Optimization of the Microwave-Assisted Carbothermical Reduction Process for Metals from Electric Arc Furnace Dust with Biochar
Abstract
:1. Introduction
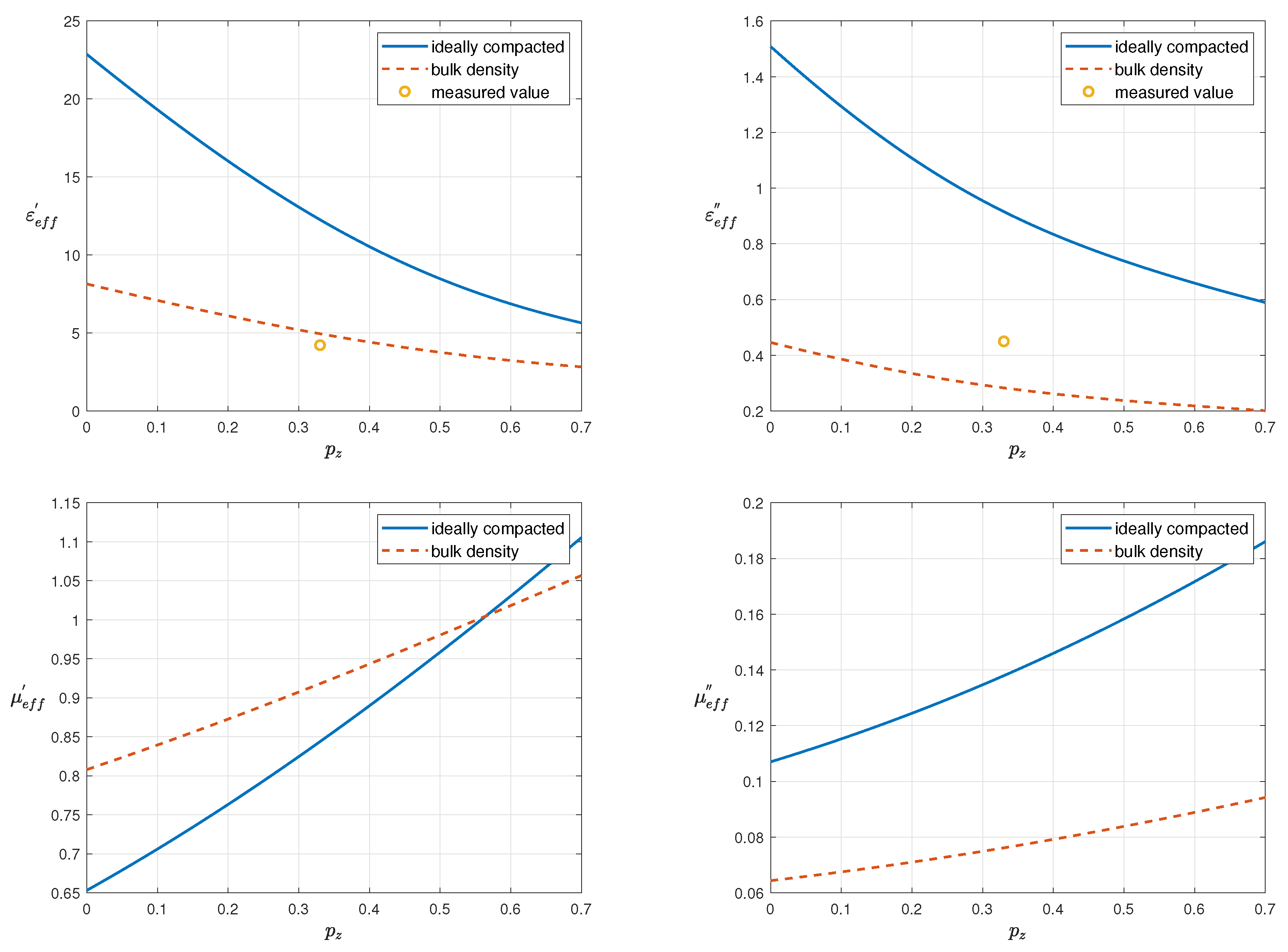
2. Effective Permittivity and Permeability of EAF Dust
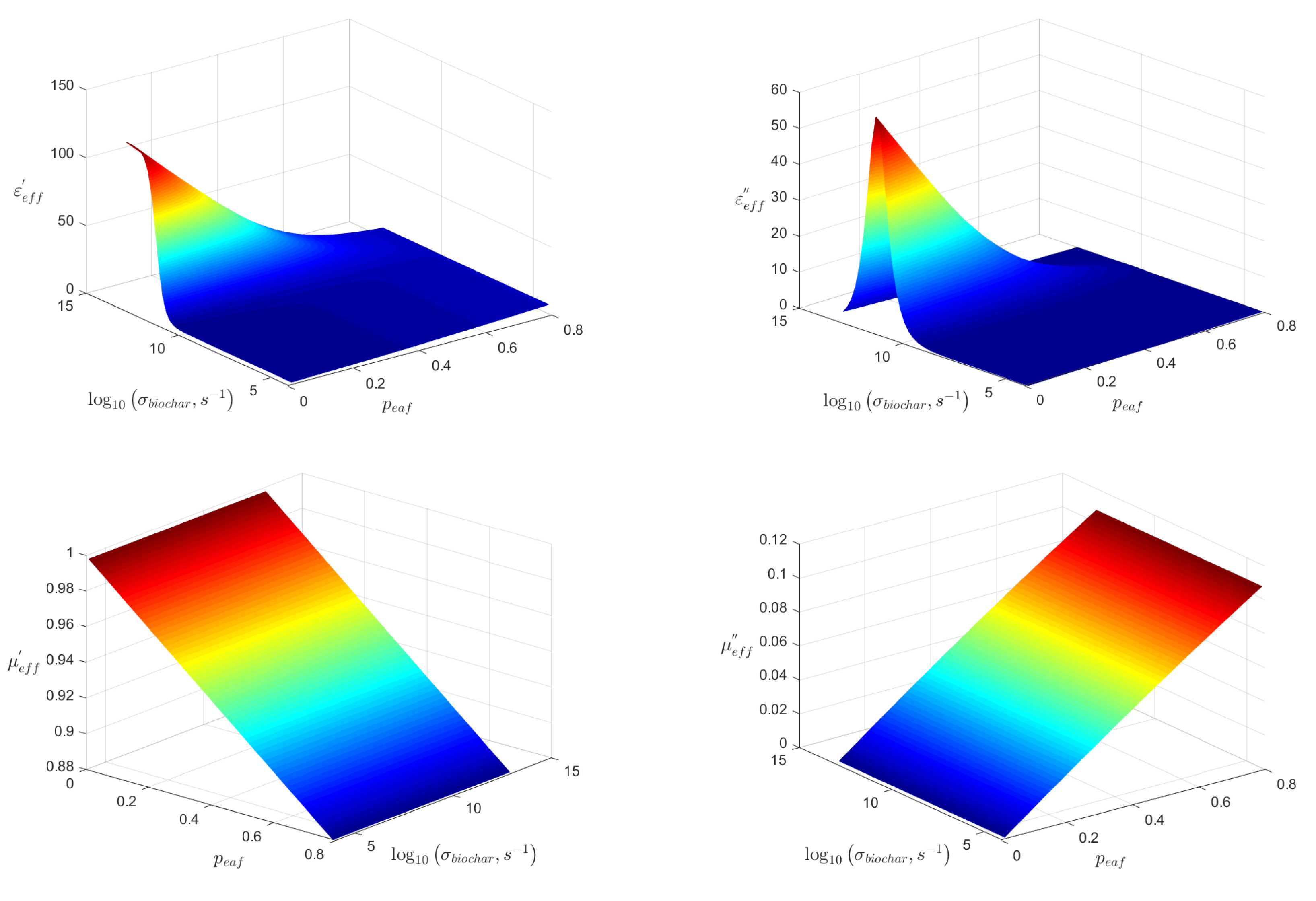
3. Effective Permittivity and Permeability of Biochar with EAF Dust Mixture
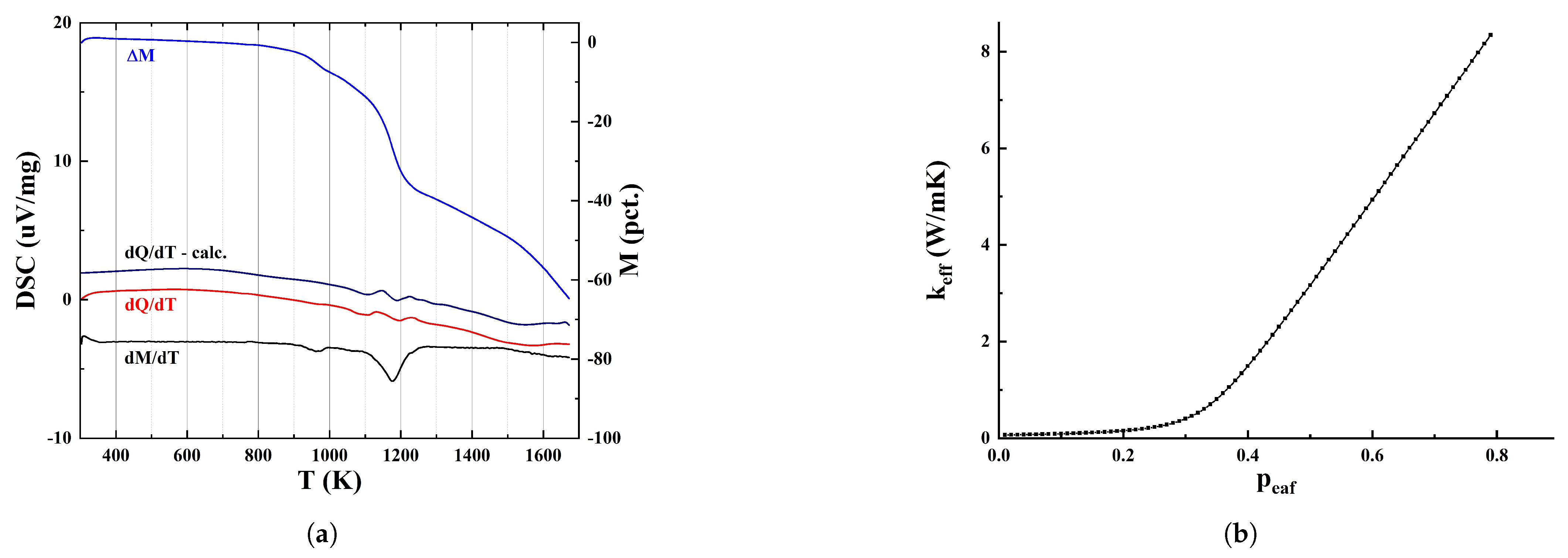
4. Effective Thermal Conductivity
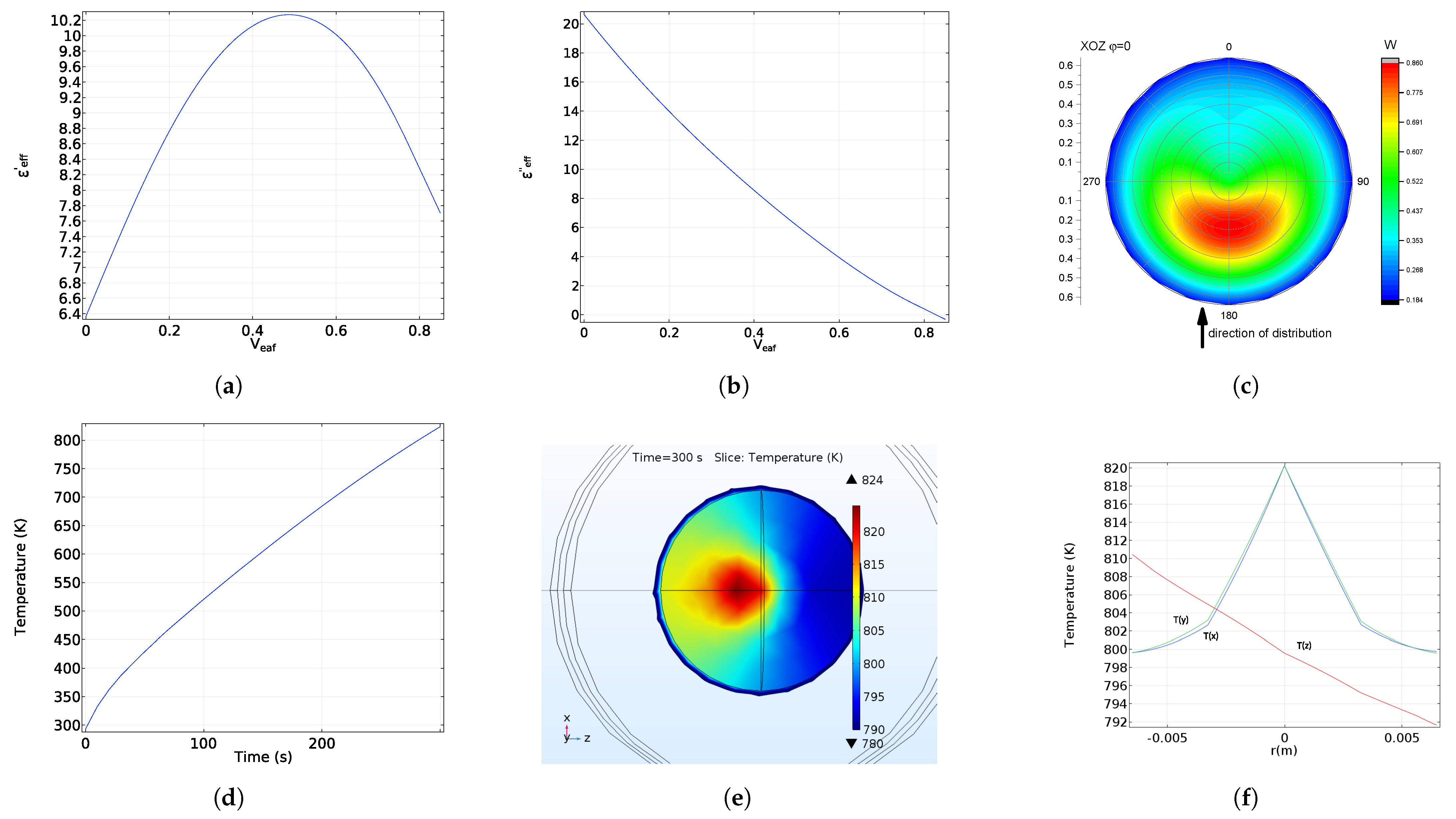
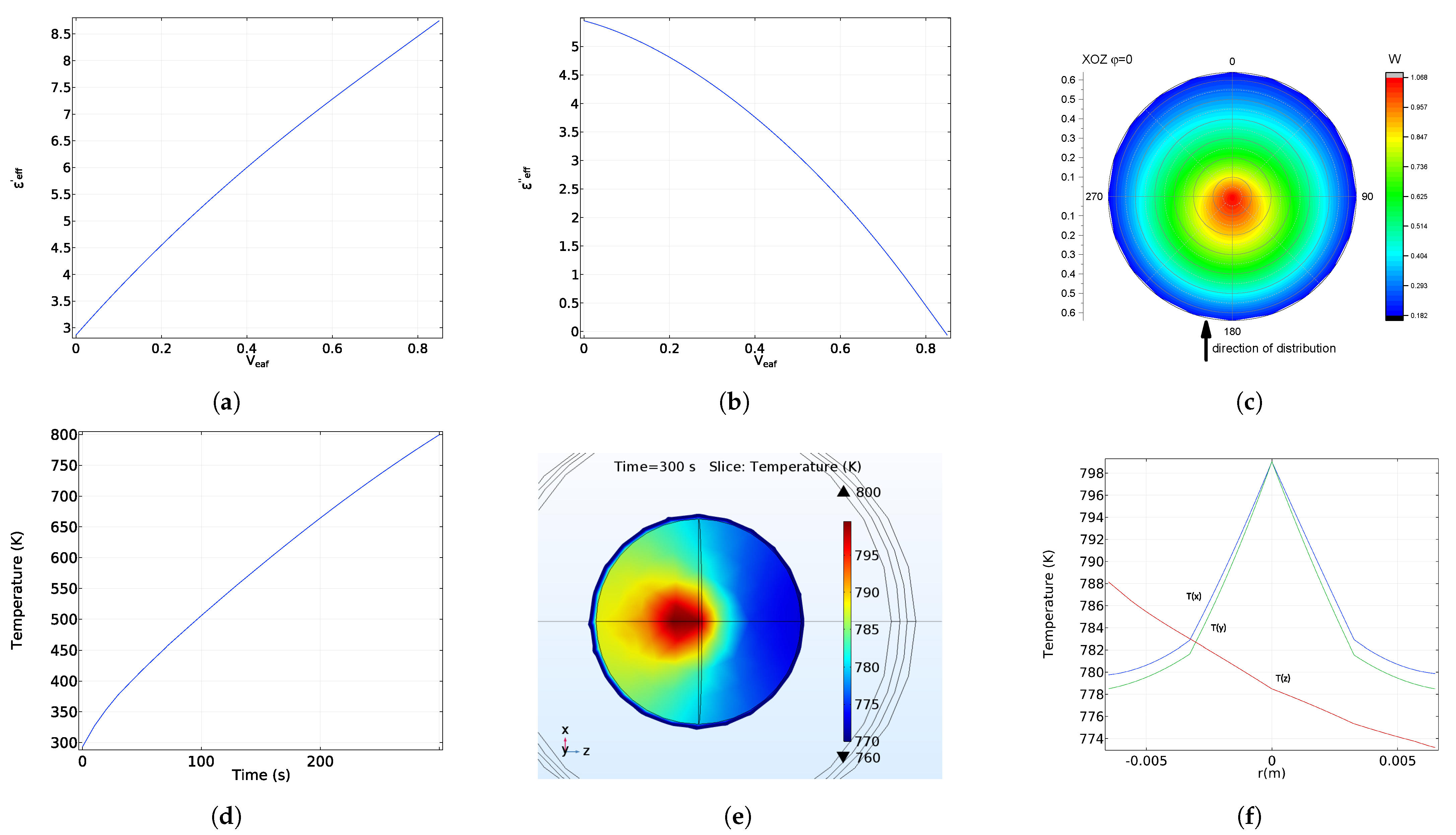
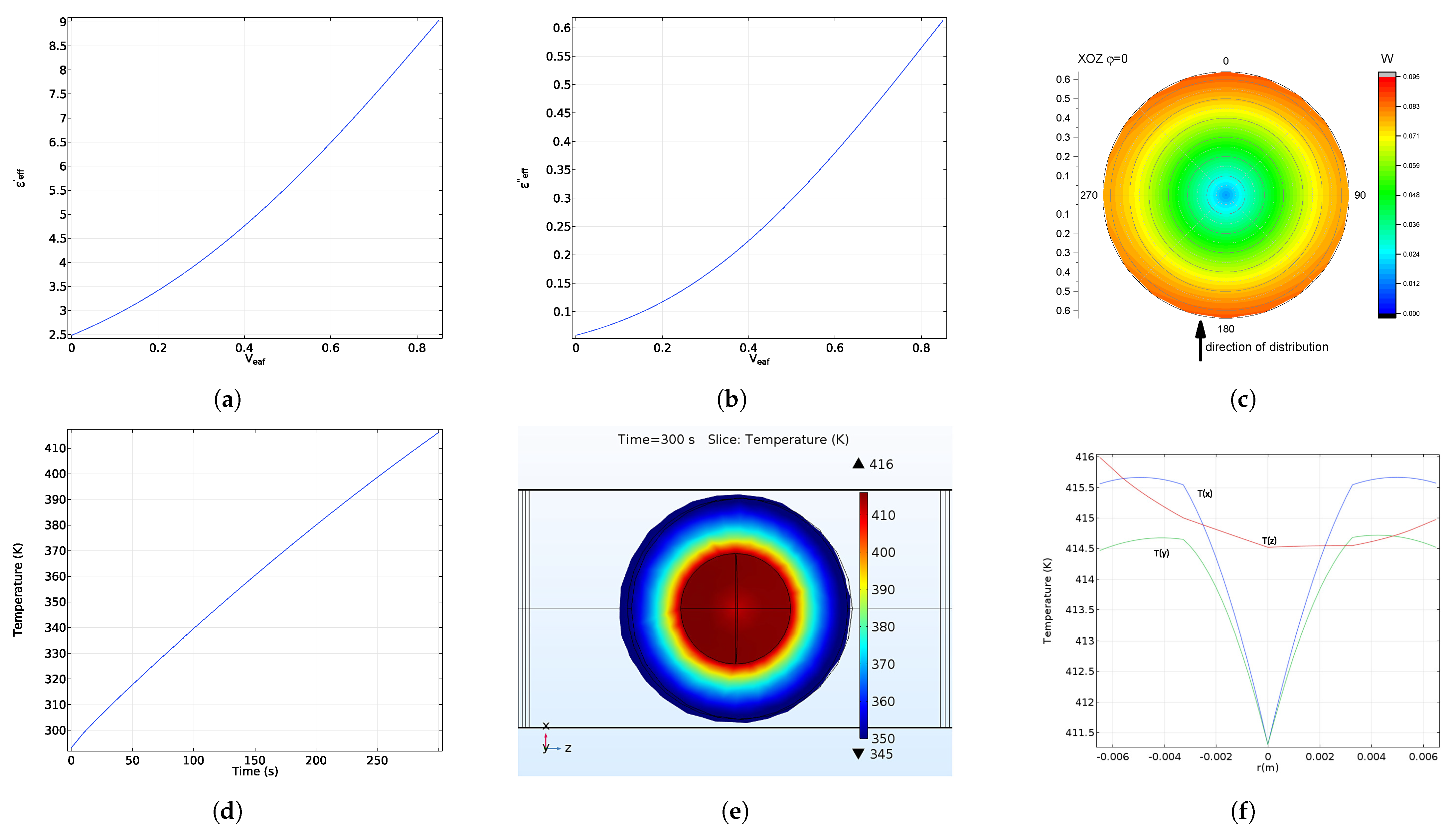
5. Electromagnetic Field and Temperature Distribution
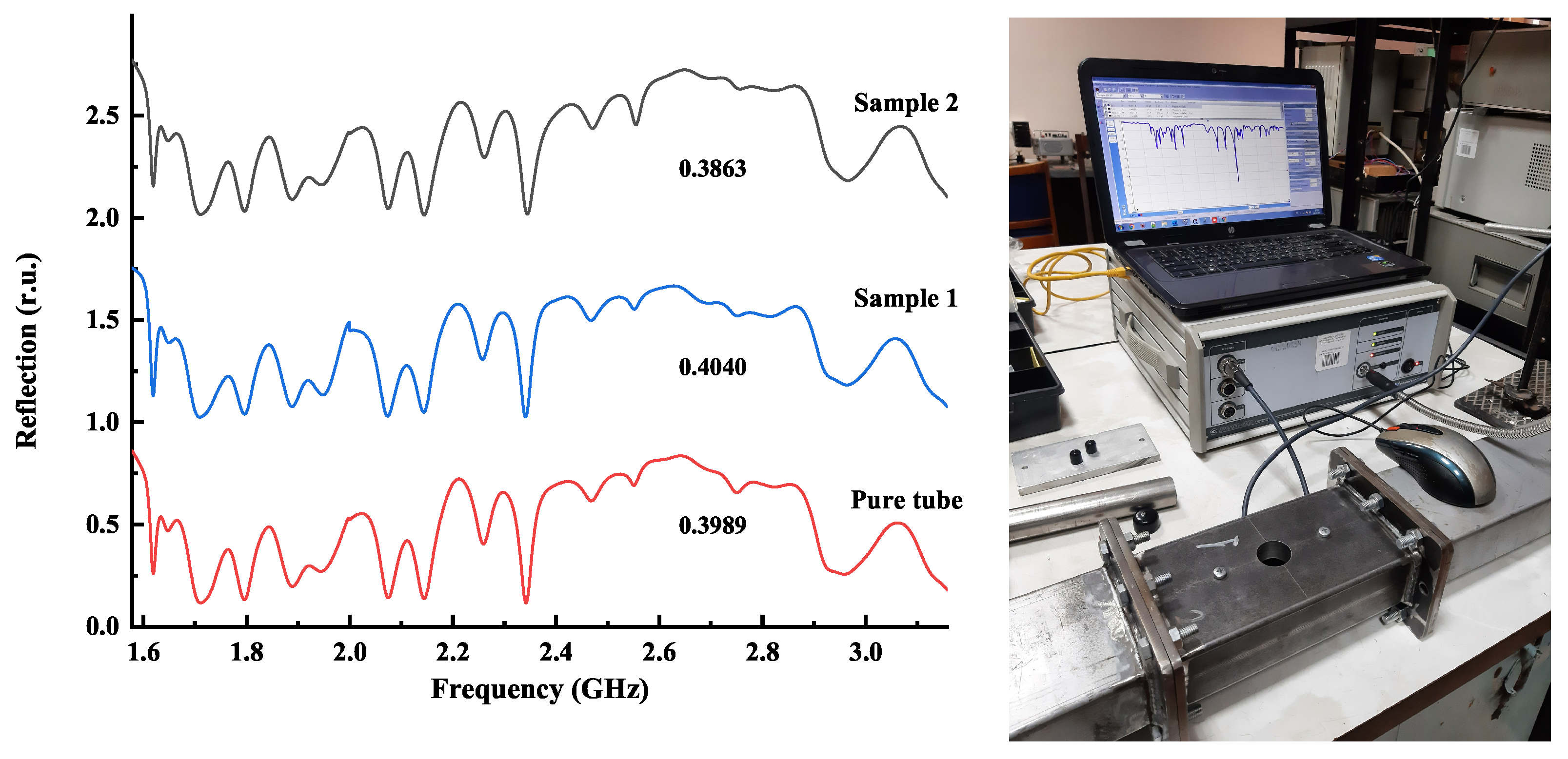
6. Microwave Reflection from Homogeneous and Radially Heterogeneous Pellets
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.Y.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Manturov, D. Prospects of the forest complex in the modern economy. Pulp Pap. Ind. 2017, 2/3, 16–17. [Google Scholar]
- Wang, L.; Peng, Z.; Lin, X.; Ye, Q.; Ye, L.; Zhang, J.; Liu, Y.; Liu, M.; Rao, M.; Li, G.; et al. Microwave-intensified treatment of low-zinc EAF dust: A route toward high-grade metallized product with a focus on multiple elements. Powder Technol. 2021, 383, 509–521. [Google Scholar] [CrossRef]
