Experimental Measurement of Vacuum Evaporation of Aluminum in Ti-Al, V-Al, Ti6Al4V Alloys by Electron Beam
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
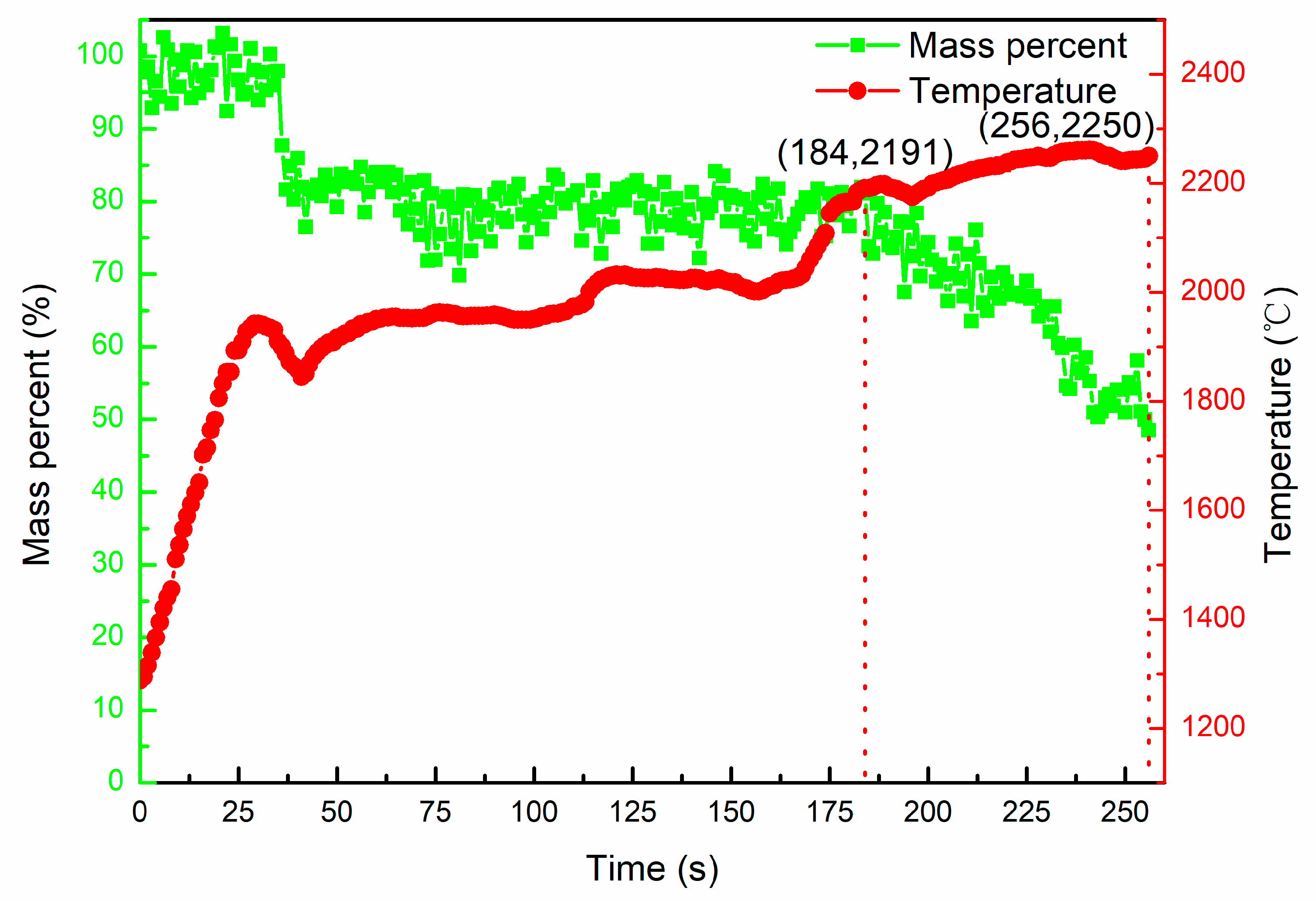
- The Al in Ti-Al alloy started to evaporate at 1753 ± 10 °C, and after heating for 500 s in the temperature range of 1750~1957 °C, the evaporative loss of aluminum was 20.6 wt.%.
