Discrete One-Stage Mechanochemical Synthesis of Titanium-Nitride in a High-Energy Mill
Abstract
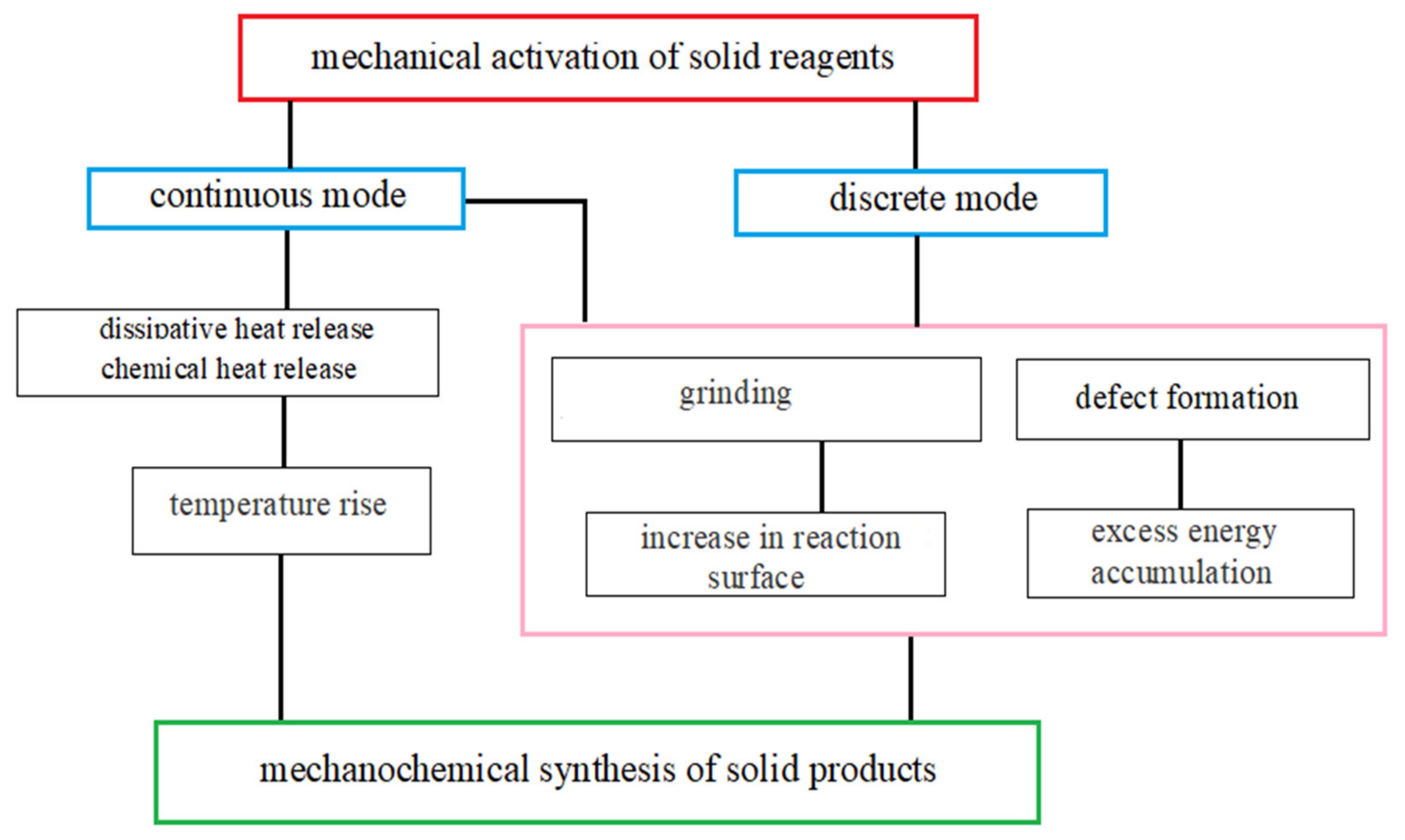
:1. Introduction
- Structural factor, which determines the grinding of substances and the formation of an interfacial area required for chemical interactions;
- Kinetic factor, resulting in the accumulation of excess energy, which is accumulated in the formed structural defects of solid reagents and decreases the activation barrier of chemical reactions;
- Temperature factor, caused by heating of milling bodies, chamber walls, and a grinding substance (dissipative heat release), as well as by heat release from the relaxation of structural defects and chemical reactions.
