Characterisation of Parameters Influencing the Phase Separation in Copper Solvent Extraction Systems Using Oxime-Type Extractants for the Field Operation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.3. Methods of Analysis
3. Results and Discussion
3.1. Physical Characteristics of the Organic Phase
3.2. Effect of Temperature and Diluent Type
3.3. Effect of Mixing Speed
3.4. Effect of O/A Ratio
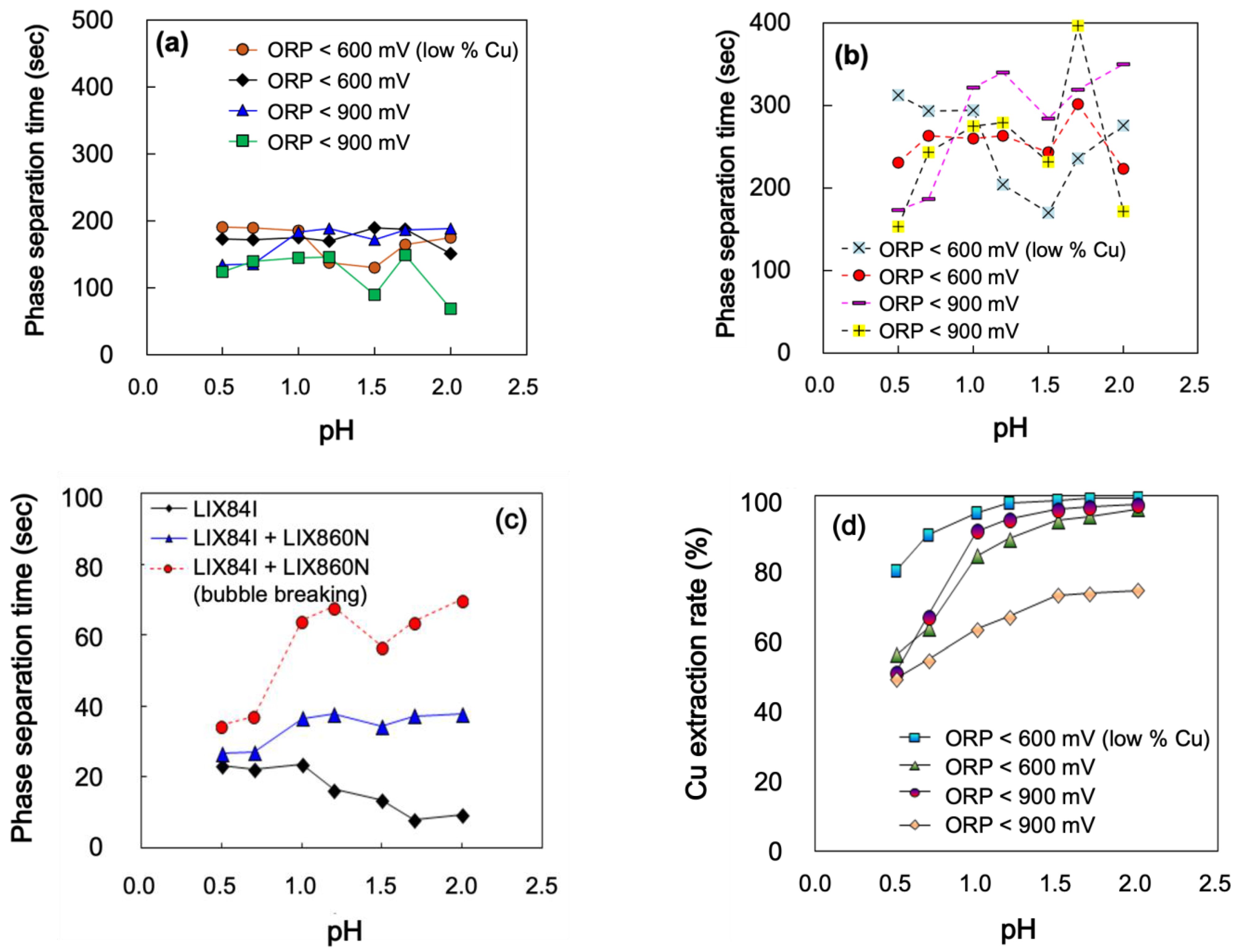
3.5. Effect of pH and ORP
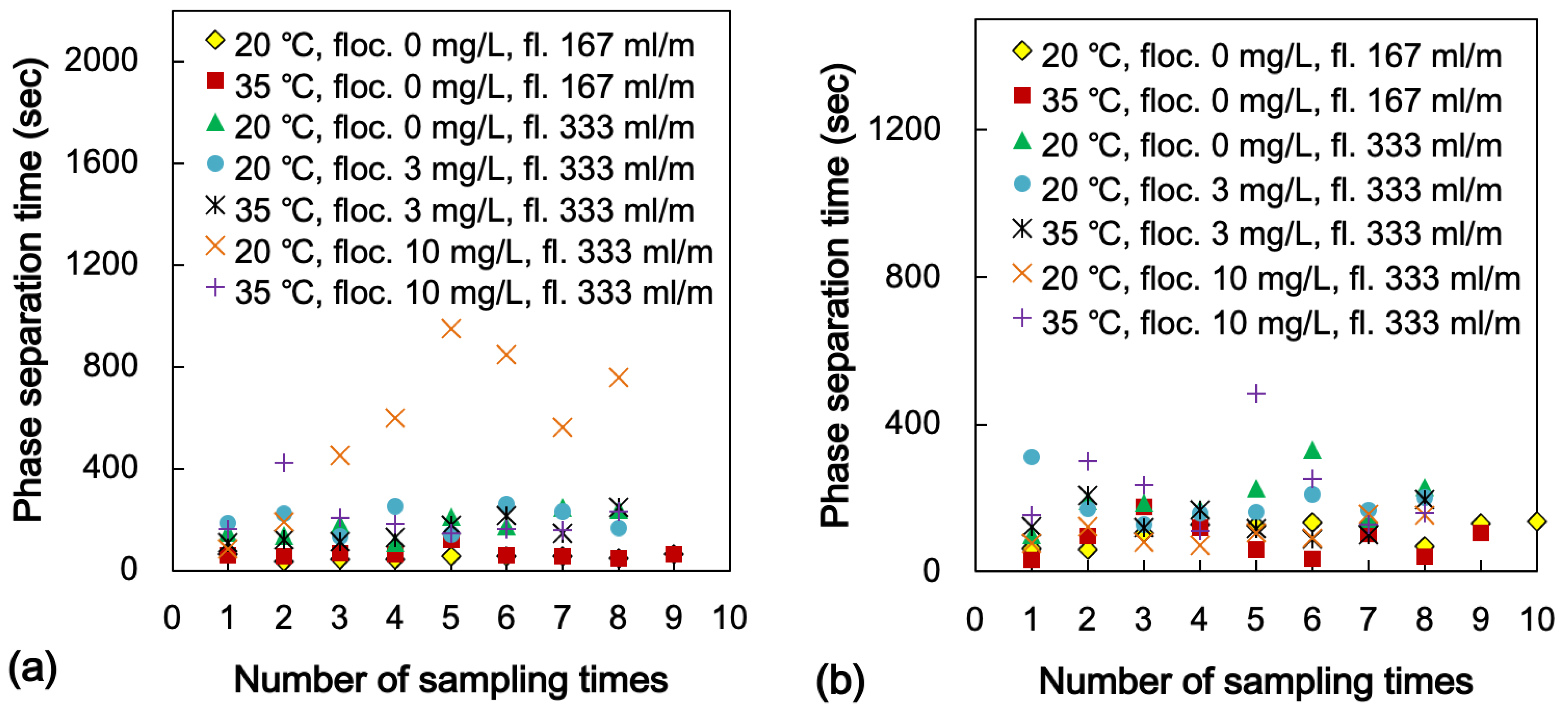
3.6. Effect of Impurities: Flocculant and Colloidal Silica
3.7. Phase Separation Behaviour in the Continuous Counter-Current System
4. Conclusions
- Typical parameters that influenced the phase separation time were identified and optimized at the temperature <35 °C, the mixing speed >2000 rpm, and the O/A ratio > 1.2;
- ORP and pH more complicatedly affect the phase separation time as using the ketoxime rich organic (Org. 1) is shown in a decreasing graph when increasing pH from 0.5 to 2.0, while the aldoxime mixed organic (Org. 2) revealed the reverse result. A high ORP (>900 mV) lowers the phase separation time slightly. However, the Cu extraction was significantly affected as a maximum of 40% lower than the optimized condition;
- The effect of the flocculant and silica level in the PLS resulted in extending the phase separation time gradually when the concentration was increased. Particularly, these were physiochemically affected by the organic and accelerated the third phase and the crud generation;
- The phase separation time using the pilot-scale mix-settlers for the continuous counter-current solvent extraction system was investigated. Increasing the temperature in the system effectively lowered the phase separation time and improved the Cu extraction. The flocculant level in the PLS affected the phase separation time dramatically. However, the generation of the crud caused a significant reduction of Cu extraction.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, T.; Sandoval, S.; Cook, P. World copper solvent extraction plants: Practices and design. JOM 2003, 55, 24–26. [Google Scholar] [CrossRef]
- Kordosky, G.A. Copper solvent extraction: The state of the art. JOM 1992, 44, 40–45. [Google Scholar] [CrossRef]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Renman, R.; Jiankang, W.; Jinghe, C. Bacterial heap-leaching: Practice in Zijinshan copper mine. Hydrometallurgy 2006, 83, 77–82. [Google Scholar] [CrossRef]