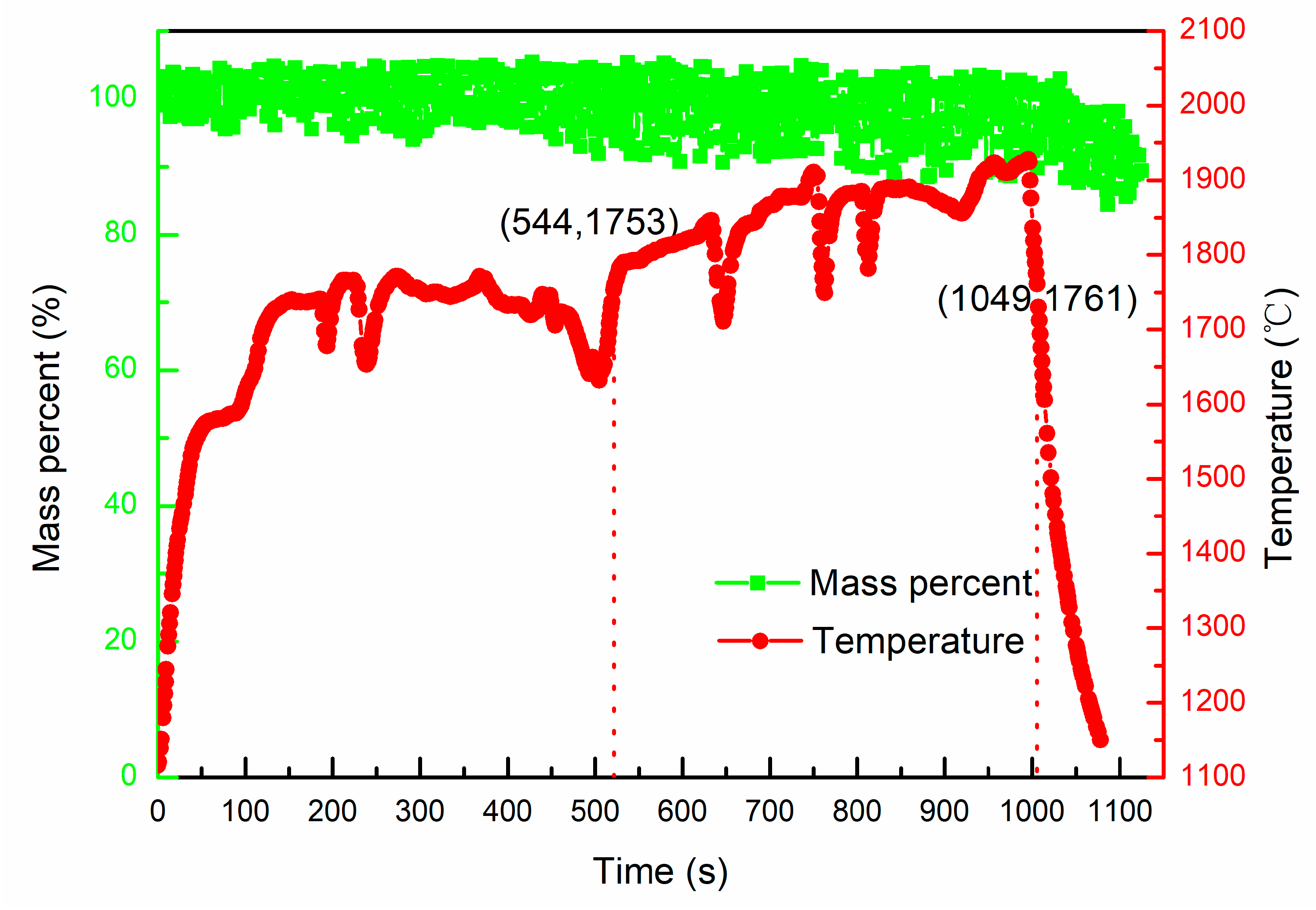
- The Al in V-Al alloy started to evaporate at 1913 ± 10 °C, and the evaporation loss of aluminum was 26.4 wt.% after heating for 543 s in the temperature range of 1893~2050 °C.