2. Experimental Procedure
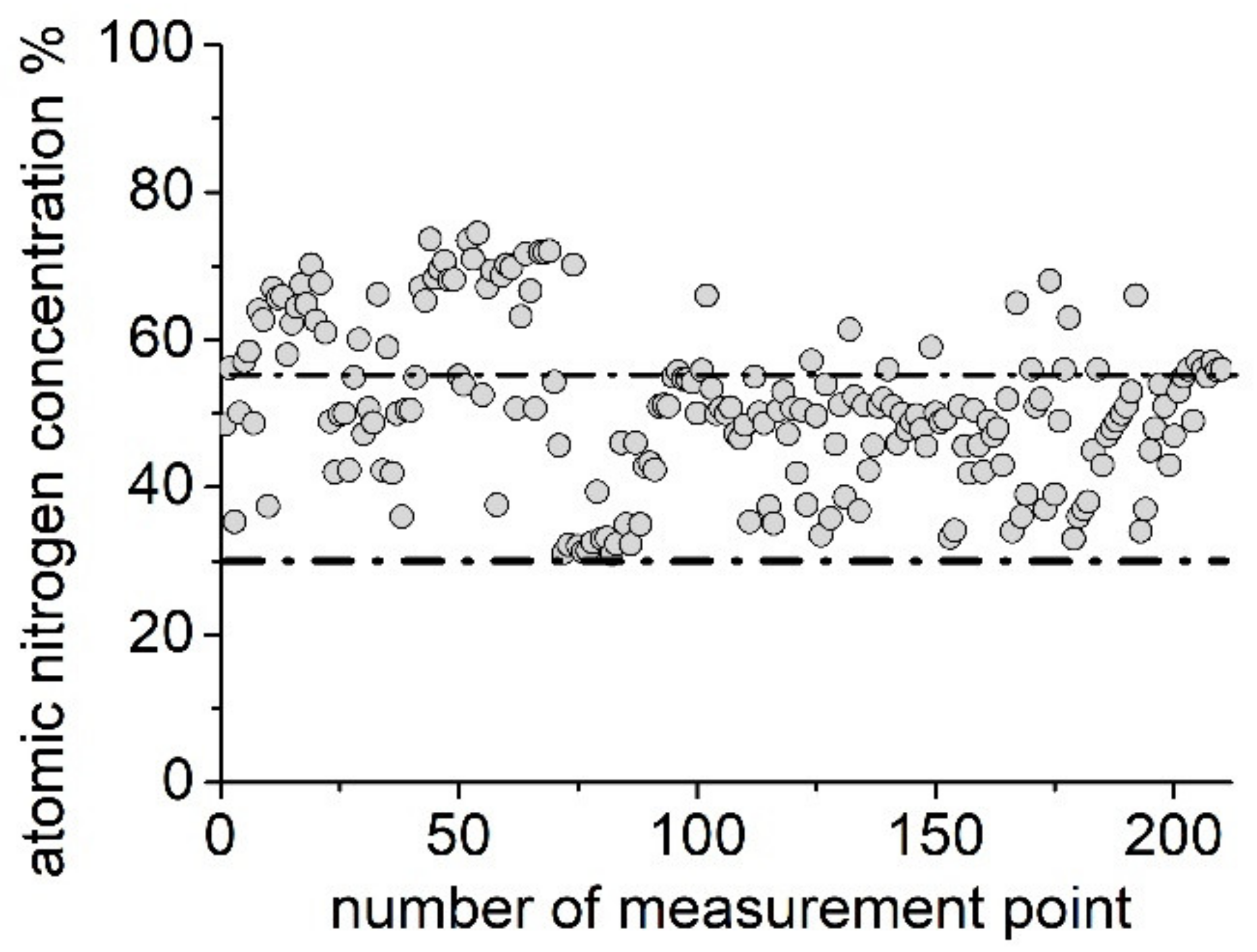
3. Mathematical Model
3.1. Thermal Balance Equation
3.2. Chemical Transformation Equation
3.3. Grinding Equation
3.4. Dynamics of Excess Energy
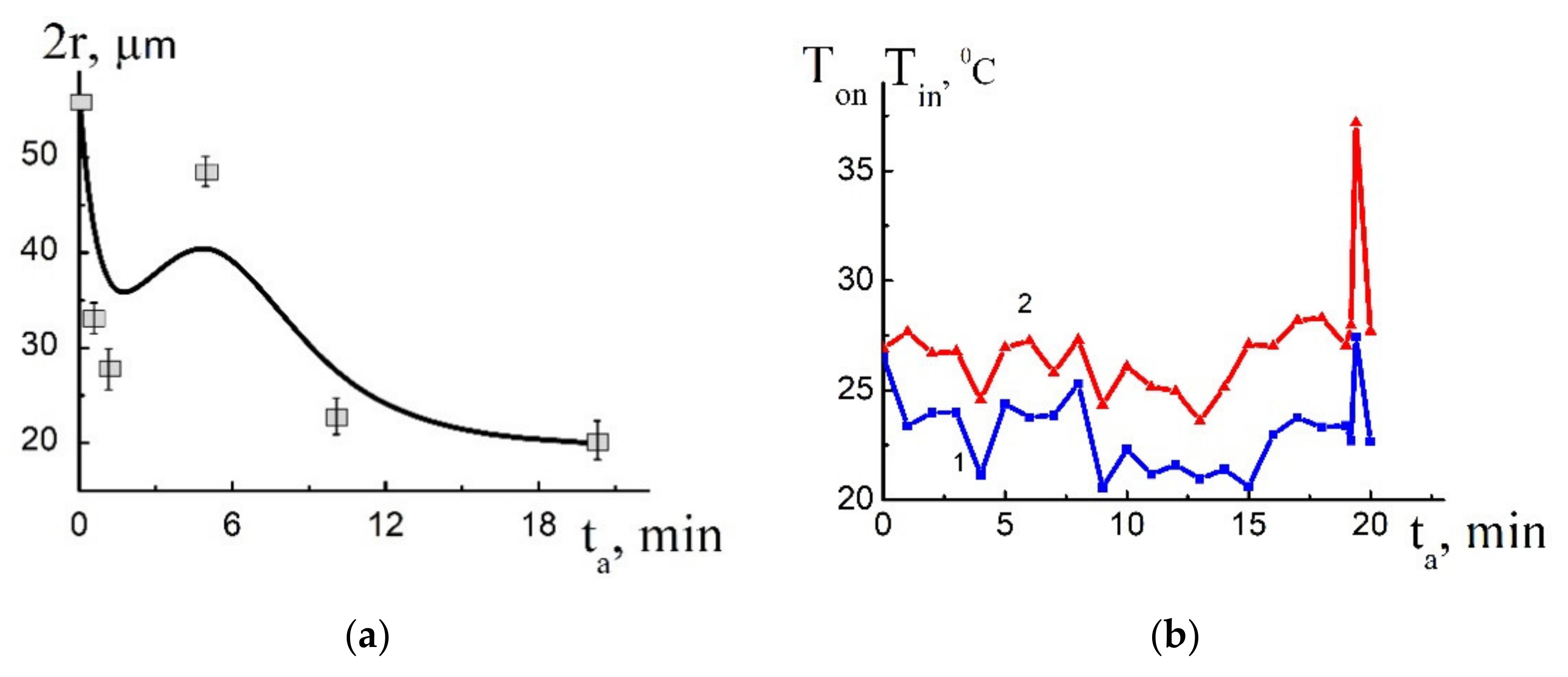
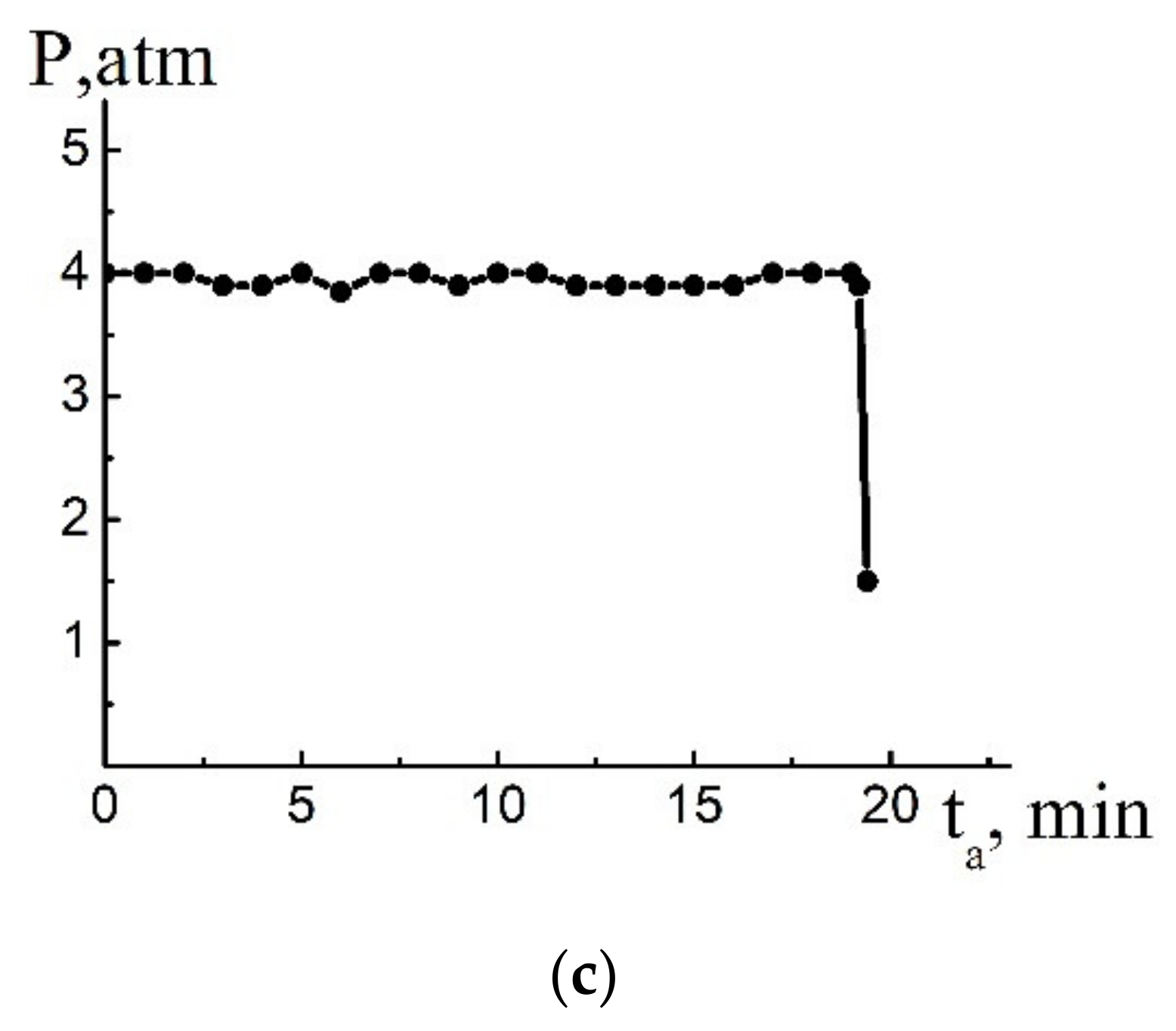
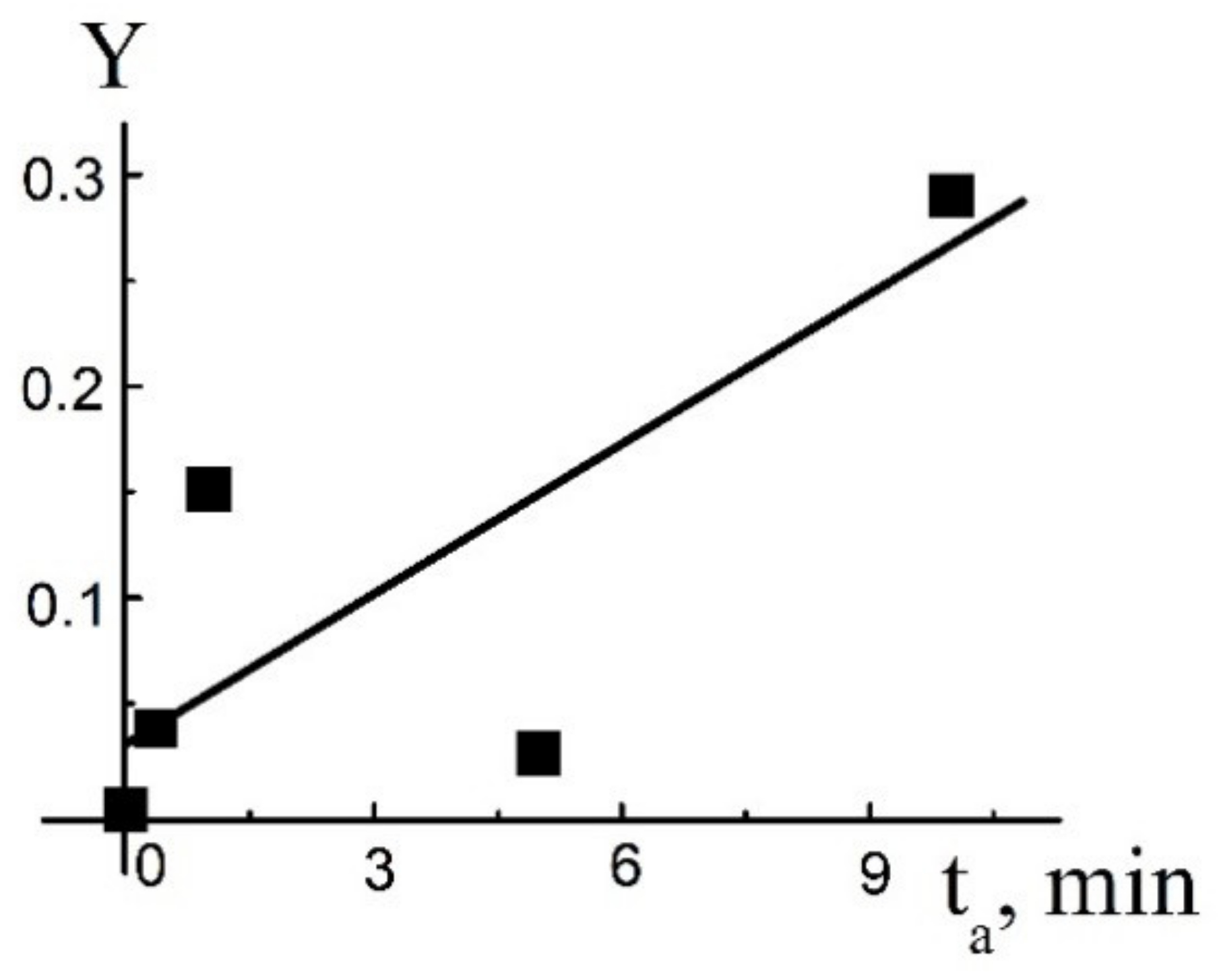
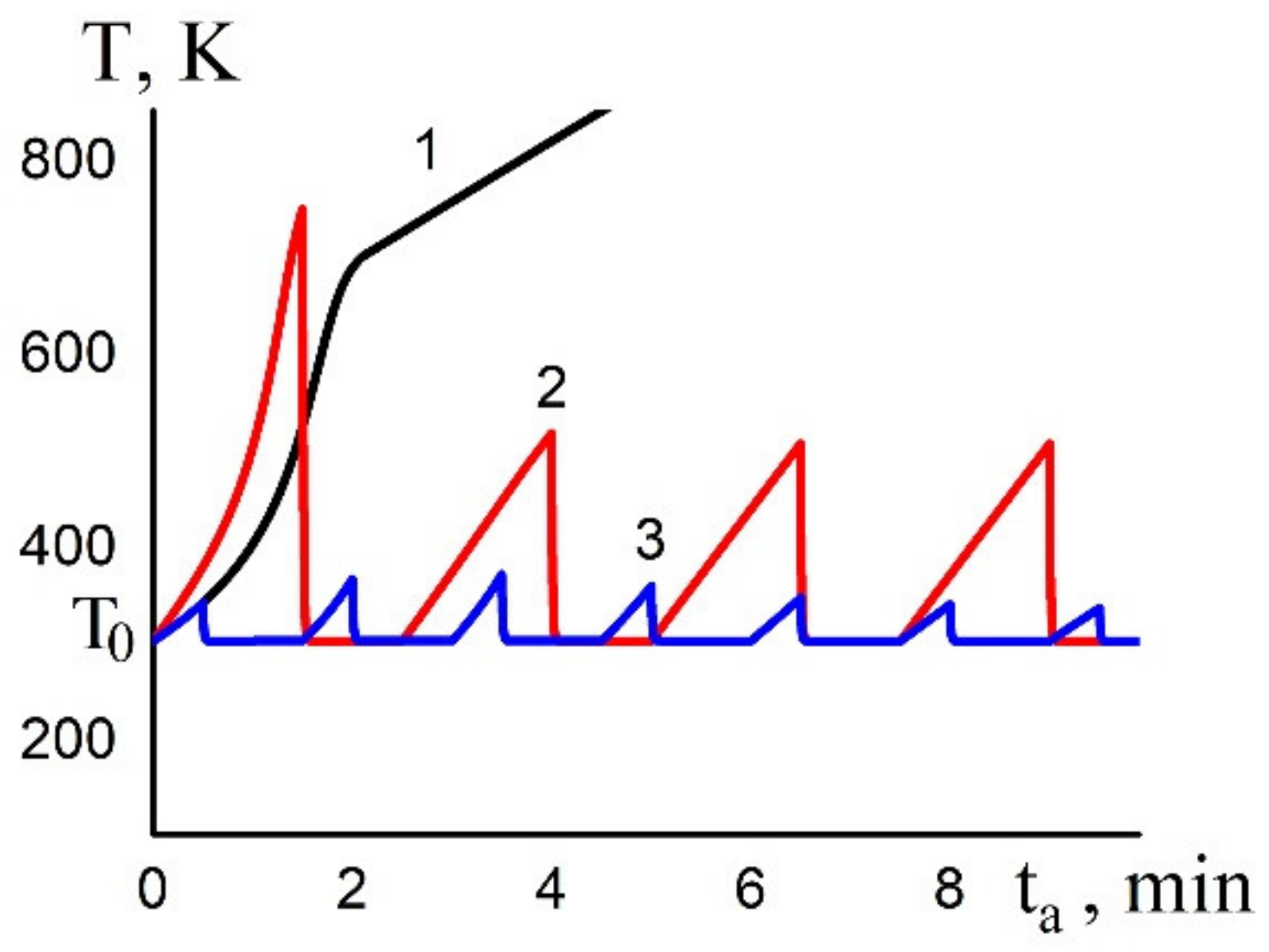
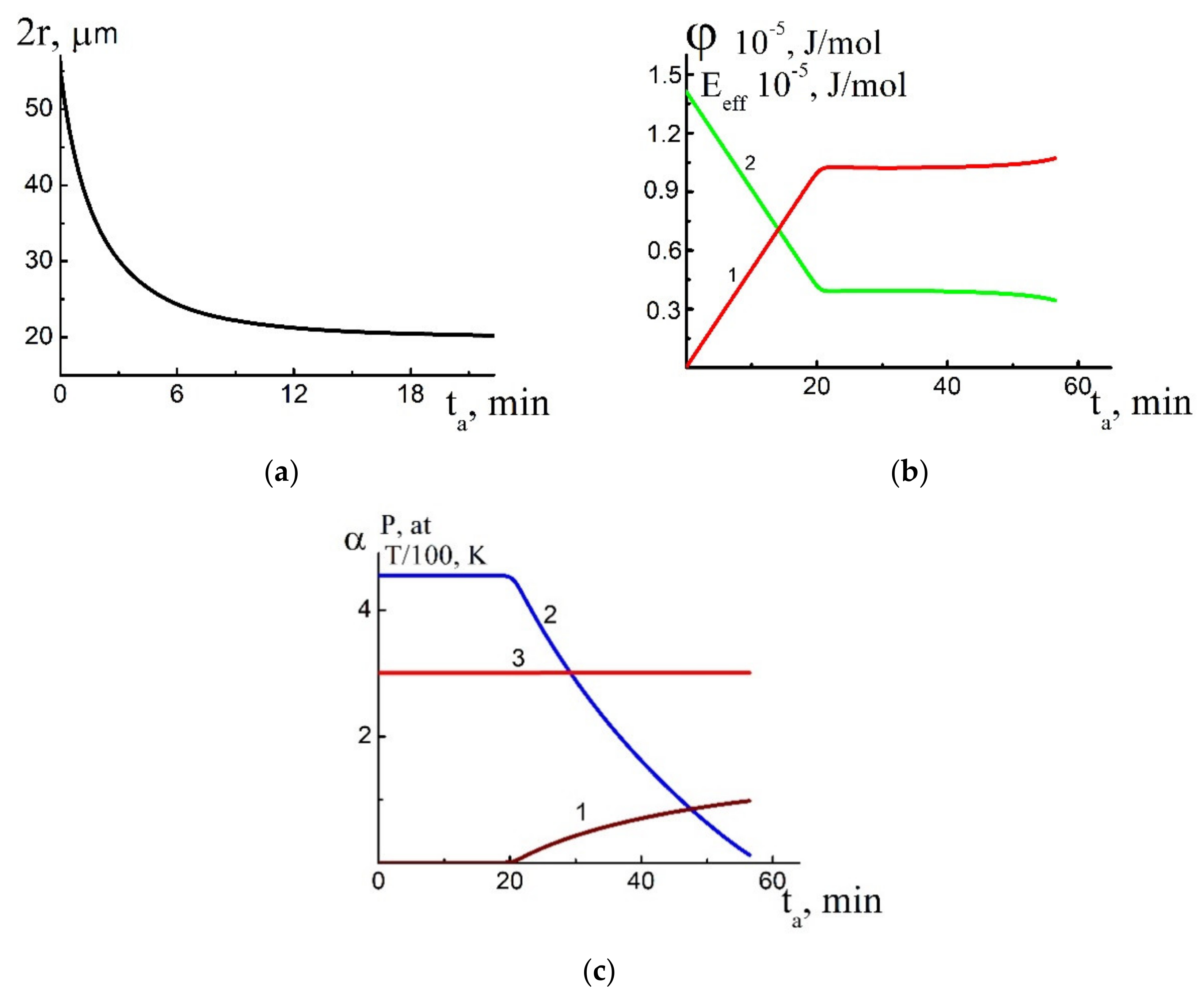
4. Solution of the Problem and Discussion
4.1. Estimation of Kinetic Parameters
4.2. Numerical Solution of the Problem
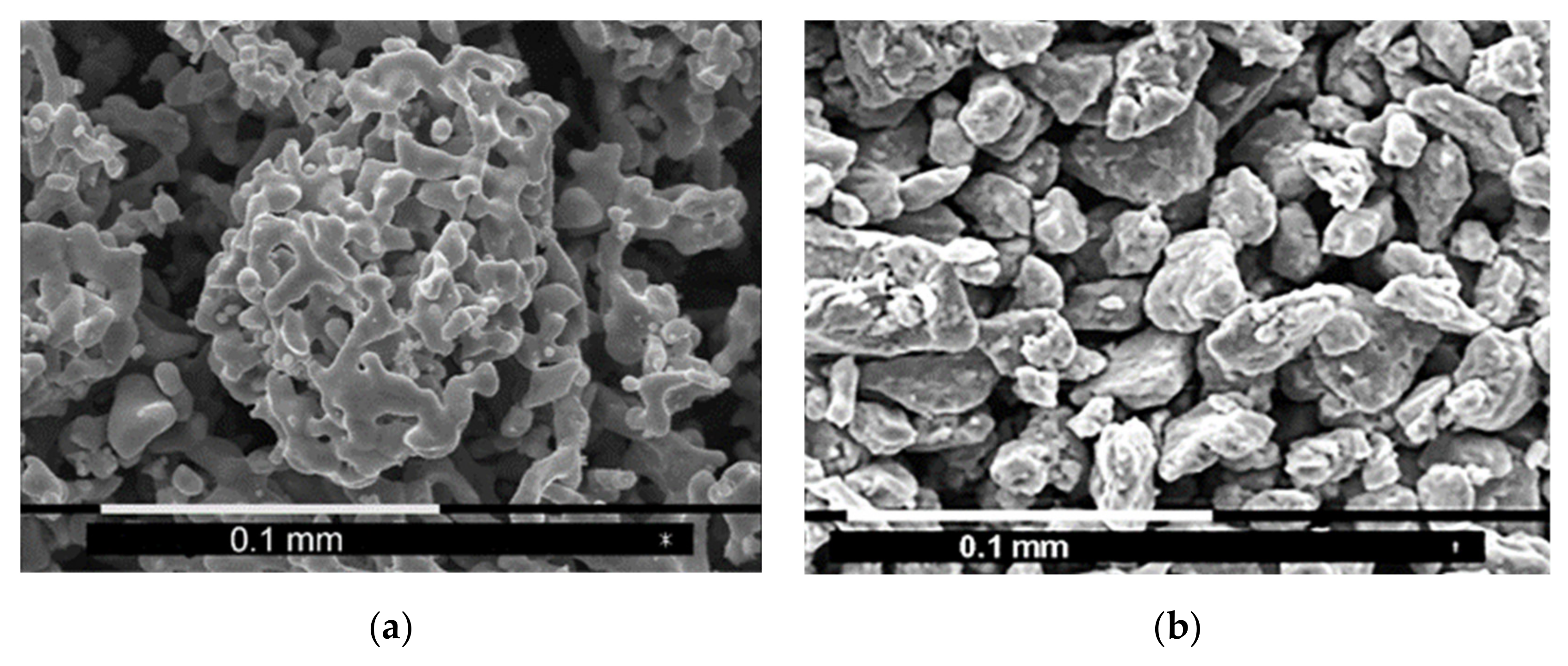
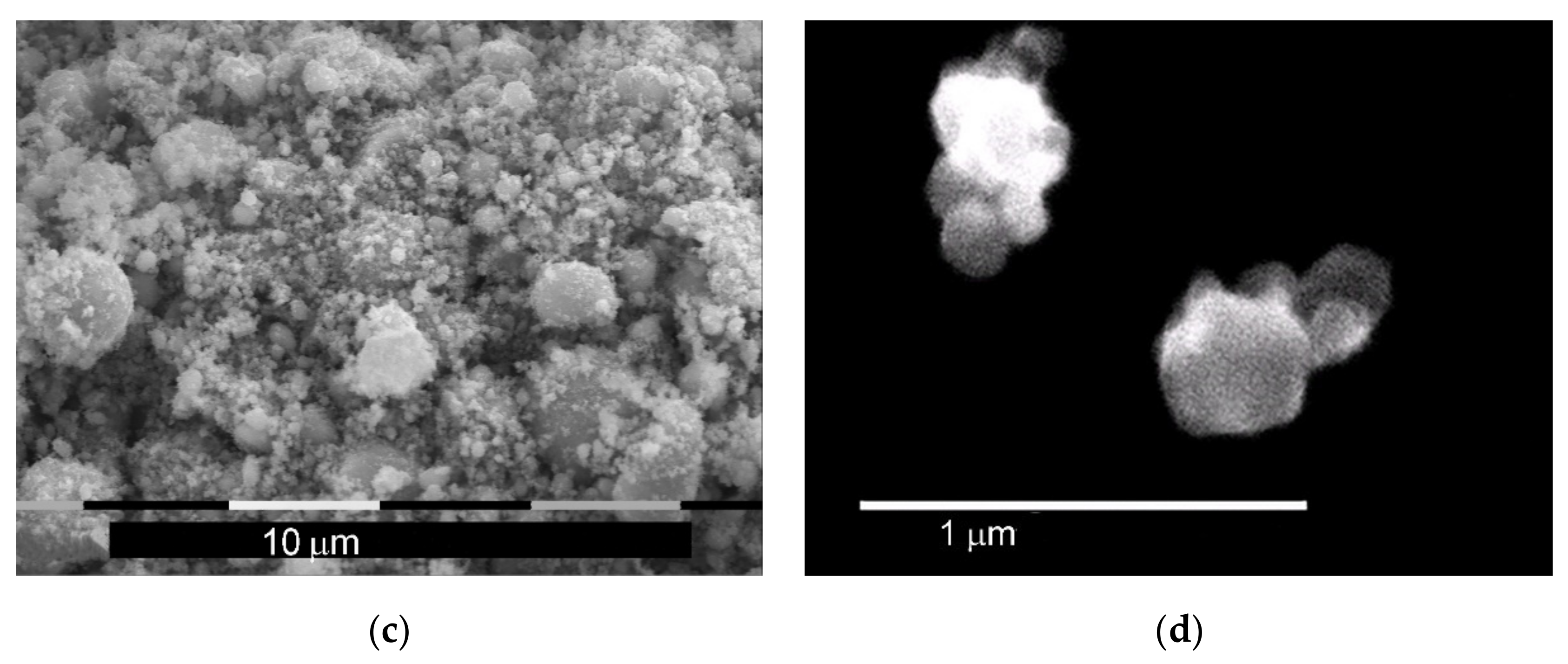
4.3. Morphology and X-ray Diffraction of the Final Product
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Rogachev, A.S. Mechanical activation of heterogeneous exothermic reactions in powder mixtures. Russ. Chem. Rev. 2019, 88, 875–900. [Google Scholar] [CrossRef]
- Andreev, D.; Vdovin, Y.; Yukhvid, V.; Golosova, O. Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure. Metals 2021, 11, 803. [Google Scholar] [CrossRef]
- Yeh, C.-L.; Chen, K.-T. Synthesis of FeSi-Al2O3 Composites by Autowave Combustion with Metallothermic Reduction. Metals 2021, 11, 258. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loubes, M.A.; González, L.C.; Tolaba, M.P. Modeling energy requirements in planetary ball milling of rice grain. Part. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- García-Garrido, C.; Ferrer, R.S.; Salvo, C.; García-Domínguez, L.; Pérez-Pozo, L.; Lloreda-Jurado, P.; Chicardi, E. Effect of Milling Parameters on the Development of a Nanostructured FCC–TiNb15Mn Alloy via High-Energy Ball Milling. Metals 2021, 11, 1225. [Google Scholar] [CrossRef]
- Hirosawa, F.; Iwasaki, T. Dependence of the dissipated energy of particles on the sizes and numbers of particles and balls in a planetary ball mill. Chem. Eng. Res. Des. 2021, 167, 84–95. [Google Scholar] [CrossRef]
- Bernauer, C.; Grohmann, S.; Angermann, P.; Dickes, D.; Holzberger, F.; Amend, P.; Zaeh, M. Investigation of the Cause-Effect Relationships between the Exothermic Reaction and the Microstructures of Reactive Ni-Al Particles Produced by High Energy Planetary Ball Milling. Metals 2021, 11, 876. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. Role of Mixing and Milling in Mechanochemical Synthesis (Review). Russ. J. Inorg. Chem. 2021, 66, 433–453. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Boldyreva, E.V.; Belenguer, A.M.; Emmerling, F.; Boldyrev, V.V. Tribochemistry, Mechanical Alloying, Mechanochemistry: What is in a Name? Front. Chem. 2021, 9, 685789. [Google Scholar] [CrossRef] [PubMed]
