Effect of Al Content on Phase Compositions of FeNiCoCrMo0.5Alx High Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
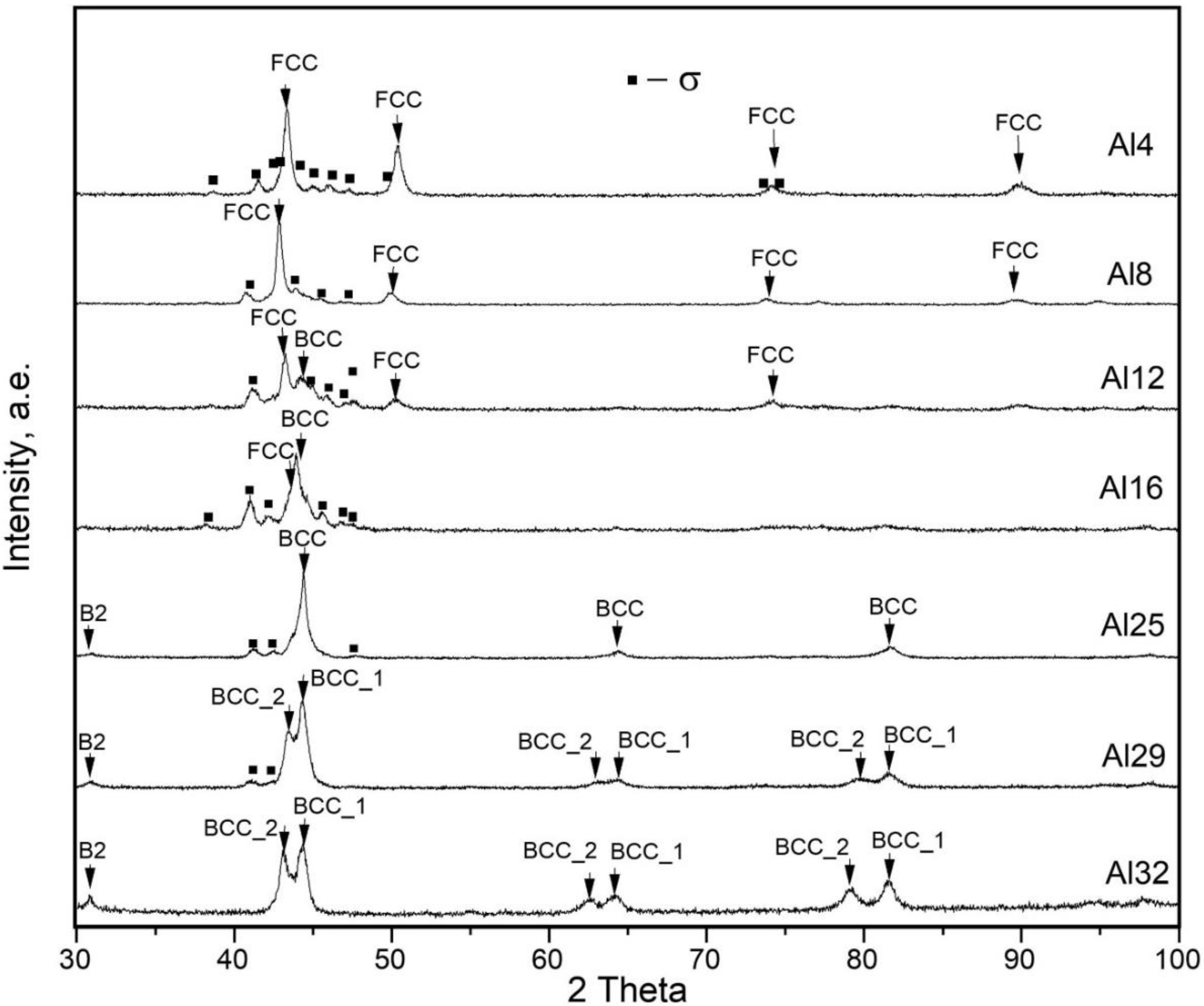
3.1. Phase Compositions (XRD Results)
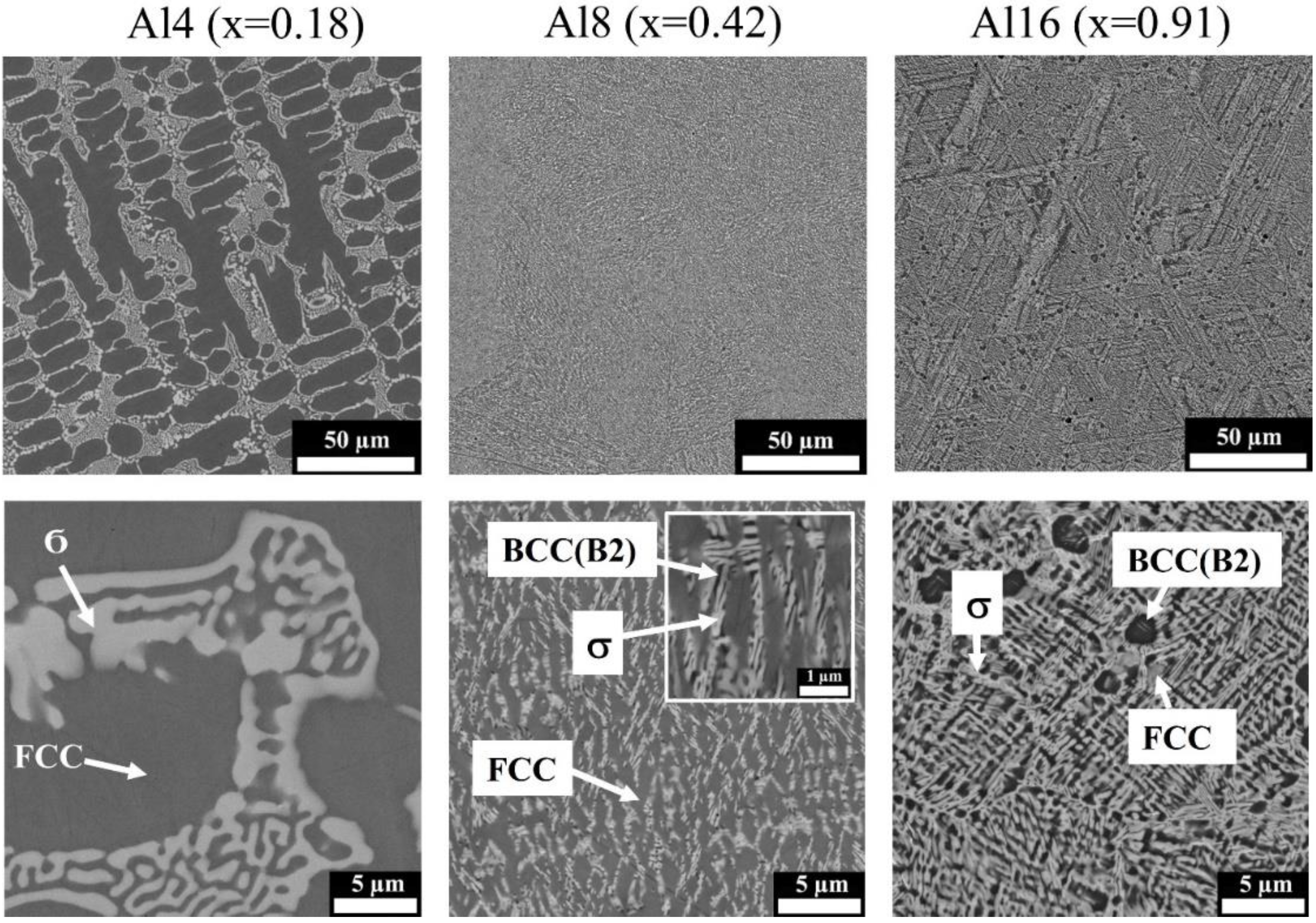
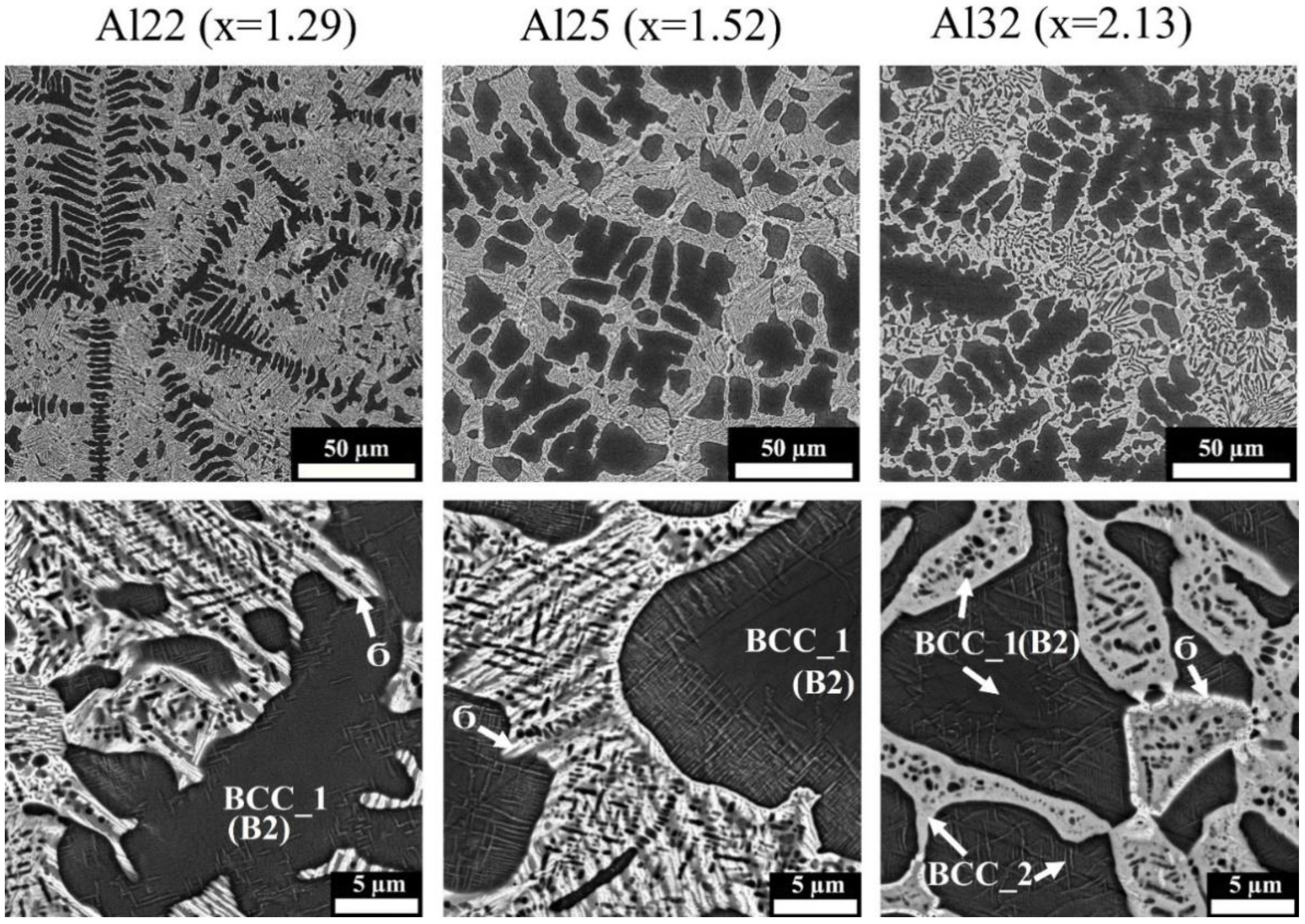
3.2. Microstructure Observation
4. Discussion
5. Properties
6. Conclusions