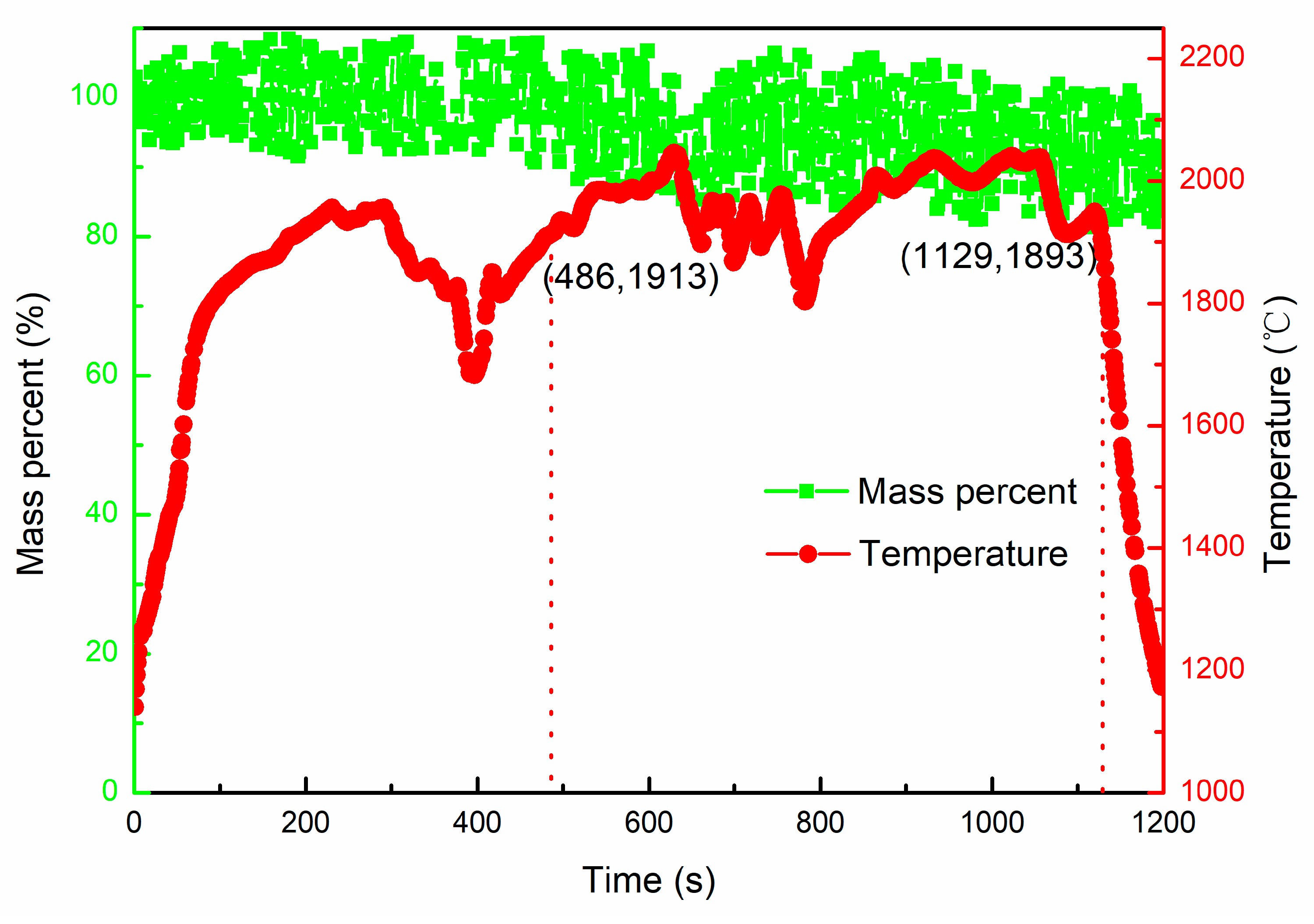
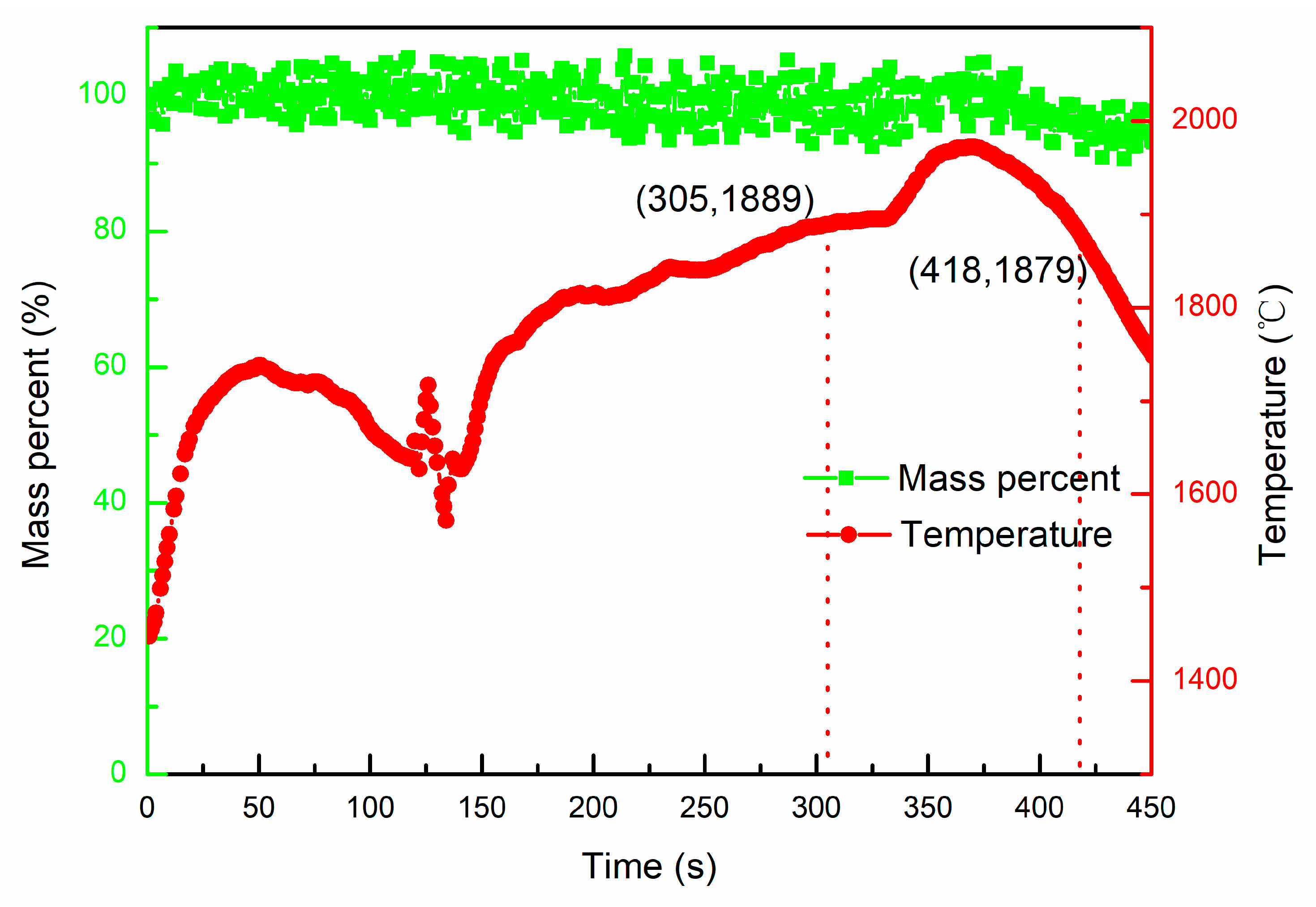
- The Al in TC4 alloy started to evaporate at 1889 ± 10 °C, and 79.6 wt.% aluminum was lost after heating for 113 s in the temperature range of 1879~1989 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, Y.; You, X.G.; Li, J.Y.; Shi, S. Application of electron beam technology in superalloy. Cailiao Gongcheng/J. Mater. Eng. 2015, 43. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Y.; You, X.; Cui, H.; Li, P.; Wang, Y.; You, Q. The denitrification behavior during electron beam smelting of FGH4096 alloy. Vacuum 2021, 189, 110212. [Google Scholar] [CrossRef]
- Liu, H.; Shi, S.; You, Q.; Zhao, L.; Tan, Y. Analysis on elemental volatilization behavior of titanium alloys during electron beam smelting. Vacuum 2018, 157, 395–401. [Google Scholar] [CrossRef]
- Du, B.; Tang, Z.H.; Zhang Z bin Zhou, W.; Wang, F.Q.; Cao, S.L. Melting technology of electron beam cold hearth melting of TC4 titanium alloy. Zhongguo Youse Jinshu Xuebao/Chin. J. Nonferrous Met. 2020, 30. [Google Scholar] [CrossRef]
- Veselovskij, A.N.; Shvyrkov, D.V.; Zorin, G.V. Experience of Titanium Sheet Fabrication from the Billet of Electron-Beam Smelting. Tsvetnye Metally, 4. 2002. Available online: https://www.researchgate.net/publication/293785383_Experience_of_titanium_sheet_fabrication_from_the_billet_of_electron-beam_smelting (accessed on 1 January 2002).
- Feng, Y.; Yan, P.; Jia, G. Application Status of Electron Beam Cold Hearth Melting Technology. Mater. China 2020, 39, 295–303. [Google Scholar] [CrossRef]