- Kochetov, N.; Sytschev, A. Effects of magnesium on initial temperature and mechanical activation on combustion synthesis in Ti–Al–Mg system. Mater. Chem. Phys. 2021, 257, 123727. [Google Scholar] [CrossRef]
- Shuck, C.E.; Manukyan, K.V.; Rouvimov, S.; Rogachev, A.S.; Mukasyan, A.S. Solid-flame: Experimental validation. Combust. Flame 2016, 163, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Delogu, F.; Takacs, L. Mechanochemistry of Ti–C powder mixtures. Acta Mater. 2014, 80, 435–444. [Google Scholar] [CrossRef]
- Shkoda, O.A.; Lapshin, O.V. Thermal Explosion in a Mechanically Activated Ti-Ni System. Experimental Data. Russ. Phys. J. 2016, 59, 1231–1234. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Shkoda, O.A. Thermal Explosion in a Mechanically Activated Ti-Ni System: Mathematical Model. Russ. Phys. J. 2017, 59, 1433–1439. [Google Scholar] [CrossRef]
- Shkoda, O.A.; Lapshin, O.V. Mechanical Activation and Thermal Treatment of Low-Energy Nb-2Si Powder Blend. I. the Experiment. Russ. Phys. J. 2019, 61, 1951–1955. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Shkoda, O.A. Mechanical Activation and Thermal Treatment of Low-Energy Nb-2Si Powder Blend. The Experiment. II. Mathematical Model. Russ. Phys. J. 2019, 61, 2209–2217. [Google Scholar] [CrossRef]
