1. Introduction
The development of modern industry, the creation of digital infrastructure and the widespread introduction of digital devices have given rise to the development of “smart” devices and technologies. In new products and assemblies, almost every material used is tailored with task-specific properties. As a result of this, functional materials have become widespread, exhibiting specific characteristics which depend on the properties required from them. Composite and gradient materials, in which the characteristics are not uniformly distributed through the volume, can provide the necessary properties in the best zones for this.
One of the groups of functional materials is shape memory alloys (SMA), which open up new possibilities in modern science and technology. With their appearance, it became possible to set in motion mechanisms and structural elements without the use of specialized actuators, motors and other devices [
1]. Nitinol (NiTi) is an SMA of titanium with nickel. This alloy was first patented in 1965 by W. Buger and F. Wang of the United States Naval Laboratory [
2]. At the end of the 1960s, the first practical application of this NiTi alloy was in aircraft construction to form a coupling in the hydraulic system of the F-14 fighter jets [
3]. Further study and research of NiTi alloys made it possible to partially expand their applicability, based on their ability to exhibit the shape memory effect, which is activated at certain temperatures and develops a certain force.
Depending on the nickel content, nitinol may show either shape memory effect (SME) and super elasticity (SE) [
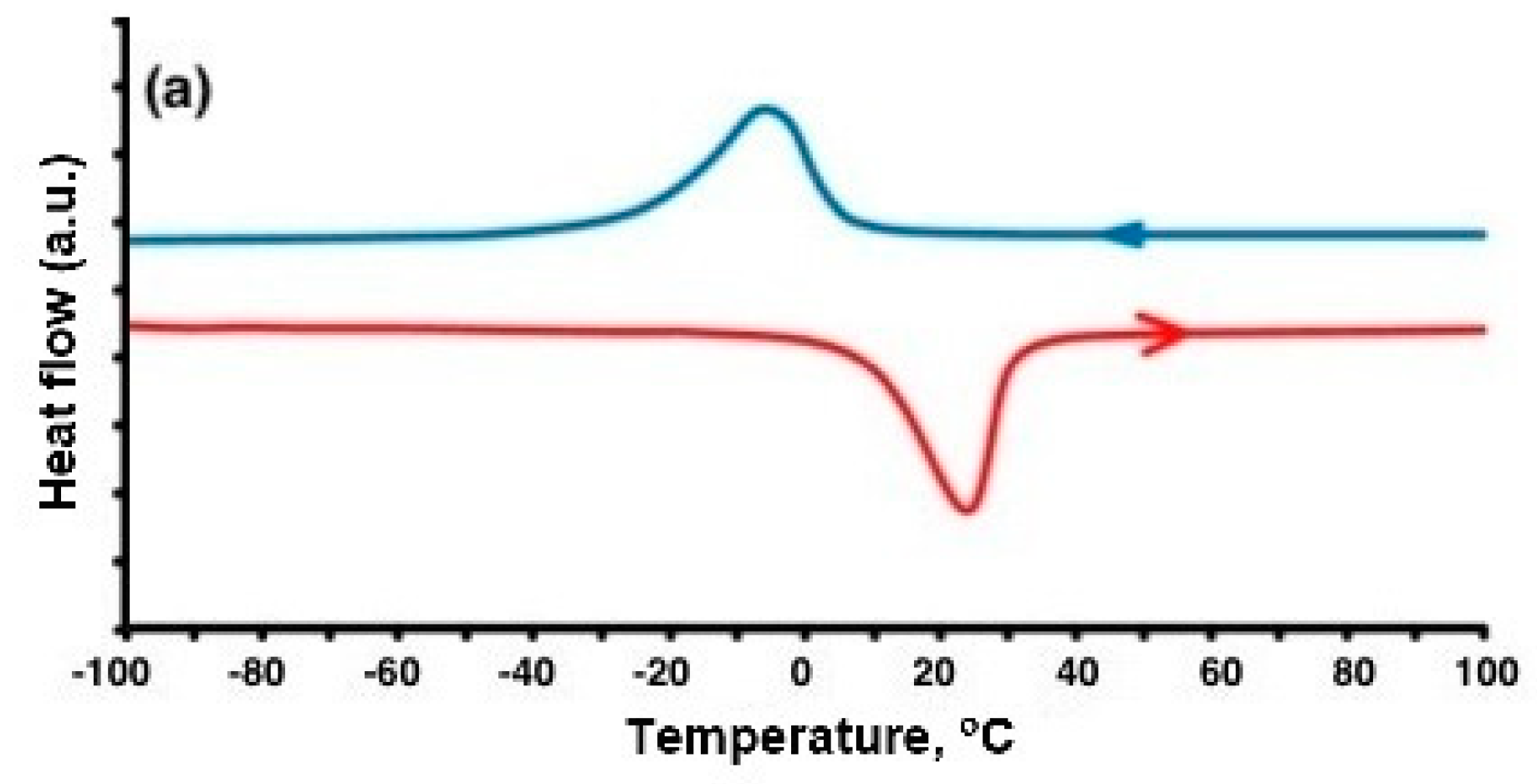
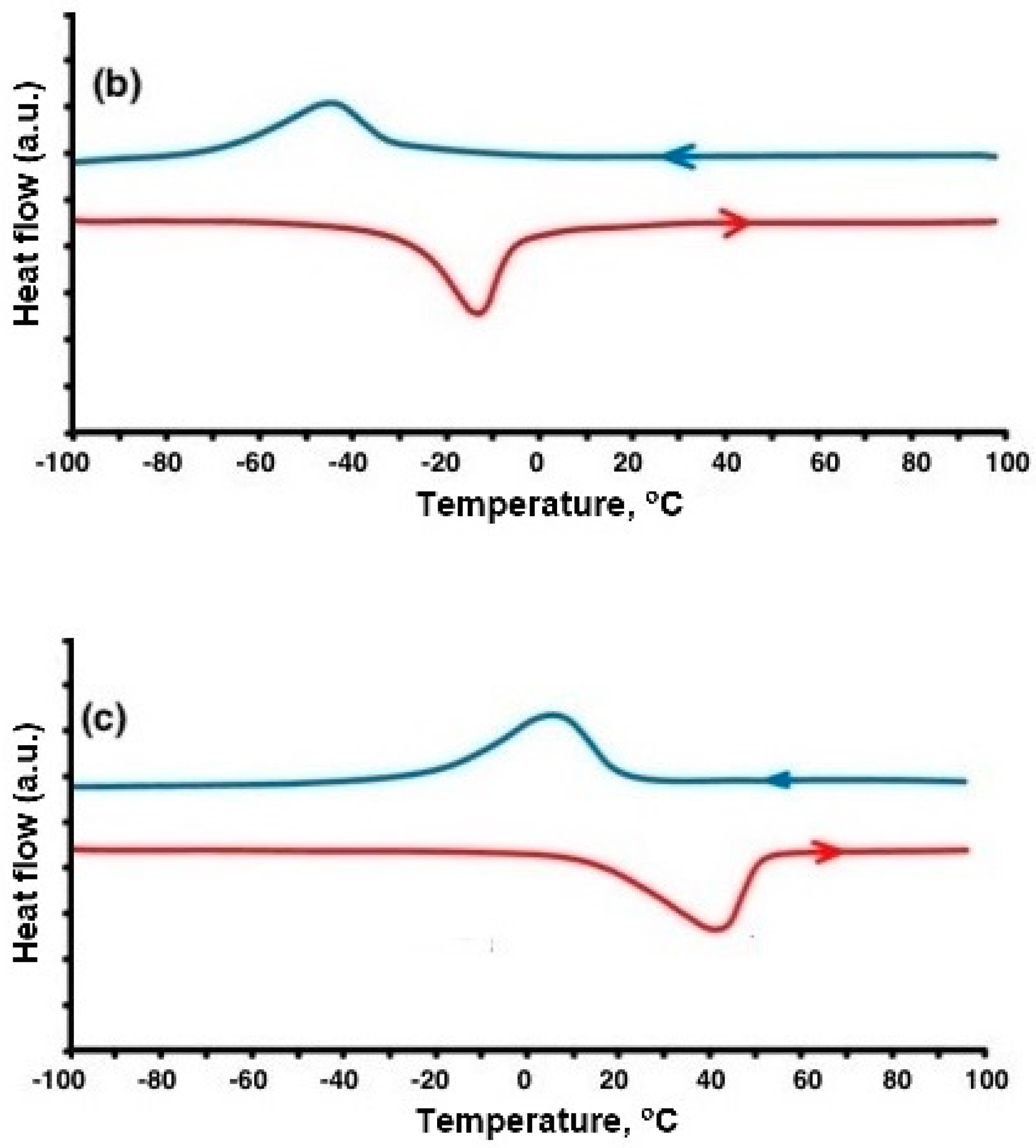
4]. Equiatomic nitinol (50 at.% Ni) exhibits SME at room temperature. In the initial state (at a low temperature and in a relaxed state), nitinol consists of twinned martensite. When a load is applied, a reorientation called detwinning begins. During unloading, this structure is retained, as well as the resulting deformation. When the material is heated above the temperature of the onset of austenite transformation (As), the transformation of martensite into austenite begins, which ends at a austenite finish temperature (Af). This restores the original shape of the product [