- Niu, S.; Zhao, L.; You, X.; Wang, Y.; Tan, Y. Evaporation for element Al in K417 Ni-based superalloy during electron beam remelting. Vacuum 2021, 187, 110073. [Google Scholar] [CrossRef]
- You, X.; Shi, S.; Tan, Y.; Zhao, L.; Zheng, J.; Zhuang, X.; Li, Y.; You, Q.; Li, P.; Long, W. The evaporation behavior of alloy elements during electron beam smelting of Inconel 718 alloy. Vacuum 2019, 169, 108920. [Google Scholar] [CrossRef]
- Wang, P.; Nai, M.L.S.; Lu, S.; Bai, J.; Zhang, B.; Wei, J. Study of Direct Fabrication of a Ti-6Al-4V Impeller on a Wrought Ti-6Al-4V Plate by Electron Beam Melting. JOM 2017, 69, 2738–2744. [Google Scholar] [CrossRef]
- Chen, B.; Du, Z.; Liu, K.; Zhang, X.; Wang, Z. Study on the denitrogenization kinetics of uranium during electron beam cold hearth refining. Vacuum 2020, 172, 109014. [Google Scholar] [CrossRef]
- Köck, W.; Paschen, P. Tantalum—Processing, properties and applications. JOM 1989, 41, 33–39. [Google Scholar] [CrossRef]
- Mimura, K.; Nanjo, M. Production of High-Purity Niobium by Carbon Reduction Smelting and Electron Beam Melting. High Temp. Mater. Process. 1988, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Li, F.; Bo, X.; Wang, X.; He, H. Preparation of High Purity Metallic Vanadium by Electron Beam Melting. Xiyou Jinshu/Chin. J. Rare Met. 2020, 44. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Galarraga, H.; Lados, D.; Dehoff, R.R.; Kirka, M.M.; Nandwana, P. Effects of the microstructure and porosity on properties of Ti-6Al-4V ELI alloy fabricated by electron beam melting (EBM). Addit. Manuf. 