- Ye, Q.; Li, G.; Peng, Z.; Augustine, R.; Perez, M.D.; Liu, Y.; Liu, M.; Rao, M.; Zhang, Y.; Jiang, T. Microwave-assisted self-reduction of EAF dust-biochar composite briquettes for production of direct reduced iron. Powder Technol. 2020, 362, 781–789. [Google Scholar] [CrossRef]
- Buzin, P.J.W.K.; Heck, N.C.; Vilela, A.C.F. EAF dust: An overview on the influences of physical, chemical and mineral features in its recycling and waste incorporation routes. J. Mater. Res. Technol. 2017, 6, 194–202. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.M. Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using mononitrilotriacetate anion and hexahydrated ferric chloride. J. Hazard. Mater. 2002, 91, 257–270. [Google Scholar] [CrossRef]
- Kim, E.; Kim, T.; Lee, J.; Kang, Y.; Morita, K. Reduction of electric arc furnace dust by graphite powders under microwave irradiation. Ironmak. Steelmak. 2012, 39, 45–50. [Google Scholar] [CrossRef]
- Xia, L.; Mao, R.; Zhang, J.; Xu, X.; Wei, M.; Yang, F. Reduction process and zinc removal from composite briquettes composed of dust and sludge from a steel enterprise. Int. J. Miner. Metall. Mater. 2015, 22, 122–131. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Improved removal of zinc from blast furnace sludge by particle size separation and microwave heating. Miner. Eng. 2018, 127, 265–276. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Utilization of blast furnace sludge for the removal of zinc from steelmaking dusts using microwave heating. Sep. Purif. Technol. 2019, 210, 867–884. [Google Scholar] [CrossRef]
- Ye, L.; Peng, Z.; Wang, L.; Anzulevich, A.; Bychkov, I.; Tang, H.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Preparation of core-shell iron ore-biochar composite pellets for microwave reduction. Powder Technol. 2018, 338, 365–375. [Google Scholar] [CrossRef]
- Ye, Q.; Li, G.; Peng, Z.; Lee, J.; Lin, X.; Rao, X.; Zhang, Y.; Jiang, T. Microwave-assisted self-reduction of composite briquettes of zinc ferrite and carbonaceous materials. Powder Technol. 2019, 342, 224–232. [Google Scholar] [CrossRef]
- Ye, Q.; Peng, Z.; Li, G.; Lee, J.; Liu, J.; Liu, M.; Wang, L.; Rao, M.; Zhang, Y. Microwave-Assisted Reduction of Electric Arc Furnace Dust with Biochar: An Examination of Transition of Heating Mechanism. ACS Sustain. Chem. Eng. 2019, 7, 9515–9524. [Google Scholar] [CrossRef]
- Anzulevich, A.; Kalganov, D.; Anzulevich, S.; Bychkov, I.; Peng, Z. Optimal microwave heating of biochar containing iron ore pellets. J. Phys. Conf. Ser. 2020, 1461, 012007. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Li, Y.; Liu, X.X. Microwave electromagentic and absorption properties of AFe2O4 (A = Ni, Mn, Zn) ferrites. In Proceedings of the International Conference on Manufacturing Construction and Energy Engineering (MCEE), Hong Kong, China, 17–18 August 2016; pp. 190–196. [Google Scholar]
- Srivastava, J.K.; Prasad, M.; Wagner, J.B., Jr. Electrical Conductivity of Silicon Dioxide Thermally Grown on Silicon. J. Electrochem. Soc. 1985, 132, 955–963. [Google Scholar] [CrossRef]
- Hotta, M.; Hayashi, M.; Nishikata, A.; Nagata, K. Complex Permittivity and Permeability of SiO2 and Fe3O4 Powders in Microwave Frequency Range between 0.2 and 13.5 GHz. ISIJ Int. 2009, 49, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.P.; Dadon, D.; Rosen, M.; Gershon, D.; Rybakov, K.I.; Birman, A.; Calame, J.P.; Levush, B.; Carmel, Y.; Hutcheon, R. Effects of anomalous permittivity on the microwave heating of zinc oxide. J. Appl. Phys. 1998, 83, 432–437. [Google Scholar] [CrossRef]
- Lunt, R.A.; Jackson, A.J.; Walsh, A. Dielectric response of Fe2O3 crystals and thin films. Chem. Phys. Lett. 2013, 586, 67–69. [Google Scholar]
- Simonyan, L.M.; Govorova, N.M. Features of dust formation in the oxygen-blowing of melts and possible uses of captured dust. Metallurgist 2011, 55, 450–458. [Google Scholar] [CrossRef]