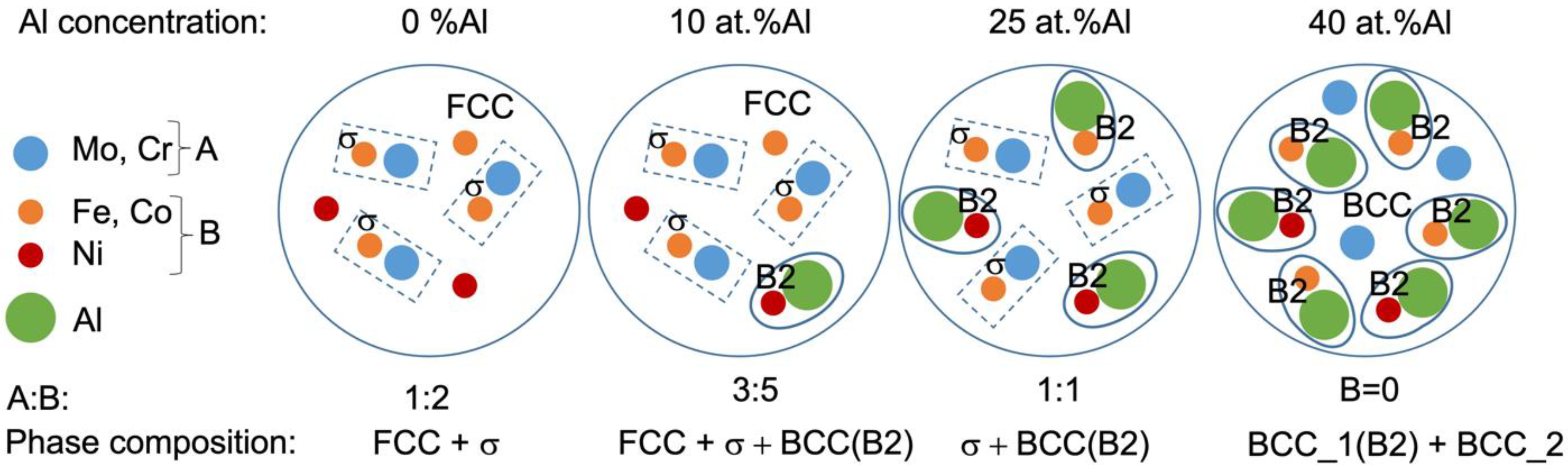
- Al dissolves in an FeCoNiCrMo0.5 FCC solid solution up to 8 at.%. At higher concentrations, Al attracts Ni, removing it from the FCC solid solution and thereby destabilizing it. At an Al content of 22 at.% and higher, corresponding to VEC ≤ 6.87, the FCC phase disappears. The Al and Ni formed the ordered BCC (B2) phase, in which Co and Fe also dissolve, occupying Ni positions.
- Additions of Al to FeCoNiCrMo0.5 strongly influence the fraction of the σ-phase. Despite Al not participating in σ-phase formation, an increase in Al concentration to about 20 at.% led to a growth in the σ-phase fraction to 50%. The increase in σ-phase was caused by an increase in the amount of B2 instead of the FCC solid solution because the solubility of Mo and Cr in B2 was less than that in the FCC solution. A further increase in Al concentration led to an excess of Mo and Cr in the solution, which formed a disordered BCC solid solution. At an Al content of 32%, the alloy consisted of two BCC phases: a solid solution enriched with Cr and Mo and ordered B2 (Ni, Co)Al-based phase.