- Miller, J.D.; Lin, C.; Garcia, C.; Arias, H. Ultimate recovery in heap leaching operations as established from mineral exposure analysis by X-ray microtomography. Int. J. Miner. Process. 2003, 72, 331–340. [Google Scholar] [CrossRef]
- Rötzer, N.; Schmidt, M. Decreasing Metal Ore Grades—Is the Fear of Resource Depletion Justified? Resources 2018, 7, 88. [Google Scholar] [CrossRef] [Green Version]
- Naldrett, A.J. World-class Ni-Cu-PGE deposits: Key factors in their genesis. Miner. Deposita 1999, 34, 227–240. [Google Scholar] [CrossRef]
- Chen, J.; Guo, X.J.; Li, H. Implementation and practice of an integrated process to recover copper from low grade ore at Zijinshan mine. Hydrometallurgy 2020, 195, 105394. [Google Scholar] [CrossRef]
- Mokmeli, M. Pre-feasibility study in hydrometallurgical treatment of low-grade chalcopyrite ores from Sarcheshmeh copper mine. Hydrometallurgy 2019, 191, 105215. [Google Scholar] [CrossRef]
- Liu, M.; Wen, J.; Tan, G.; Liu, G.; Wu, B. Experimental studies and pilot plant tests for acid leaching of low-grade copper oxide ores at the Tuwu Copper Mine. Hydrometallurgy 2016, 165, 227–232. [Google Scholar]
- Dreisinger, D.; Cheng, C.; Zhang, W.; Pranolo, Y. Development of the Boleo Process Flow Sheet and the Direct Solvent Extraction (DSX) Circuit for Cobalt and Zinc Recovery. In Proceedings of the 25th International Mineral Processing Congress (IMPC 2010), Brisbane, QLD, Australia, 6–10 September 2010; pp. 309–318. [Google Scholar]
- Cheng, C.Y.; Zhu, Z.; Pranolo, Y. The Development of a New DSX Process for the Separation of Nickel and Cobalt from Iron and Aluminium and Other Impurities, in ALTA 2011 Ni/Co/Cu; ALTA Metallurgical Services: Perth, WA, Australia, 2011; pp. 240–252. [Google Scholar]
- Cheng, C.Y.; Barnard, K.R.; Zhang, W.; Robinson, D.J. Synergistic Solvent Extraction of Nickel and Cobalt: A Review of Recent Developments. Solvent Extr. Ion Exch. 2011, 29, 719–754. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, W.; Cheng, C. A synergistic solvent extraction system for separating copper from iron in high chloride concentration solutions. Hydrometallurgy 2012, 113–114, 155–159. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, H.; Wang, Y.; Hu, F.; Zhu, S.; Zhang, S.; Zhang, Y.; Li, S.; Wang, J. Phase separation in solvent extraction of copper or nickel from acidic solution using a sulfonic acid (HDNNS) and a carboxylate ester (4PC). J. Dispers. Sci. Technol. 2019, 40, 819–827. [Google Scholar] [CrossRef]