- Anzulevich, A.P.; Bychkov, I.V.; Buchelnikov, V.D.; Anzulevich, S.N.; Kalganov, D.A.; Peng, Z. Calculation of Effective Permittivity and Permeability for Iron Ore–Biochar–Bentonite Binder Powders Mixture. In Proceedings of the 3rd International Conference on Shape Memory Alloys (SMA 2018), Chelyabinsk, Russia, 13–17 September 2018; Volume 9, pp. 136–139. [Google Scholar]
- Kalganov, D.A.; Pavlov, D.A.; Anzulevich, A.P.; Butko, L.N.; Tolkachev, V.A.; Peng, Z. Investigation of composition and properties of EAF dust for metal reduction. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1155, 012082. [Google Scholar] [CrossRef]
- Potapov, D.S.; Potapov, S.S. Mineralogy of ferrochrome production slags of Chelyabinsk Electrometallurgical Plant. Successes Mod. Nat. Sci. Conf. Mater. 2010, 8, 23–25. [Google Scholar]
- Dib, R.; Vincent, D.; Elrafhi, A. Measurement of the electromagnetic properties of thin films using a microwave resonant cavity. ACS Microw. Opt. Technol. Lett. 2019, 61, 15–19. [Google Scholar] [CrossRef]
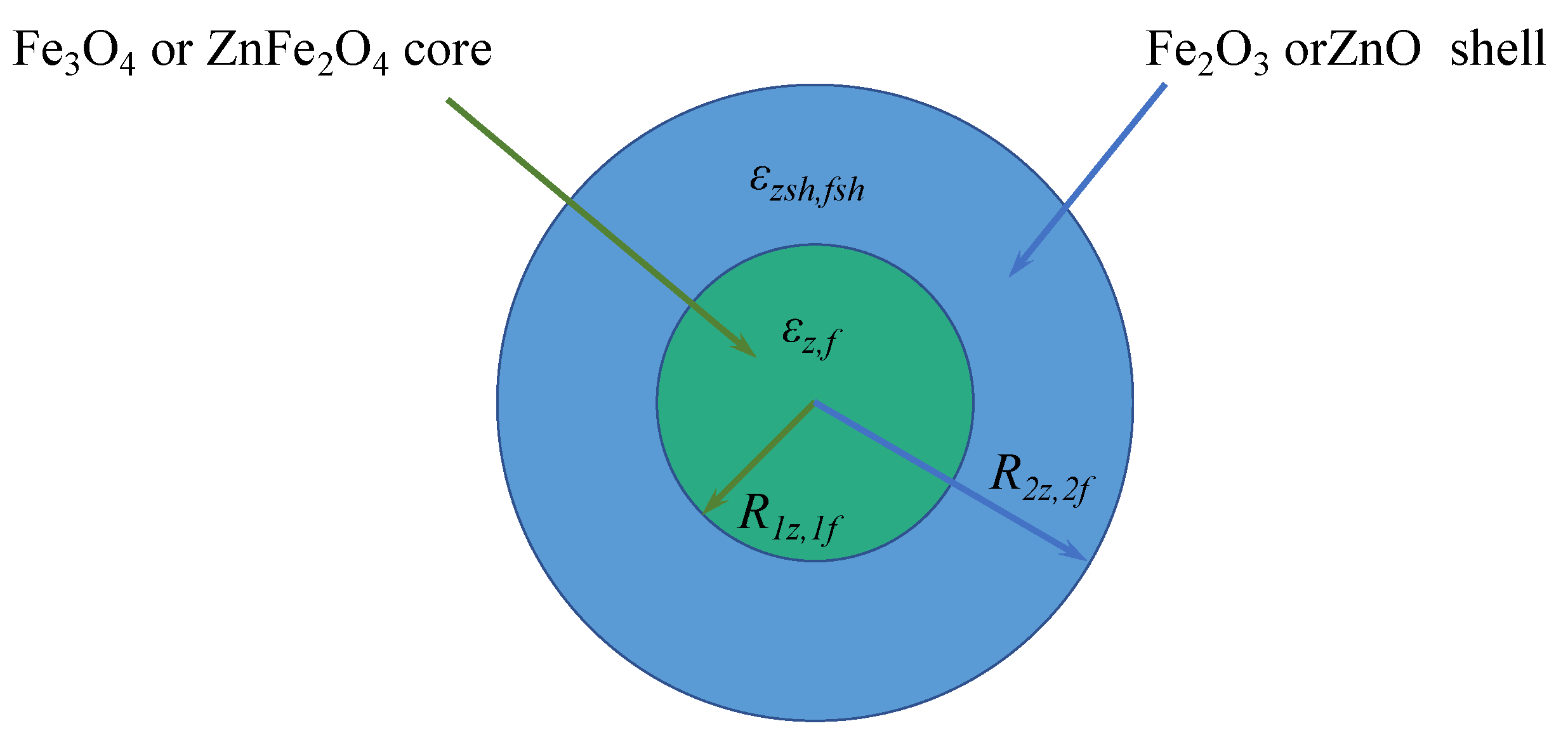