- The rise in microhardness with an increase in Al concentration to 22 at.% was caused by both an increase in the amount of σ-phase and the formation of B2 instead of a soft FCC solid solution. In a composition with 32 at.% Al, when the σ-phase almost disappeared, the microhardness reached a maximum of 630 HV, which may be explained by both an increase in the fraction of disordered BCC enriched with Mo, and by the increase in Mo concentration in it. Thus, the composition FeCoNiCrMo0.5Al2.13 had the highest hardness of 630 HV and the lowest density of 6.65 g/cm3 among the considered compositions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- López Ríos, M.; Socorro Perdomo, P.P.; Voiculescu, I. Effects of nickel content on the microstructure, microhardness and corrosion behavior of high-entropy AlCoCrFeNix alloys. Sci. Rep. 2020, 10, 21119. [Google Scholar] [CrossRef] [PubMed]
- Shun, T.-T.; Hung, W.-J. Effects of Cr Content on Microstructure and Mechanical Properties of AlCoCrxFeNi High-Entropy Alloy. Adv. Mater. Sci. Eng. 2018, 5826467. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Xiao, D.; Wu, W.; Ni, S.; Song, M. Effect of Fe on Microstructure, Phase Evolution and Mechanical Properties of (AlCoCrFeNi)100-xFex High Entropy Alloys Processed by Spark Plasma Sintering. Intermetallics 2018, 103, 1–11. [Google Scholar] [CrossRef]
- Reverte, E.; Cornide, J.; Lagos, M.A.; Campos, M.; Alvaredo, P. Microstructure Evolution in a Fast and Ultrafast Sintered Non-Equiatomic Al/Cu HEA. Metals 2021, 11, 848. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Völkl, R.; Glatzel, U.; Wanderka, N. Phase separation in equiatomic AlCoCrFeNi high-entropy alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Ozerov, M.S.; Zherebtsov, S.V.; Salishchev, G.A. Second phase formation in the CoCrFeNiMn high entropy alloy after recrystallization annealing. Mater. Lett. 2016, 185, 1–4. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Pradeep, K.G.; Kuběnová, M.; Raabe, D.; Eggeler, G.; George, E.P. Decomposition of the single-phase high-entropy alloy CrMnFeCoNi after prolonged anneals at intermediate temperatures. Acta Mater. 2016, 112, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.C.; Hsu, C.Y.; Tsai, C.W.; Wang, W.R.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. On microstructure and mechanical performance of AlCoCrFeMo0.5Nix high-entropy alloys. Intermetallics 2013, 32, 401–407. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Hao, J.; Yan, Y.; Wang, Q.; Dong, C.; Liaw, P.K. Microstructures and mechanical properties of body-centered-cubic (Al, Ti)0.7(Ni, Co, Fe, Cr)5 high entropy alloys with coherent B2/L21 nanoprecipitation. Mater. Sci. Eng. A 2018, 737, 286–296. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, B.; Li, C.; Wang, Q.; Dong, C.; Liaw, P.K.; Xu, F.; Sun, L. The BCC/B2 Morphologies in AlxNiCoFeCr High-Entropy Alloys. Metals 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Detrois, M.; Antonov, S.; Tin, S. Phase Stability and Thermodynamic Database Validation in a Set of Nonequiatomic Al-Co-Cr-Fe-Nb-Ni High-Entropy Alloys. Intermetallics 2019, 104, 103–112. [Google Scholar] [CrossRef]