2016, 10, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Galarraga, H.; Warren, R.; Lados, D.; Dehoff, R.R.; Kirka, M.M.; Nandwana, P. Effects of heat treatments on microstructure and properties of Ti-6Al-4V ELI alloy fabricated by electron beam melting (EBM). Mater. Sci. Eng. A 2017, 685, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Karmiris-Obratański, P.; Papazoglou, E.L.; Leszczyńska-Madej, B.; Zagórski, K.; Markopoulos, A.P. A Comprehensive Study on Processing Ti–6Al–4V ELI with High Power EDM. Mater. 2021, 14, 303. [Google Scholar] [CrossRef]
- Yuan, X.; Qu, X.; Yin, H.; Feng, Z.; Tang, M.; Yan, Z.; Tan, Z. Effects of Sintering Temperature on Densification, Microstructure and Mechanical Properties of Al-Based Alloy by High-Velocity Compaction. Metalls 2021, 11, 218. [Google Scholar] [CrossRef]
- Harkin, R.; Wu, H.; Nikam, S.; Quinn, J.; McFadden, S. Reuse of Grade 23 Ti6Al4V Powder during the Laser-Based Powder Bed Fusion Process. Metalls 2020, 10, 1700. [Google Scholar] [CrossRef]
- Maimaitiyili, T.; Woracek, R.; Neikter, M.; Boin, M.; Wimpory, R.C.; Pederson, R.; Strobl, M.; Drakopoulos, M.; Schäfer, N.; Bjerkén, C. Residual Lattice Strain and Phase Distribution in Ti-6Al-4V Produced by Electron Beam Melting. Materials 2019, 12, 667. [Google Scholar] [CrossRef] [Green Version]