- Anzulevich, A.P.; Bychkov, I.V.; Buchelnikov, V.D.; Peng, Z.; Huang, Z.; Xu, B.; Butko, L.N.; Anzulevich, S.N.; Moiseev, S.G. Simplified Core-Shell Model of Biochar-Iron Ore Mixture for Calculation of Effective Permittivity and Permeability. Solid State Phenom. 2018, 279, 240–244. [Google Scholar] [CrossRef]
- Ordonez-Miranda, J.; Alvarado-Gil, J.J.; Medina-Ezquivel, R. Generalized Bruggeman Formula for the Effective Thermal Conductivity of Particulate Composites with an Interface Layer. Int. J. Thermophys. 2010, 31, 975–986. [Google Scholar] [CrossRef]
- Pietrak, K.; Wisniewski, T.S. A review of models for effective thermal conductivity of composite materials. J. Power Technol. 2015, 95, 14–24. [Google Scholar]
- Tripathi, A.; Chandra, H. Performance Investigation of Automobile Radiator Operated with ZnFe2O4 Nano Fluid based Coolant. Matec Web Conf. 2015, 34, 01003. [Google Scholar] [CrossRef] [Green Version]
- Olorunyolemi, T.; Birnboim, A.; Carmel, Y.; Wilson, O.C., Jr.; Lloyd, I.K.; Smith, S.; Campbell, R. Thermal conductivity of zinc oxide: From green to sintered state. J. Am. Ceram. Soc. 2002, 85, 1249–1253. [Google Scholar] [CrossRef]
- Akiyama, T.; Ohta, H.; Takahashi, R.; Waseda, Y.; Yagi, J.I. Measurement and modeling of thermal conductivity for dense iron oxide and porous iron ore agglomerates in stepwise reduction. ISIJ Int. 1992, 32, 829–837. [Google Scholar] [CrossRef]