- Pickering, E.J.; Stone, H.J.; Jones, N.G. Fine-scale precipitation in the high-entropy alloy Al0.5CrFeCoNiCu. Mater. Sci. Eng. A 2015, 645, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Christofidou, K.A.; McAuliffe, T.P.; Mignanelli, P.M.; Stone, H.J.; Jones, N.G. On the prediction and the formation of the sigma phase in CrMnCoFeNix high entropy alloys. J. Alloys Compd. 2019, 770, 285–293. [Google Scholar] [CrossRef]
- Mukarram, M.; Munir, M.A.; Mujahid, M.; Yaqoob, K. Systematic Development of Eutectic High Entropy Alloys by Thermodynamic Modeling and Experimentation: An Example of the CoCrFeNi-Mo System. Metals 2021, 11, 1484. [Google Scholar] [CrossRef]
- Tokarewicz, M.; Grądzka-Dahlke, M. Review of Recent Research on AlCoCrFeNi High-Entropy Alloy. Metals 2021, 11, 1302. [Google Scholar] [CrossRef]
- Troparevsky, M.C.; Morris, J.; Daene, M.; Wang, Y.; Lupini, A.; Stocks, G.M. Beyond Atomic Sizes and Hume-Rothery Rules: Understanding and Predicting High-Entropy Alloys. JOM 2015, 67, 2350–2363. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Ogura, M.; Fukushima, T.; Zeller, R.; Dederichs, P.H. Structure of the High-Entropy Alloy AlxCrFeCoNi: Fcc versus Bcc. J. Alloys Compd. 2017, 715, 454–459. [Google Scholar] [CrossRef]
- Frommeyer, G.; Rablbauer, G. High temperature resistant intermetallic NiAl-based alloys with refractory metals Cr, Mo, Re. Mater. Res. Soc. Symp. Proc. 2003, 753, BB4.6.1–BB4.6.15. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb Addition on the Microstructure and Properties of AlCoCrFeNi High-Entropy Alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of Vanadium Addition on the Microstructure and Properties of AlCoCrFeNi High Entropy Alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Pickering, E.J.; Muñoz-Moreno, R.; Stone, H.J.; Jones, N.G. Precipitation in the equiatomic high-entropy alloy CrMnFeCoNi. Scr. Mater. 2016, 113, 106–109. [Google Scholar] [CrossRef]
- Hall, E.O.; Algie, S.H. The sigma phase. Int. Mater. Rev. 1966, 11, 61–88. [Google Scholar] [CrossRef]
- Lee, C.; Lee, Y.; Lee, C.; Hong, S. Precipitation behavior of the sigma phase with Ni and Mn content variations in superaustenitic stainless steel weld metal. Mater. Charact. 2018, 144, 148–154. [Google Scholar] [CrossRef]
- Nelson, J.B.; Riley, D.P. An Experimental Investigation of Extrapolation Methods in the Derivation of Accurate Unit Cell Dimensions of Crystals. Proc. Phys. Soc. 1945, 57, 160–177. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]






| Alloy Designation | Atomic Concentration, % | ΔHmix (kJmol−1) | ΔSmix (JK−1mol−1) | δ (%) | VEC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Co | Cr | Mo | Al | |||||
| Al0 (x = 0) | 21.98 | 21.97 | 21.84 | 22.22 | 11.99 | - | −4.39 | 13.18 | 3.63 | 7.97 |
| Al4 (x = 0.18) | 21.24 | 21.35 | 21.15 | 20.82 | 11.62 | 3.82 | −6.14 | 14.03 | 4.32 | 7.799 |
| Al8 (x = 0.42) | 20.09 | 20.16 | 20.01 | 20.33 | 10.98 | 8.42 | −8.04 | 14.47 | 4.93 | 7.555 |