- White, J.D.E.; Reeves, R.V.; Son, S.F.; Mukasyan, A.S. Thermal Explosion in Al−Ni System: Influence of Mechanical Activation. J. Phys. Chem. A 2009, 113, 13541–13547. [Google Scholar] [CrossRef]
- Shteinberg, A.S.; Lin, Y.-C.; Son, S.; Mukasyan, A. Kinetics of High Temperature Reaction in Ni-Al System: Influence of Mechanical Activation. J. Phys. Chem. A 2010, 114, 6111–6116. [Google Scholar] [CrossRef]
- Aly, Y.; Schoenitz, M.; Dreizin, E.L. Ignition and combustion of mechanically alloyed Al–Mg powders with customized particle sizes. Combust. Flame 2013, 160, 835–842. [Google Scholar] [CrossRef]
- Aly, Y.; Dreizin, E.L. Ignition and combustion of Al·Mg alloy powders prepared by different techniques. Combust. Flame 2015, 162, 1440–1447. [Google Scholar] [CrossRef]
- Terry, B.; Son, S.; Groven, L.J. Altering combustion of silicon/polytetrafluoroethylene with two-step mechanical activation. Combust. Flame 2015, 162, 1350–1357. [Google Scholar] [CrossRef]
- Jin, H.-B.; Yang, Y.; Chen, Y.-X.; Lin, Z.-M.; Li, J.-T. Mechanochemical-Activation-Assisted Combustion Synthesis of alpha-Si3N4. J. Am. Ceram. Soc. 2006, 89, 1099–1102. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Li, J.-T.; Du, J.-S. Cost effective combustion synthesis of silicon nitride. Mater. Res. Bull. 2008, 43, 1598–1606. [Google Scholar] [CrossRef]