- Del Guercio, G.; Galati, M.; Saboori, A.; Fino, P.; Iuliano, L. Microstructure and Mechanical Performance of Ti–6Al–4V Lattice Structures Manufactured via Electron Beam Melting (EBM): A Review. Acta Met. Sin. 2020, 33, 183–203. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, A.; Klöden, B.; Luft, J.; Weißgärber, T.; Kieback, B. Process window for electron beam melting of Ti-6Al-4V. Powder Met. 2015, 58, 246–249. [Google Scholar] [CrossRef]
- Safdar, A.; Wei, L.-Y. Microstructures of Electron Beam Melted (EBM) Biomaterial Ti-6Al-4V. MRS Proc. 2008, 1145. [Google Scholar] [CrossRef]
- Karlsson, J.; Norell, M.; Ackelid, U.; Engqvist, H.; Lausmaa, J. Surface oxidation behavior of Ti–6Al–4V manufactured by Electron Beam Melting (EBM®). J. Manuf. Process. 2015, 17, 120–126. [Google Scholar] [CrossRef]
- Kolamroudi, M.K.; Asmael, M.; Ilkan, M.; Kordani, N. Developments on Electron Beam Melting (EBM) of Ti–6Al–4V: A Review. Trans. Indian Inst. Met. 2021, 74, 783–790. [Google Scholar] [CrossRef]
- Soundarapandiyan, G.; Khan, R.; Johnston, C.; Chen, B.; Fitzpatrick, M. Effect of postprocessing thermal treatments on electron-beam powder bed–fused Ti6Al4V. Mater. Des. Process. Commun. 2020, 3. [Google Scholar] [CrossRef] [Green Version]
- Pasang, T.; Tavlovich, B.; Yannay, O.; Jackson, B.; Fry, M.; Tao, Y.; Turangi, C.; Wang, J.-C.; Jiang, C.-P.; Sato, Y.; et al. Directionally-Dependent Mechanical Properties of Ti6Al4V Manufactured by Electron Beam Melting (EBM) and Selective Laser Melting (SLM). Materials 2021, 14, 3603. [Google Scholar] [CrossRef]
- Azgomi, N.; Tetteh, F.; Duntu, S.H.; Boakye-Yiadom, S. Effect of Heat Treatment on the Microstructural Evolution and Properties of 3D-Printed and Conventionally Produced Medical-Grade Ti6Al4V ELI Alloy. Met. Mater. Trans. A 2021, 52, 3382–3400. [Google Scholar] [CrossRef]
