Effects of Fe/Si Stoichiometry on Formation of Fe3Si/FeSi-Al2O3 Composites by Aluminothermic Combustion Synthesis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
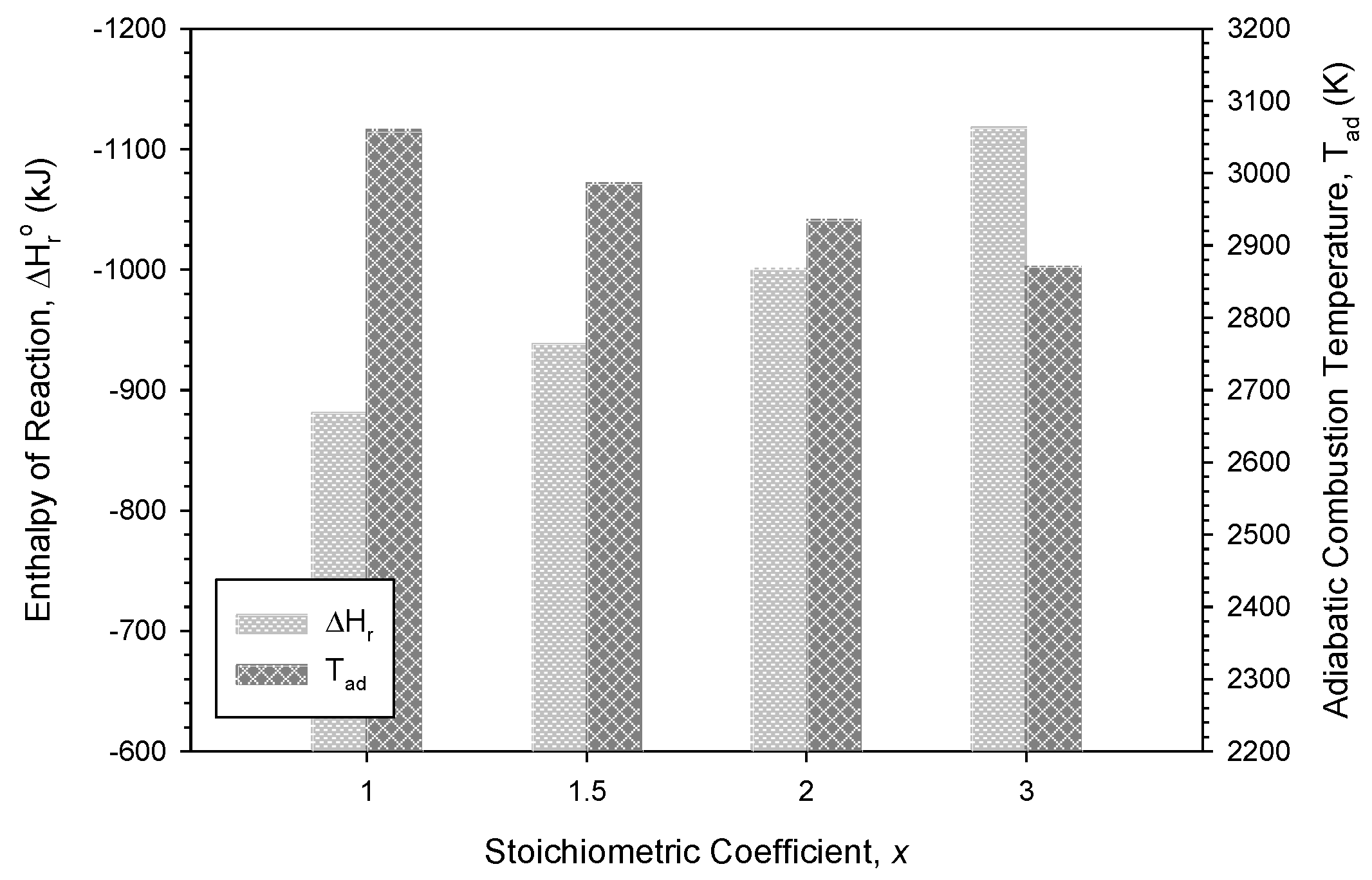
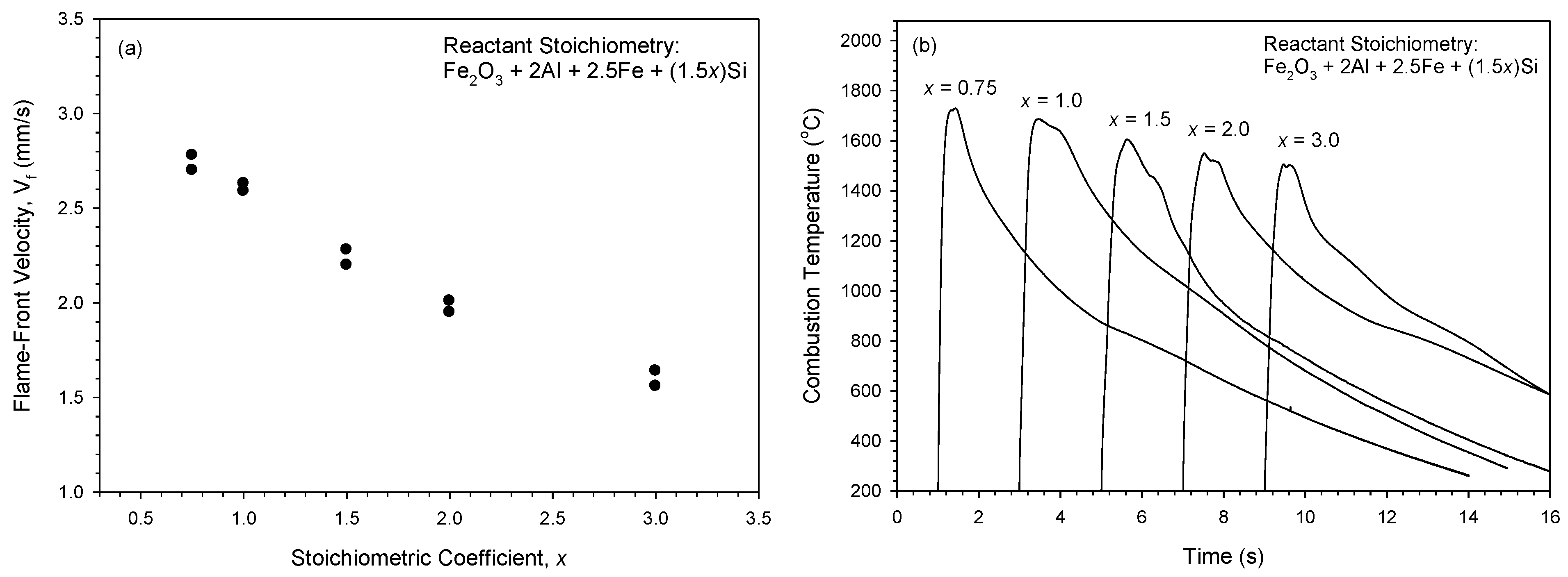
3.1. Combustion Exothermicity and Combustion Wave Kinetics
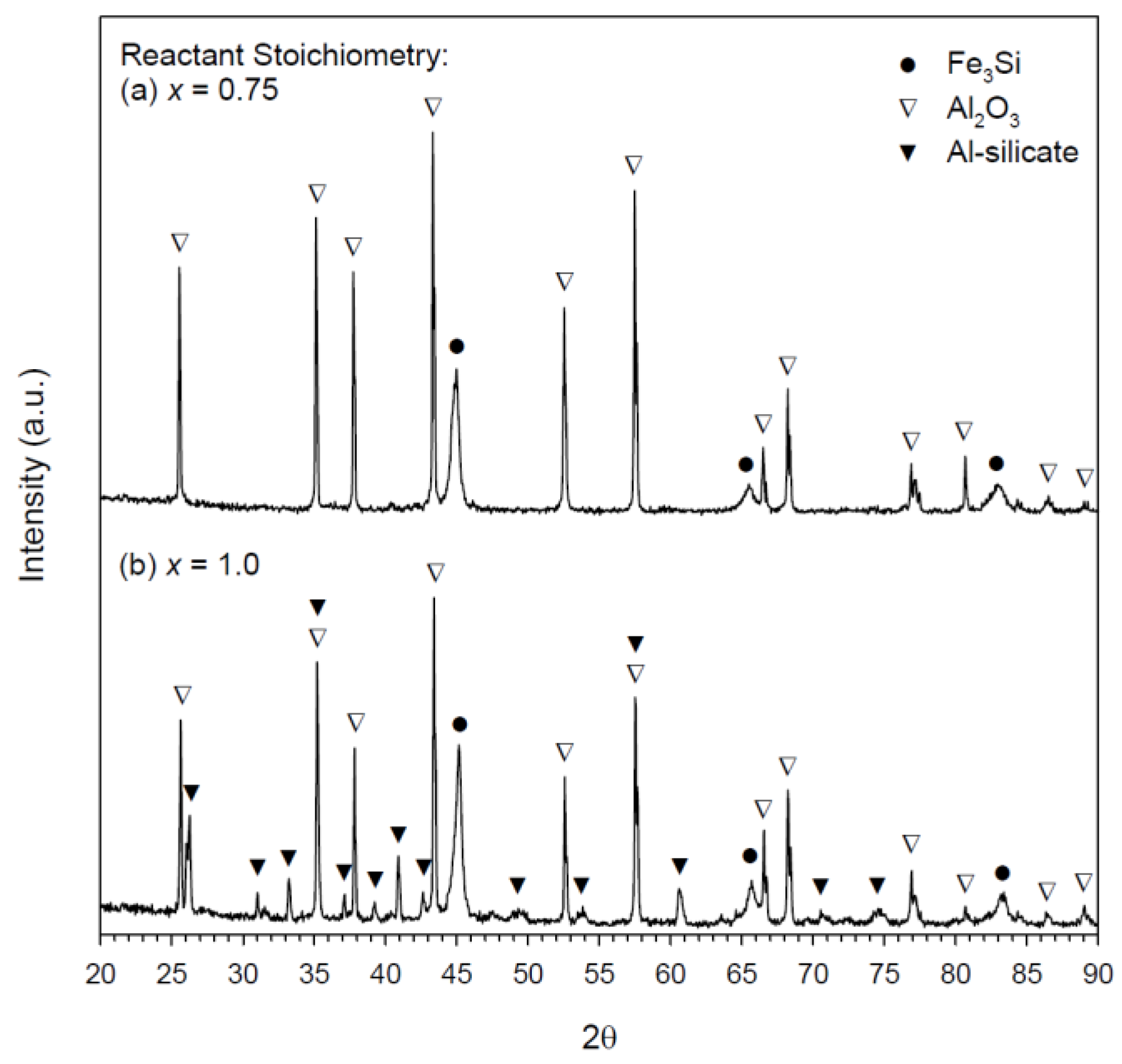
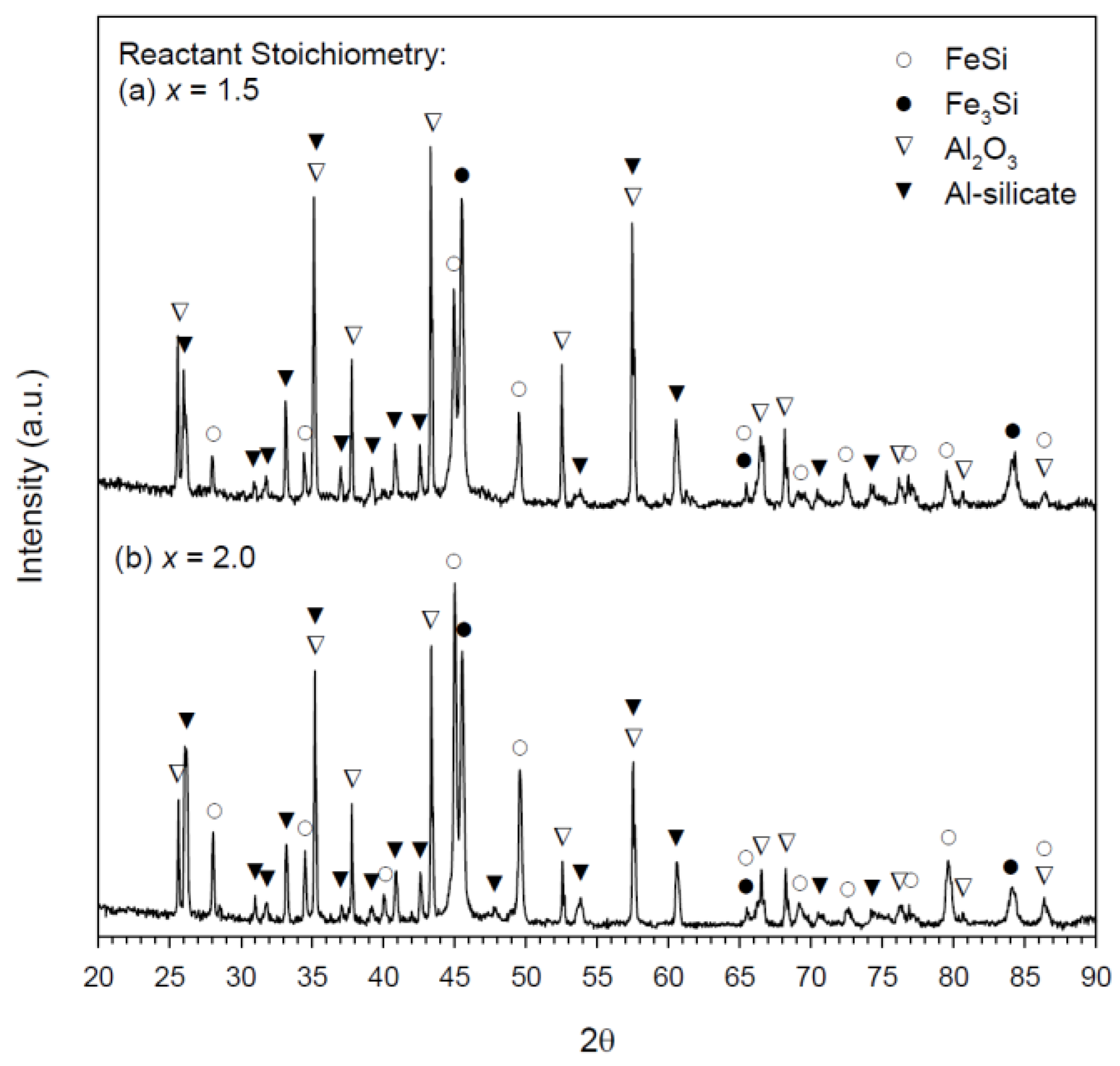
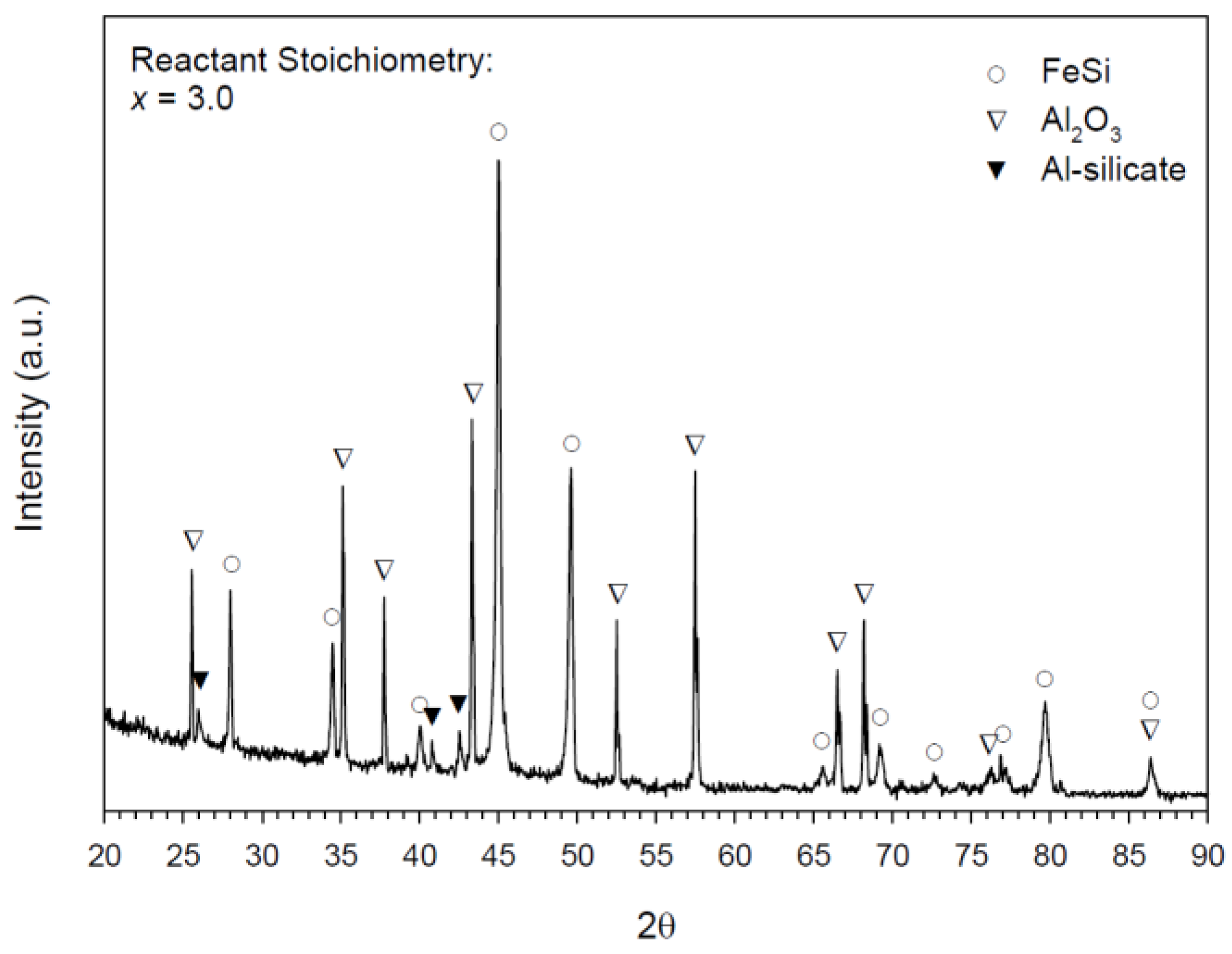
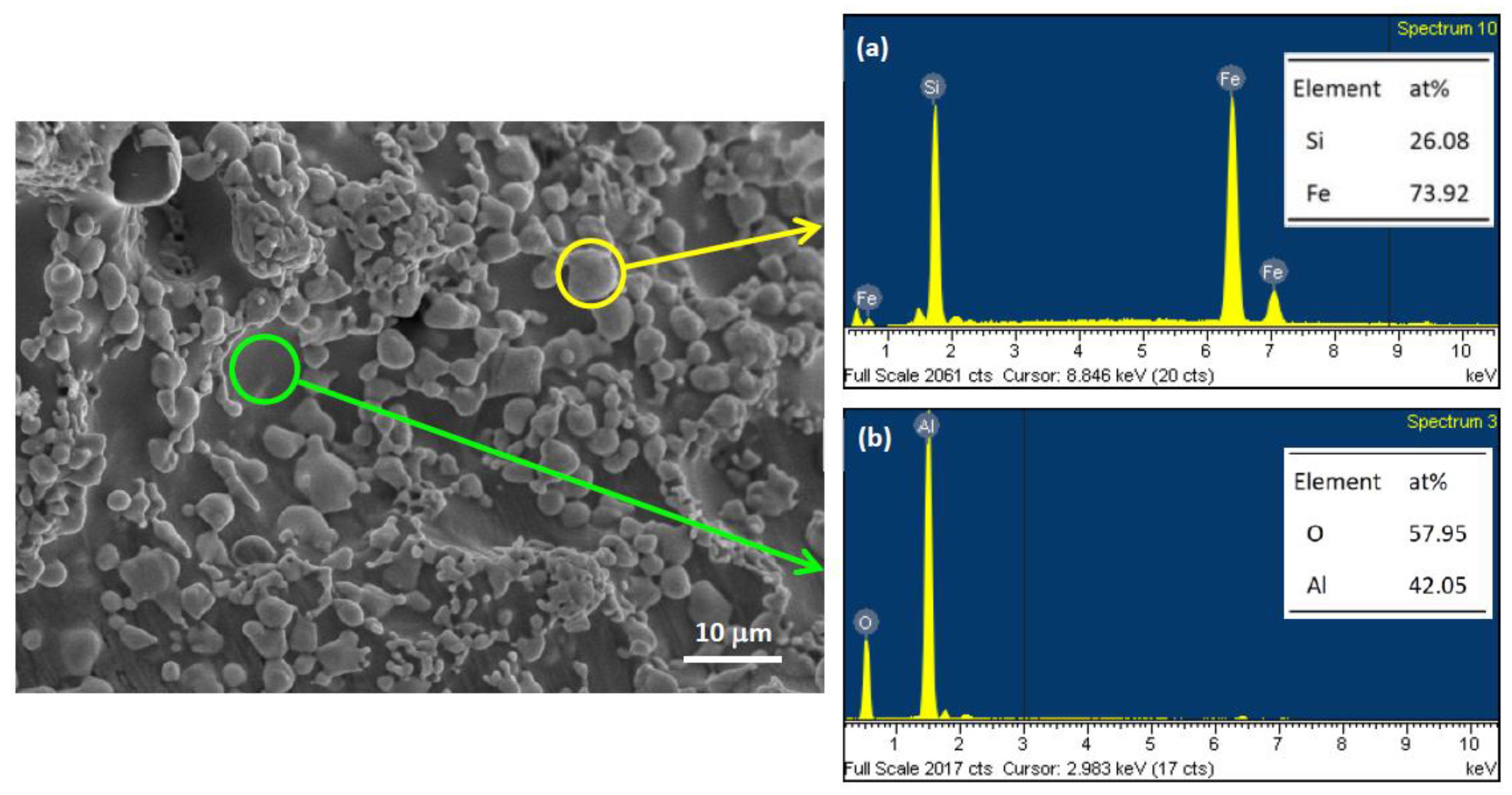
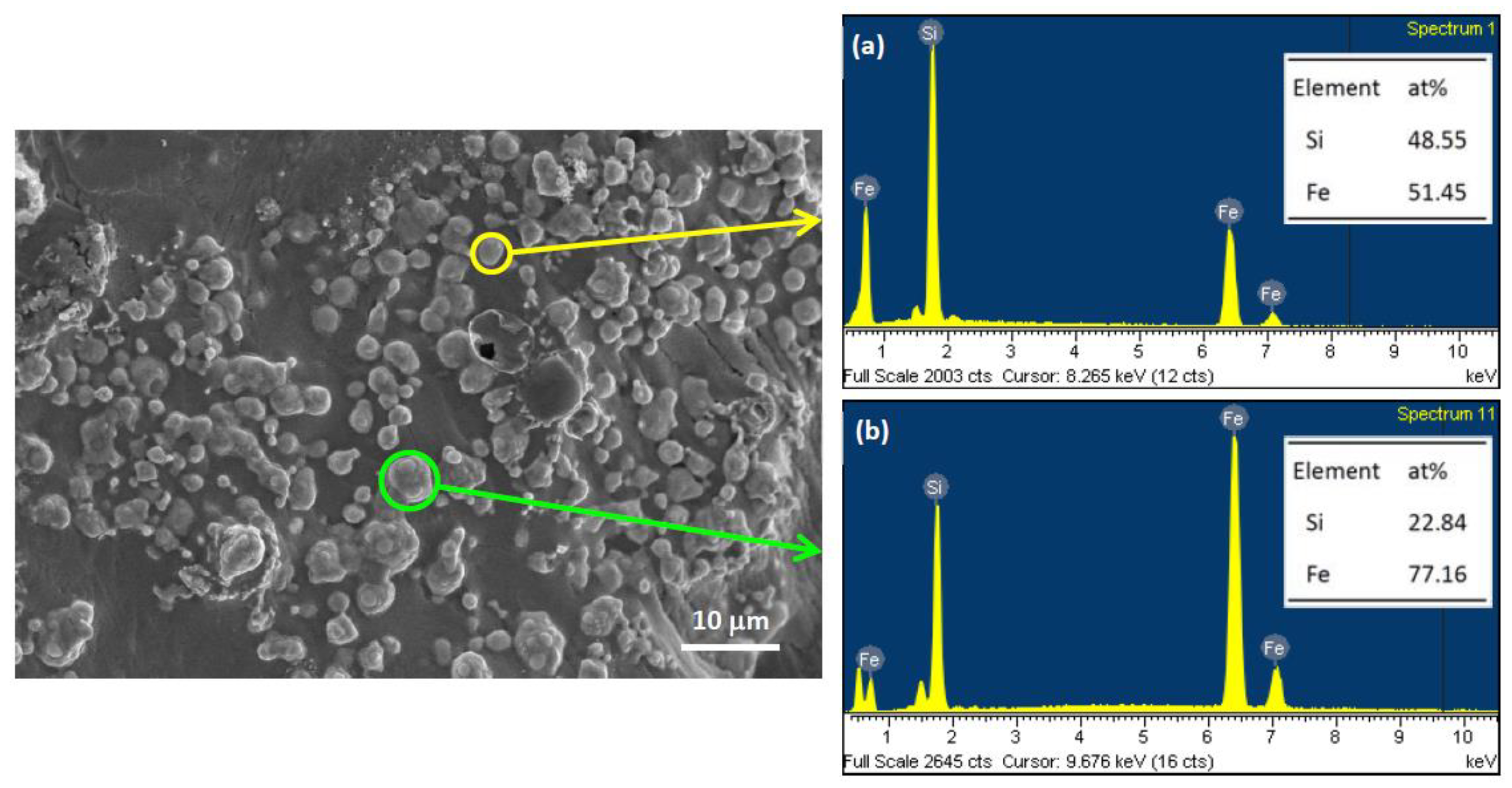
3.2. Composition and Microstructure of Synthesized Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murarka, S.P. Silicide thin films and their applications in microelectronics. Intermetallics 1995, 3, 173–186. [Google Scholar] [CrossRef]
- Petrovic, J.J.; Vasudevan, A.K. Key developments in high temperature structural silicides. Mater. Sci. Eng. A 1999, 261, 1–5. [Google Scholar] [CrossRef]