5]. At temperatures above Af, the effect of superelasticity appears. With an increase in the nickel content, the values of the transformation temperatures decrease, and at some values of the content, the alloy can exhibit the effect of super elasticity at room temperature [
4]. Currently, alloys of the NiTi system with SME or SE are used in the aerospace industry [
6], medicine [
7], there are various developments and applications in the automotive industry and other devices [
8,
9]. NiTi alloys can play various roles including temperature sensors, thermo-force actuators, thermomechanical connectors, various implants with SME, stents and tightening bone fixators [
4,
5,
10].
Despite a certain share of NiTi system alloys in industry, their use is still extremely limited. This is primarily due to the high cost and complexity of production, and the serious and complex influence of the processing parameters on the final alloy properties (including how it performs). These factors create certain difficulties in obtaining NiTi products in complex shapes when classical processing methods are used [
11,
12].
The emergence and development of additive manufacturing technologies has changed the approach in manufacturing NiTi system alloys and the products made from them [
13]. Additive technologies have made it possible to manufacture products of complex shape without additional machining from powders of various alloys. Concerning the manufacture of products from NiTi system powders, the term 4D printing is used [
14]. According to [
15], the term 4D printing relates to the technique of using additive manufacturing on stimulus-responsive active materials which results in a physical or chemical change in their composition with time [