- Mølgaard, J.; Smeltzer, W.W. Thermal conductivity of magnetite and hematite. J. Appl. Phys. 1971, 42, 3644–3647. [Google Scholar] [CrossRef]
- Yao, D.J.; Lai, W.C.; Chien, H.C. Temperature dependence of thermal conductivity for silicon dioxide. Int. Conf. Micro/Nanoscale Heat Transf. 2008, 42924, 435–439. [Google Scholar]
- Horai, K.I.; Simmons, G. Thermal conductivity of rock-forming minerals. Earth Planet. Sci. Lett. 1969, 6, 359–368. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Ye, Q.; Peng, Z.; Ye, L.; Wang, L.; Augustine, R.; Lee, J.; Liu, Y.; Liu, M.; Rao, M.; Li, G.; et al. Thermodynamic Analysis of Carbothermic Reduction of Electric Arc Furnace Dust. In Proceedings of the 10th International Symposium on High-Temperature Metallurgical Processing, San Antonio, TX, USA, 10–14 March 2019; pp. 117–124. [Google Scholar]



| Weight Fraction, % | Model Volume Fraction, % | |||||
|---|---|---|---|---|---|---|
| ZnFe2O4 | 3.7 | 1.528 × 109 | 1.25 | 0.55 | 5–50 | 23 |
| ZnO | 5.822 | 1.798 × 105 | 1 | 1 | 1–25 | 8 |
| Fe3O4 | 57.355 | 1.079 × 1010 | 1.2 | 0.2 | 10–60 | 25 |
| Fe2O3 | 14.922 | 8.988 × 104 | 1 | 1 | 10–25 | 12 |
| SiO2 | 6 | 1.011 × 101 | 1 | 1 | 1–10 | 2 |