- Liu, G.; Chen, K.; Zhou, H.; Li, J.; Pereira, C.; Ferreira, J. Mechanical-activation-assisted combustion synthesis of α-SiAlON in air. Mater. Res. Bull. 2007, 42, 989–995. [Google Scholar] [CrossRef]
- Lapshin, O.; Ivanova, O. Macrokinetics of mechanochemical synthesis in heterogeneous systems: Mathematical model and evaluation of thermokinetic constants. Mater. Today Commun. 2021, 28, 102671. [Google Scholar] [CrossRef]
- Smolyakov, V.K.; Lapshin, O.V.; Maksimov, Y.M. Macrokinetics of Mechanosynthesis in Solid-Gas Systems. I. Mathematical Simulation. Combust. Explos. Shock Waves 2005, 41, 554–565. [Google Scholar] [CrossRef]
- Smolyakov, V.K.; Itin, V.I.; Golobokov, N.N.; Kasatskii, N.G.; Lapshin, O.V.; Maksimov, Y.M.; Terekhova, O.G.; Shkoda, O.A. Macrokinetics of Mechanosynthesis in Solid-Gas Systems. II. Experimental Studies. Analysis of Results. Combust. Explos. Shock Waves 2005, 41, 566–572. [Google Scholar] [CrossRef]
- Córdoba, J.; Alcalá, M.; Avilés, M.; Sayagues, M.J.; Gotor, F. New production of TiCxN1−x-based cermets by one step mechanically induced self-sustaining reaction: Powder synthesis and pressureless sintering. J. Eur. Ceram. Soc. 2008, 28, 2085–2098. [Google Scholar] [CrossRef]