16,
17,
18,
19,
20].
For an actuator based on the SME, its behavior greatly depends on the geometric shape of the main element, and its chemical composition. There are several works [
21,
22,
23,
24,
25,
26,
27,
28,
29], which noted a change in the chemical composition of the NiTi alloys during laser processing. This was caused by a decrease in the concentration of nickel due to evaporation. Under present methods, routes to carefully control the chemical composition of nitinol alloy are yet to be found. There are several studies devoted to the study of the influence of the SLM process parameters on the composition of elements in the NiTi alloy.
There have been studies testing the influence of various parameter sets with different single parameters but same volume energy density [
23,
28,
29]. In paper [
23] it was shown that the Ni content in all of the NiTi fabricated samples was 55.3–55.4 wt%, which showed no obvious difference when compared with the starting NiTi powders (55.4 wt%). At the same time, in [
28,
29] a significant difference was found between the chemical composition of the samples with same energy density.
In [
24] investigations were carried out of the dependence of the composition and properties of two NiTi alloys depending on the energy density of the parameter set used. It was found that the temperatures of phase transformations increase almost monotonically with the volumetric energy density used for printing due to the Ni evaporation during the melting process. Consequently, it is shown that it is possible to fine-tune the chemical composition and functional properties of NiTi alloys with a high nickel content by varying the processing parameters, which was implemented in [
25]. However, with an increase in the energy density to a certain value, the formation of keyhole porosity begins, which reduces the total density of the samples and increases the number of defects [
30]. This limits the ability to control the composition of the samples.