- DeMott, R.; Haghdadi, N.; Liao, X.; Ringer, S.P.; Primig, S. 3D characterization of microstructural evolution and variant selection in additively manufactured Ti-6Al-4 V. J. Mater. Sci. 2021, 56, 14763–14782. [Google Scholar] [CrossRef]
- Yilmaz, B.; Al Rashid, A.; Mou, Y.A.; Evis, Z.; Koç, M. Bioprinting: A review of processes, materials and applications. Bioprinting 2021, 23, e00148. [Google Scholar] [CrossRef]
- Harun, W.; Manam, N.; Kamariah, M.; Sharif, S.; Zulkifly, A.H.; Ahmad, I.; Miura, H. A review of powdered additive manufacturing techniques for Ti-6al-4v biomedical applications. Powder Technol. 2018, 331, 74–97. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent development in beta titanium alloys for biomedical applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Powell, A.; Pal, U.; van den Avyle, J.; Damkroger, B.; Szekely, J. Analysis of multicomponent evaporation in electron beam melting and refining of titanium alloys. Met. Mater. Trans. A 1997, 28, 1227–1239. [Google Scholar] [CrossRef]
- Ivanchenko, V.G.; Ivasishin, O.M.; Semiatin, S.L. Evaluation of evaporation losses during electron-beam melting of Ti-Al-V alloys. Met. Mater. Trans. A 2003, 34, 911–915. [Google Scholar] [CrossRef]
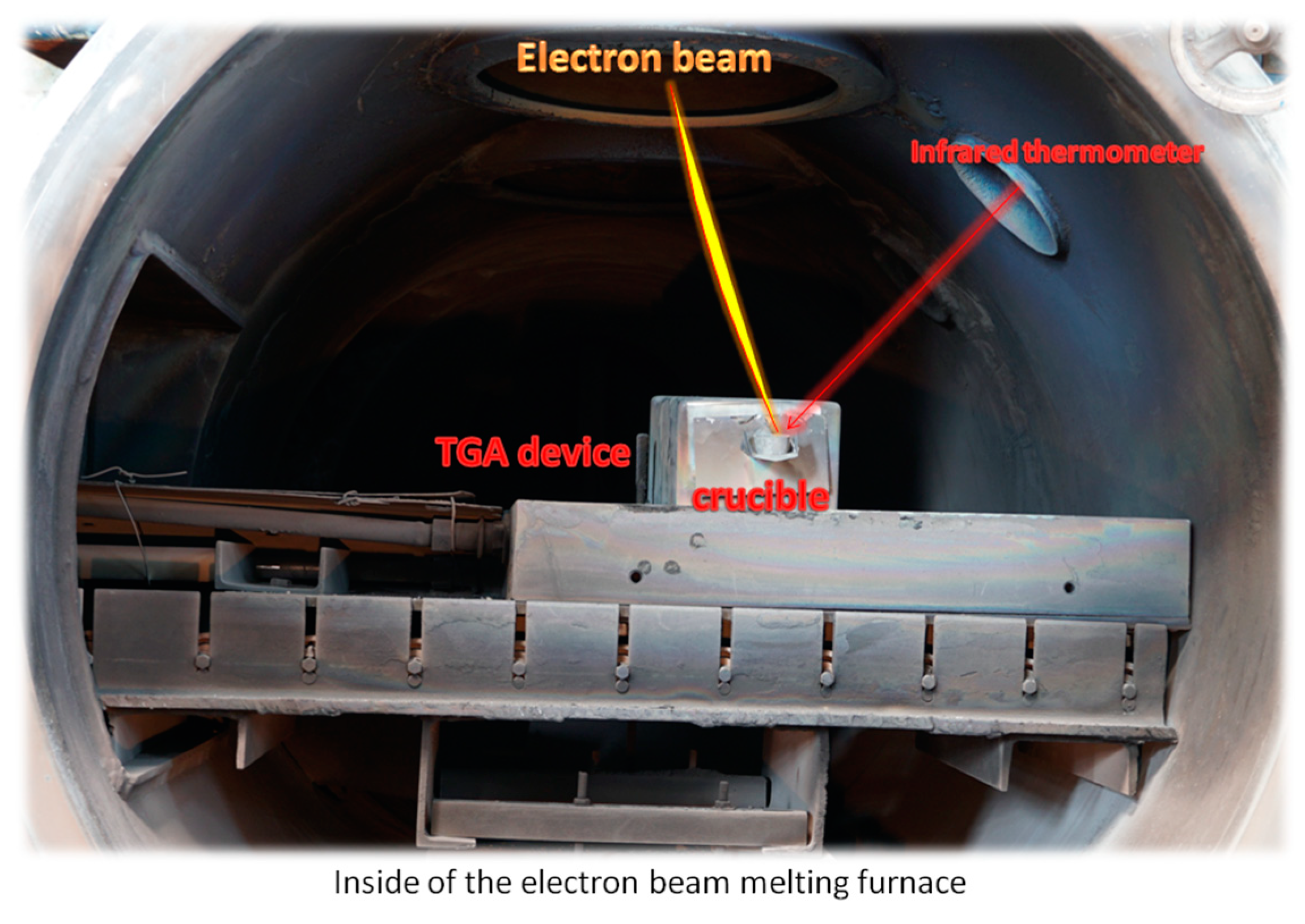


| Material | Constituents (wt.) | Shape |
|---|---|---|
| Ti | 99.9% | small pieces |
| V | 99.9% | small pieces |
| Al | 99.9% | chunk |
| Ti-Al (64:36) | Ti: 64%, Al: 36% | small pieces |
| V-Al (65:35) | V: 65%, Al: 35% | small pieces |