- Liu, X.R.; Qiu, G.Z.; Hu, Y.H.; Yang, J. Effect of Lix984N content on phase disengagement dynamics in copper-SX. Trans. Nonferrous Met. Soc. China 2003, 13, 963–967. [Google Scholar]
- Kedari, C.S.; Coll, T.; Fortuny, A.; Sastre, A.M. Third Phase Formation in the Solvent Extraction System Ir(IV)—Cyanex 923. Solvent Extr. Ion Exch. 2005, 23, 545–559. [Google Scholar] [CrossRef]
- Lu, Z.; Dourdain, S.; Pellet-Rostaing, S. Understanding the Effect of the Phase Modifier n-Octanol on Extraction, Aggregation, and Third-Phase Appearance in Solvent Extraction. Langmuir 2020, 36, 12121–12129. [Google Scholar] [CrossRef] [PubMed]
- Swami, K.R.; Venkatesan, K.A.; Antony, M.P. Role of Phase Modifiers in Controlling the Third-phase Formation during the Solvent Extraction of Trivalent Actinides. Solvent Extr. Ion Exch. 2019, 37, 500–517. [Google Scholar] [CrossRef]
- Musadaidzwa, J.M.; Tshiningayamwe, E.I. Skorpion zinc solvent extraction: The upset conditions. J. South Afr. Inst. Min. Metall. 2009, 109, 691–695. [Google Scholar]
- Priest, C.; Hashmi, S.F.; Zhou, J.; Sedev, R.; Ralston, J. Microfluidic Solvent Extraction of Metal Ions from Industrial Grade Leach Solutions: Extraction Performance and Channel Aging. J. Flow Chem. 2013, 3, 76–80. [Google Scholar] [CrossRef]
- Kriel, F.H.; Holzner, G.; Grant, R.A.; Woollam, S.; Ralston, J.; Priest, C. Microfluidic solvent extraction, stripping, and phase disengagement for high-value platinum chloride solutions. Chem. Eng. Sci. 2015, 138, 827–833. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, J.; Nam, J.O.; Kang, S.M.; Jeong, S.G.; Lee, C. Microfluidic Design of Complex Emulsions. ChemPhysChem 2014, 15, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Aota, A.; Nonaka, M.; Hibara, A.; Kitamori, T. Countercurrent Laminar Microflow for Highly Efficient Solvent Extraction. Angew. Chem. Int. Ed. 2007, 46, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Brugarolas, T.; Tu, F.; Lee, D. Directed assembly of particles using microfluidic droplets and bubbles. Soft Matter 2013, 9, 9046–9058. [Google Scholar] [CrossRef]
- Bidari, E.; Irannejad, M.; Gharabaghi, M. Solvent extraction recovery and separation of cadmium and copper from sulphate solution. J. Environ. Chem. Eng. 2013, 1, 1269–1274. [Google Scholar] [CrossRef]