Thus, the purpose of this work was to study the effect of selective laser melting (SLM) process parameters on changes in the Ni content and density of the NiTi alloys with different initial chemical compositions. Also, determination of the possibility to effectively control the chemical composition of samples without using high-energy parameter sets, which lead to the formation of defects in the samples.
2. Materials and Methods
Commercial powders of NiTi alloys were gas atomized and supplied by CNPC Powder (CNPC Powder, Shanghai, China) and were used as the initial material. For this study, a sample with a near-equiatomic composition, and a sample with a high nickel content were selected, designated as NiTi 50.0 and NiTi 50.7, respectively (composition of initial powders 50.3% of Ni for NiTi 50.0 and 51.02 for NiTi 50.7). The particle size distribution was measured with a Fritsch Analysette 22 Laser Particle Sizer (Fritsch GmbH, Idar-Oberstein, Germany). The particle sizes were d10 = 22.4 µm, d50 = 39.4 µm, d90 = 66.3 µm for 50.0 Ni, and d10 = 26.7 µm, d50 = 49.6 µm, d90 = 84.6 µm for 50.7 Ni. Samples were prepared using an Aconity3D MIDI (Aconity3D GmbH, Herzogenrath, Germany) selective laser melting machine. The machine is equipped with a laser with a power of one kilowatt. To study the chemical composition of powders and compact samples, a TESCAN Mira 3 LMU scanning electron microscope (TESCAN, Brno, Czech Republic) with an EDX X-max 80 energy dispersive spectrometer manufactured by Oxford Instruments are used. Measurements were carried out over 10 areas of the sample microsection, after which the average value and standard deviation of the composition were calculated. The density of the samples was carried out using the Archimedes method. Two cubic samples were made for each parameter set. Each sample was tested three times, and the average of all measurements was taken as the density value of the sample. Sample microstructure was studied on pre-polished and etched microsections of the specimens using a Leica DMI 5000 optical microscope (Leica Microsystems, Wetzlar, Germany). Differential scanning calorimetry (DSC) measurements were used to study the transformation temperatures.
To study the process of selective laser melting and the dependence of the density and chemical composition of the manufactured samples on the values of process parameters, the values presented in
Table 1 were used. A range of laser power values from 175 to 225 watts with a step of 25 watts and a range scanning speeds from 800 to 1050 mm/s with a step of 125 mm/s were selected. Laser beam diameter for all parameter sets was ~80 µm. When the parameter sets were formed, the power and speed of laser radiation were simultaneously increased so as to keep the volumetric energy density constant (60 J/mm
3). In addition to the three-parameter sets, there were two parameter sets with constant laser power were used, differing only in the speed of laser radiation. For them, the average value (200 watts) was chosen as the power, and the extreme values (for the A4 mode—1050 mm/s, for the A5 mode—800 mm/s) were chosen as the laser scanning speed. The volumetric energy density at the selected parameters of power and scanning speed, and the distance between the laser hatches equal to 0.120 mm, is in the range of ~53–69 J/mm
3.
Based on these parameter sets, further sets were selected, to investigate their influence on the chemical composition of samples. The selected parameter sets are shown in
Table 2.
Parameter sets were selected in such a way as to conduct a study of the bearing of various parameters on the SLM process and to identify those that had the greatest impact. In parameter sets 1, 2, 5, and 6 the scanning speed of the laser beam was changed. In parameter sets 1 and 3, the laser radiation power was changed. Parameter sets 1 and 4 differ from each other in that they use different distances between the individual hatches.