- Calka, A.; Williams, J. Synthesis of Nitrides by Mechanical Alloying. Mater. Sci. Forum 1992, 88–90, 787–794. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Reactive Ball Milling to Fabricate Nanocrystalline Titanium Nitride Powders and Their Subsequent Consolidation Using SPS. J. Mater. Eng. Perform. 2017, 26, 2954–2962. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Sumiyama, K.; Aoki, K.; Suzuki, K. Morphological and structural evolutions of nonequilibrium titanium-nitride alloy powders produced by reactive ball milling. J. Mater. Res. 1992, 7, 888–893. [Google Scholar] [CrossRef]
- Oghenevweta, J.E.; Wexler, D.; Calka, A. Understanding reaction sequences and mechanisms during synthesis of nanocrystalline Ti2N and TiN via magnetically controlled ball milling of Ti in nitrogen. J. Mater. Sci. 2017, 53, 3064–3077. [Google Scholar] [CrossRef] [Green Version]
- Shkoda, O.A.; Lapshin, O.V. Features of mechanochemical synthesis in the system of solid reagent—Active gas. J. Phys. Conf. Ser. 2018, 1115, 042014. [Google Scholar] [CrossRef]
- Smolyakov, V.K.; Lapshin, O.V.; Maksimov, Y.M. Nonisothermal Interaction of Powders with a Reactive Gaseous Medium During Grinding. Combust. Explos. Shock Waves 2003, 39, 659–669. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. (Eds.) Smithells Metals Reference Book, 8th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2004; 2072p. [Google Scholar]