| Al12 (x = 0.64) | 19.22 | 19.31 | 19.15 | 19.38 | 10.53 | 12.41 | −9.42 | 14.64 | 5.35 | 7.359 |
| Al16 (x = 0.91) | 18.40 | 18.45 | 18.32 | 18.17 | 10.07 | 16.69 | −10.98 | 14.74 | 5.71 | 7.161 |
| Al22 (x = 1.29) | 17.21 | 17.28 | 17.14 | 16.86 | 9.43 | 22.08 | −12.47 | 14.67 | 6.06 | 6.887 |
| Al25 (x = 1.52) | 16.56 | 16.54 | 16.55 | 16.55 | 8.65 | 25.15 | −13.2 | 14.54 | 6.21 | 6.735 |
| Al29 (x = 1.84) | 15.71 | 15.68 | 15.73 | 15.7 | 8.26 | 28.92 | −14.0 | 14.34 | 6.37 | 6.546 |
| Al32 (x = 2.13) | 14.98 | 15.03 | 14.99 | 15.01 | 8.04 | 31.95 | −14.43 | 14.17 | 6.44 | 6.392 |
| Alloy | Phase/Lattice Parameter, Å |
|---|---|
| Al0 | FCC/3.5960; σ-phase |
| Al4 | FCC/3.6158; σ-phase |
| Al8 | FCC/3.6248; BCC/2.8908; σ-phase |
| Al12 | FCC/3.6151; BCC/2.8893; σ-phase |
| Al16 | FCC/3.5885; BCC/2.8880; σ-phase |
| Al22 | BCC(B2)/2.8823; σ-phase |
| Al25 | BCC(B2)/2.8852; BCC_2/2.9098; σ-phase |
| Al29 | BCC_1(B2)/2.8863; BCC_2/2.9434; σ-phase |
| Al32 | BCC_1(B2)/2.8893; BCC_2/2.9636 |
| Alloy | Phase | Atomic Concentration, % | |||||
|---|---|---|---|---|---|---|---|
| Fe | Ni | Co | Cr | Mo | Al | ||
| Al4 | FCC | 22 | 22 | 22 | 21 | 9 | 4 |
| σ | 18 | 14 | 18 | 25 | 21 | 4 | |
| Al8 | FCC | 21 | 22 | 21 | 19 | 8 | 8 |
| BCC(B2) * | 18 | 24 | 20 | 17 | 7 | 14 | |
| σ * | 19 | 17 | 19 | 23 | 15 | 6 | |
| Al12 | FCC | 24 | 19 | 22 | 21 | 7 | 8 |
| BCC(B2) | 17 | 25 | 19 | 16 | 5 | 18 | |
| σ | 19 | 16 | 19 | 20 | 16 | 9 | |
| Al16 | BCC(B2) | 15 | 28 | 17 | 14 | 4 | 21 |
| σ | 20 | 16 | 19 | 22 | 13 | 11 | |
| Al22 | BCC(B2) | 13 | 24 | 18 | 10 | 3 | 31 |
| Interdendritic | 21 | 11 | 17 | 26 | 13 | 11 | |
| Al25 | BCC_1(B2) | 14 | 20 | 18 | 13 | 5 | 30 |
| Interdendritic | 19 | 14 | 18 | 20 | 10 | 18 | |
| Al29 | BCC_1(B2) | 14 | 20 | 18 | 12 | 5 | 32 |
| Interdendritic | 22 | 7 | 13 | 26 | 15 | 16 | |
| Al32 | BCC_1(B2) | 13 | 19 | 17 | 9 | 5 | 37 |
| BCC_2 | 20 | 6 | 11 | 24 | 20 | 19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semikolenov, A.; Shalnova, S.; Klinkov, V.; Andreeva, V.; Salynova, M.; Larionova, T.; Tolochko, O. Effect of Al Content on Phase Compositions of FeNiCoCrMo0.5Alx High Entropy Alloy. Metals 2021, 11, 1734. https://doi.org/10.3390/met11111734
Semikolenov A, Shalnova S, Klinkov V, Andreeva V, Salynova M, Larionova T, Tolochko O. Effect of Al Content on Phase Compositions of FeNiCoCrMo0.5Alx High Entropy Alloy. Metals. 2021; 11(11):1734. https://doi.org/10.3390/met11111734
Chicago/Turabian StyleSemikolenov, Anton, Svetlana Shalnova, Victor Klinkov, Valentina Andreeva, Maria Salynova, Tatiana Larionova, and Oleg Tolochko. 2021. "Effect of Al Content on Phase Compositions of FeNiCoCrMo0.5Alx High Entropy Alloy" Metals 11, no. 11: 1734. https://doi.org/10.3390/met11111734
APA StyleSemikolenov, A., Shalnova, S., Klinkov, V., Andreeva, V., Salynova, M., Larionova, T., & Tolochko, O. (2021). Effect of Al Content on Phase Compositions of FeNiCoCrMo0.5Alx High Entropy Alloy. Metals, 11(11), 1734. https://doi.org/10.3390/met11111734