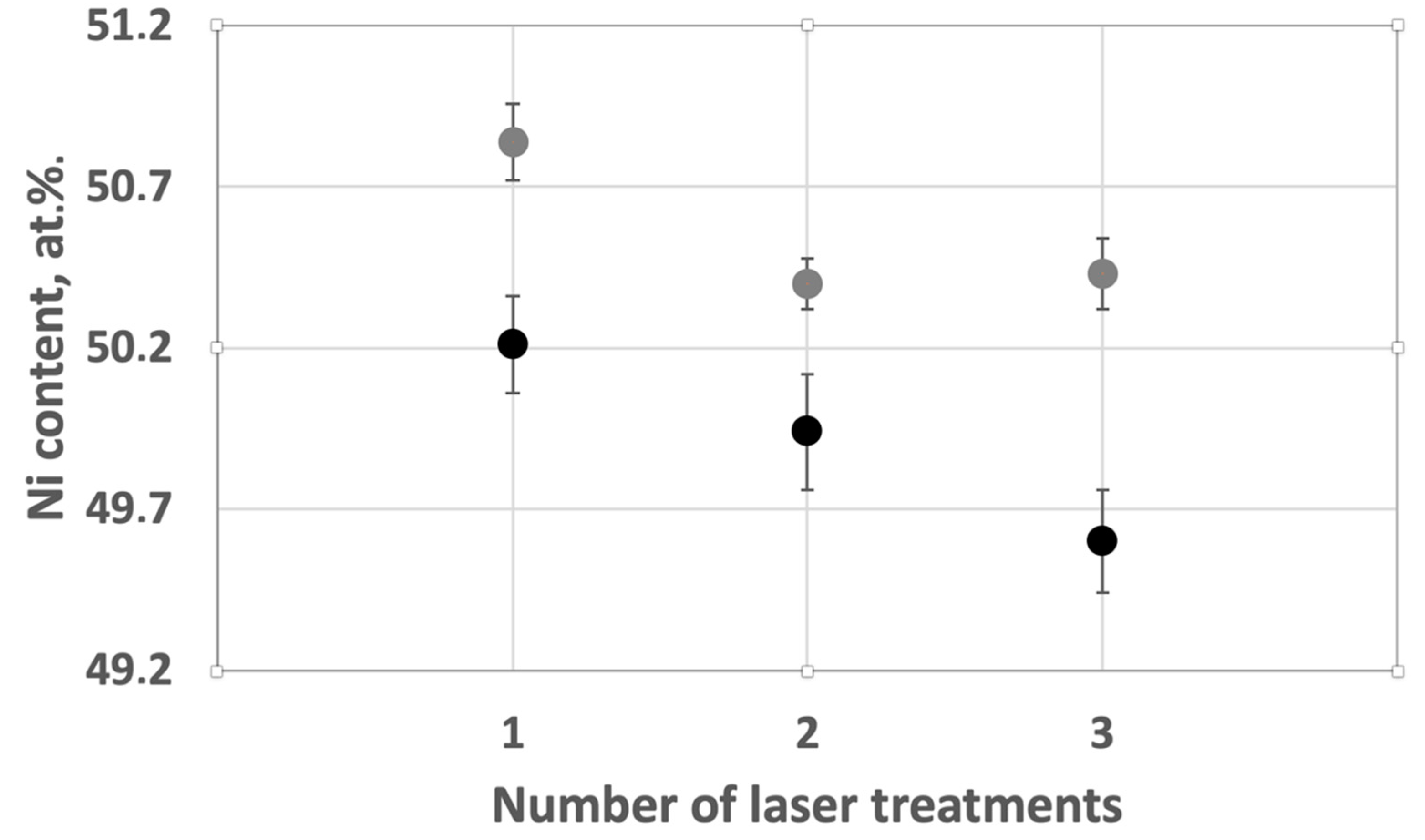
To investigate the possibility of changing the energy density for each layer, without using high-energy process parameters, a study of multiple laser processing was carried out. The essence of this method was that after the main laser treatment was carried out, reprocessing was started according to the same parameters and according to the same strategy. Thus, for the first time, the main melting of the powder material and the formation of a continuous layer occurred. During the second time, the laser treatment went along the previously-formed layer with no additional powder material applied. The process parameters used to study the effect of laser treatment are shown in
Table 3. Parameter set 6 with the lowest value of VED and parameter set 4 with the highest non-destructive VED value were selected. The VED value of parameter set 6 allows 3 repeated scans, but the VED value of parameter set 4 is too high and overheating of the sample surface occurs during triple scanning.
Parameter set 4_2 is a modification of 4, but with double laser treatment. Similarly, parameter set 6_2 is double processing of 6, and 6_3 is a parameter set 6 with triple processing.