- Levashov, E.A.; Kurbatkina, V.V.; Rogachev, A.S.; Kochetov, N.A. Mechanoactivation of SHS systems and processes. Int. J. Self-Propagating High-Temp. Synth. 2007, 16, 46–50. [Google Scholar] [CrossRef]
- Wriedt, H.A.; Murray, J.L. The N-Ti (Nitrogen-Titanium) system. Bull. Alloy. Phase Diagr. 1987, 8, 378–388. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapshin, O.; Shkoda, O.; Ivanova, O.; Zelepugin, S. Discrete One-Stage Mechanochemical Synthesis of Titanium-Nitride in a High-Energy Mill. Metals 2021, 11, 1743. https://doi.org/10.3390/met11111743
Lapshin O, Shkoda O, Ivanova O, Zelepugin S. Discrete One-Stage Mechanochemical Synthesis of Titanium-Nitride in a High-Energy Mill. Metals. 2021; 11(11):1743. https://doi.org/10.3390/met11111743
Chicago/Turabian StyleLapshin, Oleg, Olga Shkoda, Oksana Ivanova, and Sergey Zelepugin. 2021. "Discrete One-Stage Mechanochemical Synthesis of Titanium-Nitride in a High-Energy Mill" Metals 11, no. 11: 1743. https://doi.org/10.3390/met11111743
APA StyleLapshin, O., Shkoda, O., Ivanova, O., & Zelepugin, S. (2021). Discrete One-Stage Mechanochemical Synthesis of Titanium-Nitride in a High-Energy Mill. Metals, 11(11), 1743. https://doi.org/10.3390/met11111743