- Aguilar, M.; Cortina, J.L. Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kordosky, G.A. Copper recovery using leach/solvent extraction/electrowinning technology: Forty years of innovation, 2.2 million tonnes of copper annually. J. South Afr. Inst. Min. Metall. 2002, 102, 445–450. [Google Scholar]
- Sole, K.C.; Feather, A.M. Solvent Extraction of Copper from High-Tenor Pressure Leach Solutions Using New Modified Aldoximes. In Proceedings of the Copper-Cobre 2003: Hydrometallurgy of Copper Book 2, Santiago, Chile, 30 November–3 December 2003; pp. 691–706. [Google Scholar]
- Delegard, C.H.; Casella, A.J. Literature Review: Crud Formation at the Liquid/Liquid Interface of TBP-Based Solvent-Extraction Processes; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2016. [Google Scholar]
- Testard, F.; Bauduin, P.; Zemb, T.; Berthon, L. Phase Formation in Liquid/Liquid Extraction: A Colloidal Approach. In Ion Exchange and Solvent Extraction; Moyer, B.A., Ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 48. [Google Scholar]










| Element | Unit | Cu | Co | Zn | Mn | Fe | Si |
|---|---|---|---|---|---|---|---|
| PLS | mg/L | 4150 | 81 | 429 | 2940 | 2425 | 122 |
| Organic phase | Org. 1: LIX84I (10%) in ISD-159 (90%) Org. 2: LIX84I (5.6%) and LIX860N (4.4%) in ISD-159 (90%) Org. 3: LIX84I (5.6%) and LIX860N (4.4%) in Orfom SX-12 CT (90%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.; Lee, G.S.; Kim, H.R.; Kim, J.-G. Characterisation of Parameters Influencing the Phase Separation in Copper Solvent Extraction Systems Using Oxime-Type Extractants for the Field Operation. Metals 2021, 11, 1785. https://doi.org/10.3390/met11111785
Seo S, Lee GS, Kim HR, Kim J-G. Characterisation of Parameters Influencing the Phase Separation in Copper Solvent Extraction Systems Using Oxime-Type Extractants for the Field Operation. Metals. 2021; 11(11):1785. https://doi.org/10.3390/met11111785
Chicago/Turabian StyleSeo, Sangyun, Gwang Seop Lee, Hye Rim Kim, and Jong-Gwan Kim. 2021. "Characterisation of Parameters Influencing the Phase Separation in Copper Solvent Extraction Systems Using Oxime-Type Extractants for the Field Operation" Metals 11, no. 11: 1785. https://doi.org/10.3390/met11111785
APA StyleSeo, S., Lee, G. S., Kim, H. R., & Kim, J.-G. (2021). Characterisation of Parameters Influencing the Phase Separation in Copper Solvent Extraction Systems Using Oxime-Type Extractants for the Field Operation. Metals, 11(11), 1785. https://doi.org/10.3390/met11111785