- Viennois, R.; Tao, X.; Jund, P.; Tedenac, J.C. Stability and thermoelectric properties of transition-metal silicides. J. Electron. Mater. 2011, 40, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Chirkin, A.D.; Lavrenko, V.O.; Talash, V.M. High-temperature and electrochemical oxidation of transition metal silicides. Powder Metall. Metal. Ceram. 2009, 48, 330–345. [Google Scholar] [CrossRef]
- Chen, X.; Liang, C. Transition metal silicides: Fundamentals, preparation and catalytic applications. Catal. Sci. Technol. 2019, 9, 4785–4820. [Google Scholar] [CrossRef]
- Kauss, O.; Obert, S.; Bogomol, I.; Wablat, T.; Siemensmeyer, N.; Naumenko, K.; Krüger, M. Temperature Resistance of Mo3Si: Phase stability, microhardness, and creep properties. Metals 2021, 11, 564. [Google Scholar] [CrossRef]
- Dahal, N.; Chikan, V. Phase-Controlled synthesis of iron silicide (Fe3Si and FeSi2) nanoparticles in solution. Chem. Mater. 2010, 22, 2892–2897. [Google Scholar] [CrossRef]
- Wu, J.; Chong, X.Y.; Jiang, Y.H.; Feng, J. Stability, electronic structure, mechanical and thermodynamic properties of Fe-Si binary compounds. J. Alloys Compd. 2017, 693, 859–870. [Google Scholar] [CrossRef]
- Kubaschewski von Goldbeck, O. Iron-Binary Phase Diagrams; Springer-Verlag: Berlin/Heidelberg, Germany, 1982; pp. 136–139. [Google Scholar]
- Zhang, Y.; Ivey, D.G. Fe3Si formation in Fe-Si diffusion couples. J. Mater. Sci. 1998, 33, 3131–3135. [Google Scholar] [CrossRef]
- Gras, C.; Gaffet, E.; Bernard, F.; Niepce, C.J. Enhancement of self-sustaining reaction by mechanical activation: Case of an Fe-Si system. Mater. Sci. Eng. A 1999, 264, 94–107. [Google Scholar] [CrossRef]