| CaMgSiO4 | 8.5 | 6.92 × 10−1 | 1 | 1 | 15–60 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzulevich, A.; Butko, L.; Kalganov, D.; Pavlov, D.; Tolkachev, V.; Fedii, A.; Buchelnikov, V.; Peng, Z. Optimization of the Microwave-Assisted Carbothermical Reduction Process for Metals from Electric Arc Furnace Dust with Biochar. Metals 2021, 11, 1765. https://doi.org/10.3390/met11111765
Anzulevich A, Butko L, Kalganov D, Pavlov D, Tolkachev V, Fedii A, Buchelnikov V, Peng Z. Optimization of the Microwave-Assisted Carbothermical Reduction Process for Metals from Electric Arc Furnace Dust with Biochar. Metals. 2021; 11(11):1765. https://doi.org/10.3390/met11111765
Chicago/Turabian StyleAnzulevich, Anton, Leonid Butko, Dmitrii Kalganov, Dmitrii Pavlov, Valentin Tolkachev, Alexander Fedii, Vasiliy Buchelnikov, and Zhiwei Peng. 2021. "Optimization of the Microwave-Assisted Carbothermical Reduction Process for Metals from Electric Arc Furnace Dust with Biochar" Metals 11, no. 11: 1765. https://doi.org/10.3390/met11111765
APA StyleAnzulevich, A., Butko, L., Kalganov, D., Pavlov, D., Tolkachev, V., Fedii, A., Buchelnikov, V., & Peng, Z. (2021). Optimization of the Microwave-Assisted Carbothermical Reduction Process for Metals from Electric Arc Furnace Dust with Biochar. Metals, 11(11), 1765. https://doi.org/10.3390/met11111765








