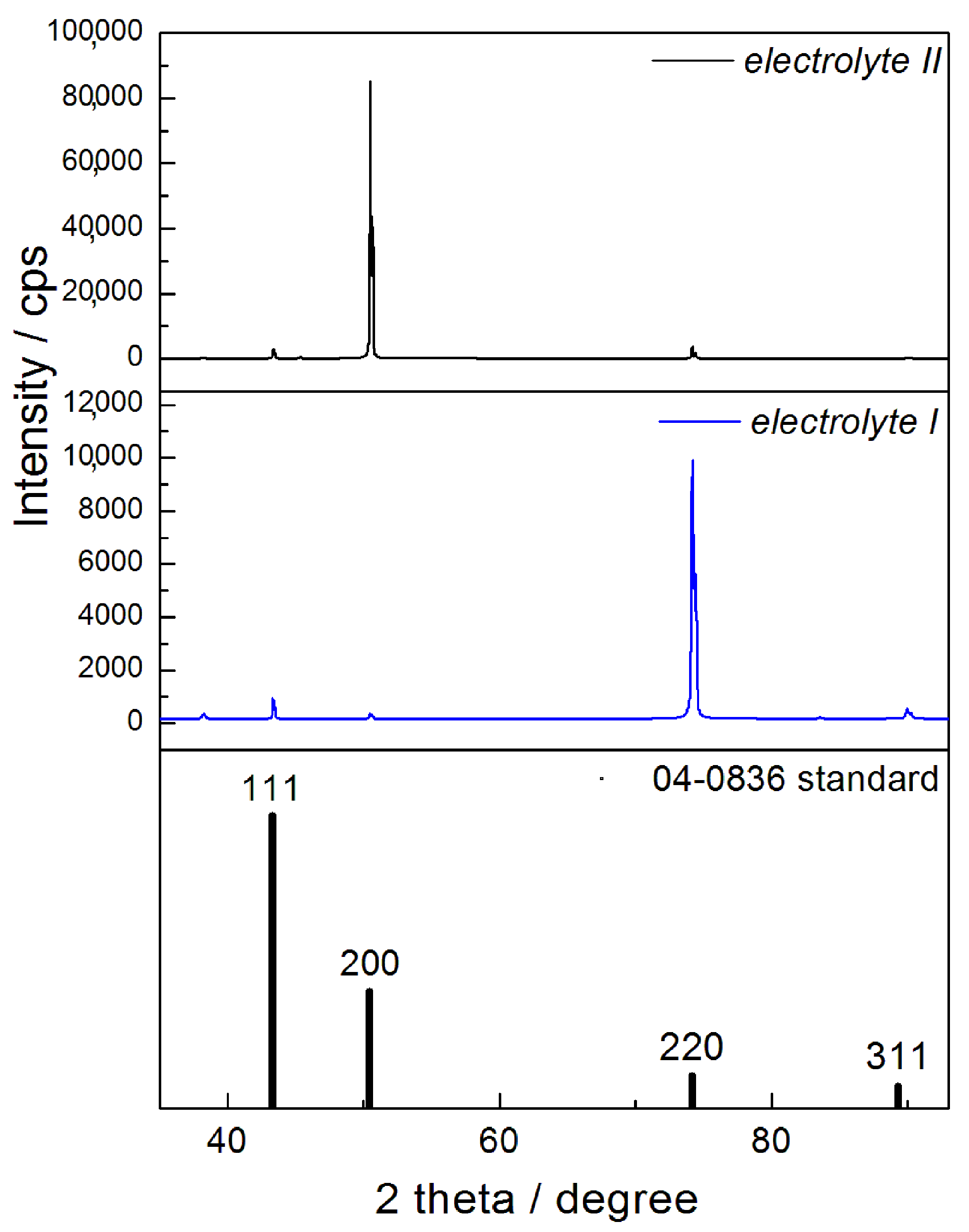
| No. | The Type of Electrolyte | The Type of Cathode | The Coating Thickness (δ/µm) |
|---|---|---|---|
| 1. | electrolyte I | brass | 20 |
| 2. | electrolyte II | brass | 20 |
| 3. | electrolyte I | Si(111) | 20 |
| 4. | electrolyte II | Si(111) | 20 |
| 5. | electrolyte I | Si(111) | 40 |
| 6. | electrolyte II | Si(111) | 40 |
| Plane | R (in %) | Rs | TC | RTC (in %) | |||
|---|---|---|---|---|---|---|---|
| (hkl) | RI | RII | (in %) | TCI | TCII | RTCI | RTCII |
| (111) | 7.9 | 3.31 | 54.6 | 0.145 | 0.061 | 1.71 | 1.47 |
| (200) | 3.2 | 92.12 | 25.1 | 0.127 | 3.67 | 1.49 | 88.31 |
| (220) | 84.0 | 4.05 | 10.9 | 7.71 | 0.37 | 90.67 | 8.90 |
| (311) | 4.9 | 0.52 | 9.4 | 0.521 | 0.055 | 6.13 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, I.O.; Lamovec, J.S.; Vasiljević-Radović, D.G.; Vasilić, R.; Radojević, V.J.; Nikolić, N.D. Implementation of the Chicot–Lesage Composite Hardness Model in a Determination of Absolute Hardness of Copper Coatings Obtained by the Electrodeposition Processes. Metals 2021, 11, 1807. https://doi.org/10.3390/met11111807
Mladenović IO, Lamovec JS, Vasiljević-Radović DG, Vasilić R, Radojević VJ, Nikolić ND. Implementation of the Chicot–Lesage Composite Hardness Model in a Determination of Absolute Hardness of Copper Coatings Obtained by the Electrodeposition Processes. Metals. 2021; 11(11):1807. https://doi.org/10.3390/met11111807
Chicago/Turabian StyleMladenović, Ivana O., Jelena S. Lamovec, Dana G. Vasiljević-Radović, Rastko Vasilić, Vesna J. Radojević, and Nebojša D. Nikolić. 2021. "Implementation of the Chicot–Lesage Composite Hardness Model in a Determination of Absolute Hardness of Copper Coatings Obtained by the Electrodeposition Processes" Metals 11, no. 11: 1807. https://doi.org/10.3390/met11111807
APA StyleMladenović, I. O., Lamovec, J. S., Vasiljević-Radović, D. G., Vasilić, R., Radojević, V. J., & Nikolić, N. D. (2021). Implementation of the Chicot–Lesage Composite Hardness Model in a Determination of Absolute Hardness of Copper Coatings Obtained by the Electrodeposition Processes. Metals, 11(11), 1807. https://doi.org/10.3390/met11111807