- Gras, C.; Bernsten, N.; Bernard, F.; Gaffet, E. The mechanically activated combustion reaction in the Fe-Si system: In situ time-resolved synchrotron investigations. Intermetallics 2002, 10, 271–282. [Google Scholar] [CrossRef]
- Gras, C.; Zink, N.; Bernard, F.; Gaffet, E. Assisted self-sustaining combustion reaction in the Fe-Si system: Mechanical and chemical activation. Mater. Sci. Eng. A 2007, 456, 270–277. [Google Scholar] [CrossRef]
- Zakeri, M.; Rahimipour, M.R.; Sadrnezhad, S.K. In situ synthesis of FeSi-Al2O3 nanocomposite powder by mechanical alloying. J. Alloys Compd. 2010, 492, 226–230. [Google Scholar] [CrossRef]
- Guan, J.; Chen, X.; Zhang, L.; Wang, J.; Liang, C. Rapid preparation and magnetic properties of Fe3Si-Al2O3 nanocomposite by mechanical alloying and heat treatment. Phys. Status Solidi A 2016, 213, 1585–1591. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Guan, J.; Wang, J.; Liang, C. Thermally induced phase transition and magnetic properties of Fe–FeSi2 with core–shell structure. Phys. Status Solidi A 2013, 210, 2710–2715. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, K.T. Synthesis of FeSi–Al2O3 composites by autowave combustion with metallothermic reduction. Metals 2021, 11, 258. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, K.T. Fabrication of FeSi/α-FeSi2–based composites by metallothermically assisted combustion synthesis. J. Aust. Ceram. Soc. 2021. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Combustion processes that synthesize materials. J. Mater. Process. Technol. 1996, 56, 222–241. [Google Scholar] [CrossRef]
- Bertolino, N.; Anselmi-Tamburini, U.; Maglia, F.; Spinolo, G.; Munir, Z.A. Combustion synthesis of Zr-Si intermetallic compounds. J. Alloys Compd. 1999, 288, 238–248. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H. Combustion synthesis of MoSi2 and MoSi2-Mo5Si3 composites. J. Alloys Compd. 2007, 438, 165–170. [Google Scholar] [CrossRef]
- Yeh, C.L.; Peng, J.A. Combustion synthesis of MoSi2-Al2O3 composites from thermite-based reagents. Metals 2016, 6, 235. [Google Scholar] [CrossRef] [Green Version]
- Maung, S.T.M.; Chanadee, T.; Niyomwas, S. Two reactant systems for self-propagating high-temperature synthesis. J. Aust. Ceram. Soc. 2019, 55, 873–882. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Acker, J.; Bohmhammel, K.; van den Berg, G.J.K.; van Miltenburg, J.C.; Kloc, C. Thermodynamic properties of iron silicides FeSi and α-FeSi2. J. Chem. Thermodyn. 1999, 31, 1523–1536. [Google Scholar] [CrossRef]
- Yeh, C.L.; Lin, J.Z. Combustion synthesis of Cr-Al and Cr-Si intermetallics with Al2O3 additions from Cr2O3-Al and Cr2O3-Al-Si reaction systems. Intermetallics 2013, 33, 126–133. [Google Scholar] [CrossRef]