| TC4 | Ti: 89%, Al: 5.8%, V: 3.9% | chunk |
| Parameter | Value |
|---|---|
| Scan pattern | point focused |
| Point size | 3~4 mm |
| Beam power | 3~20 kW |
| Vapor pressure | (1.5~3) × 10−2 Pa |
| Cooling water temperature | 20 °C |
| Material | Electron Beam Power | Heating Rate (°C/s) | Cooling Rate (°C/s) |
|---|---|---|---|
| Al substance | 4.2 kW | 53 | - |
| Ti substance | 5.7 kW (<160 s) 9.3 kW (≥160 s) | 74 | - |
| V substance | 8.1 kW (<320 s) 7.8 kW (≥320 s) | 94 | 11 |
| Ti-Al alloy | 4.2 kW (<500 s) 6.9 kW (≥500 s) | 40 | 15 |
| V-Al alloy | 3.6 kW (<400 s) 6.3 kW (≥400 s) | 22 | 11 |
| TC4 alloy | 4.2 kW (<140 s) 7.2 kW (≥140 s) | 65 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, Z.; Liu, W. Experimental Measurement of Vacuum Evaporation of Aluminum in Ti-Al, V-Al, Ti6Al4V Alloys by Electron Beam. Metals 2021, 11, 1688. https://doi.org/10.3390/met11111688
Wang D, Liu Z, Liu W. Experimental Measurement of Vacuum Evaporation of Aluminum in Ti-Al, V-Al, Ti6Al4V Alloys by Electron Beam. Metals. 2021; 11(11):1688. https://doi.org/10.3390/met11111688
Chicago/Turabian StyleWang, Dawei, Zhiguo Liu, and Wenrui Liu. 2021. "Experimental Measurement of Vacuum Evaporation of Aluminum in Ti-Al, V-Al, Ti6Al4V Alloys by Electron Beam" Metals 11, no. 11: 1688. https://doi.org/10.3390/met11111688
APA StyleWang, D., Liu, Z., & Liu, W. (2021). Experimental Measurement of Vacuum Evaporation of Aluminum in Ti-Al, V-Al, Ti6Al4V Alloys by Electron Beam. Metals, 11(11), 1688. https://doi.org/10.3390/met11111688