- Wang, L.L.; Munir, Z.A.; Maximov, Y.M. Thermite reactions: Their utilization in the synthesis and processing of materials. J. Mater. Sci. 1993, 28, 3693–3708. [Google Scholar] [CrossRef]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and properties of mullite—A review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]
- Zaki, Z.I.; Mostafa, N.Y.; Ahmed, Y.M.Z. Synthesis of dense mullite/MoSi2 composite for high temperature applications. Int. J. Refract. Met. Hard Mater. 2014, 45, 23–30. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chou, C.C. Formation of NbB2/mullite composites by combustion synthesis involving metallothermic reduction of Nb2O5 and B2O3. Ceram. Int. 2015, 41, 14592–14596. [Google Scholar] [CrossRef]


| Stoichiometric Coefficients | ||
|---|---|---|
| x | m | n |
| 0.75 | 1.5 | 0 |
| 1.0 | 1.5 | 0 |
| 1.5 | 1.125 | 1.125 |
| 2.0 | 0.75 | 2.25 |
| 3.0 | 0 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Chen, K.-T.; Shieh, T.-H. Effects of Fe/Si Stoichiometry on Formation of Fe3Si/FeSi-Al2O3 Composites by Aluminothermic Combustion Synthesis. Metals 2021, 11, 1709. https://doi.org/10.3390/met11111709
Yeh C-L, Chen K-T, Shieh T-H. Effects of Fe/Si Stoichiometry on Formation of Fe3Si/FeSi-Al2O3 Composites by Aluminothermic Combustion Synthesis. Metals. 2021; 11(11):1709. https://doi.org/10.3390/met11111709
Chicago/Turabian StyleYeh, Chun-Liang, Kuan-Ting Chen, and Tzong-Hann Shieh. 2021. "Effects of Fe/Si Stoichiometry on Formation of Fe3Si/FeSi-Al2O3 Composites by Aluminothermic Combustion Synthesis" Metals 11, no. 11: 1709. https://doi.org/10.3390/met11111709
APA StyleYeh, C.-L., Chen, K.-T., & Shieh, T.-H. (2021). Effects of Fe/Si Stoichiometry on Formation of Fe3Si/FeSi-Al2O3 Composites by Aluminothermic Combustion Synthesis. Metals, 11(11), 1709. https://doi.org/10.3390/met11111709







