Influence of Anodization on the Fatigue and Corrosion-Fatigue Behaviors of the AZ31B Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
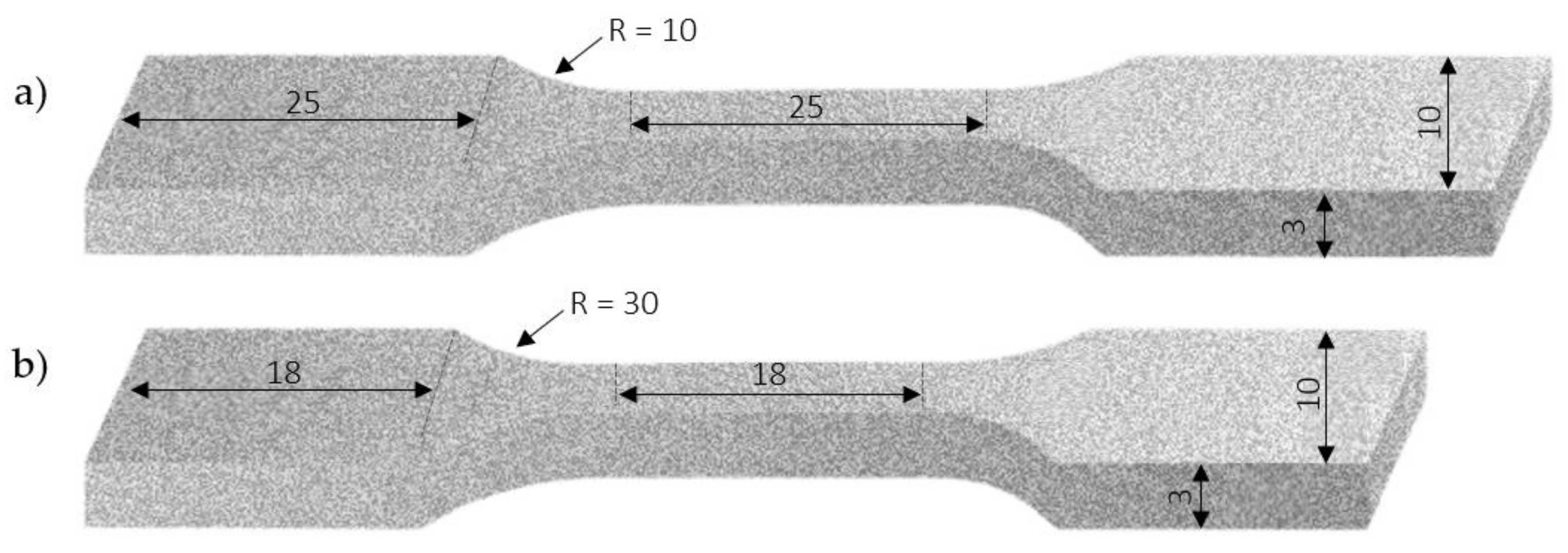
2.1. Material and Specimen Preparation
2.2. Anodizing Treatment
2.3. Coating Morphology and Corrosion Test
2.4. Tensile Test
2.5. Fatigue and Corrosion Fatigue Tests
2.6. Fracture Surface Analysis
3. Results and Discussion
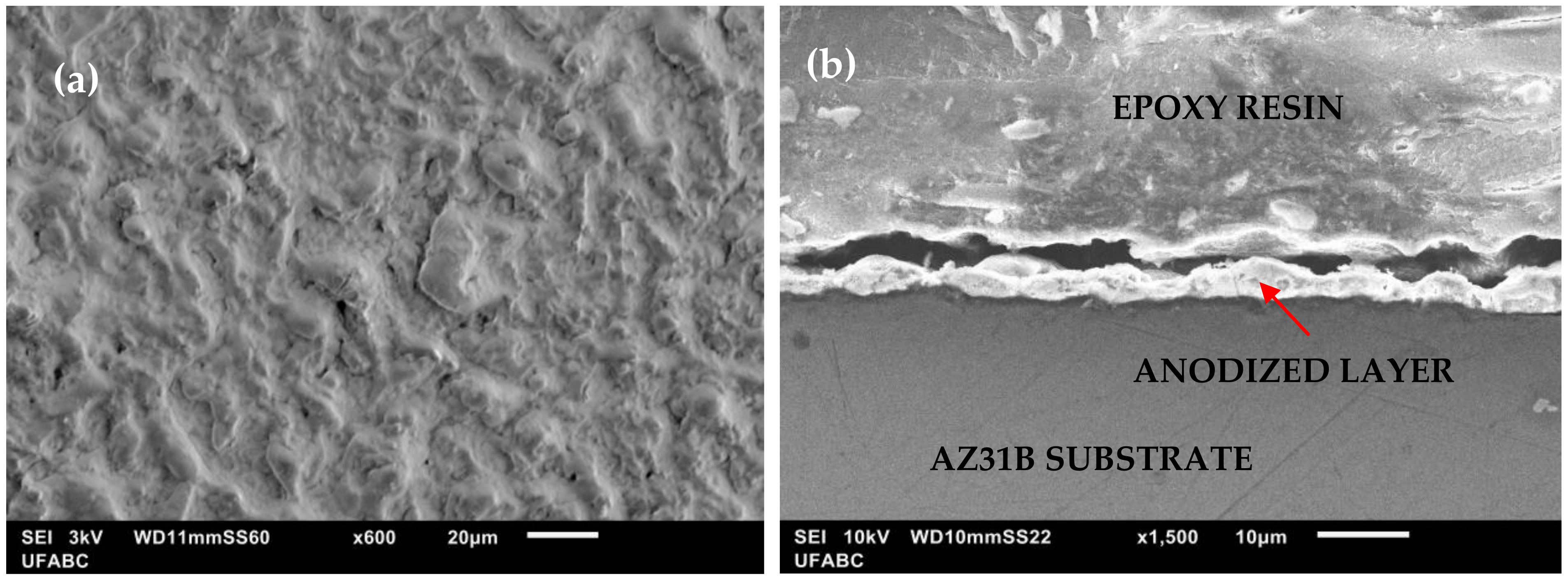
3.1. Anodized Layer Morphology and Corrosion Test
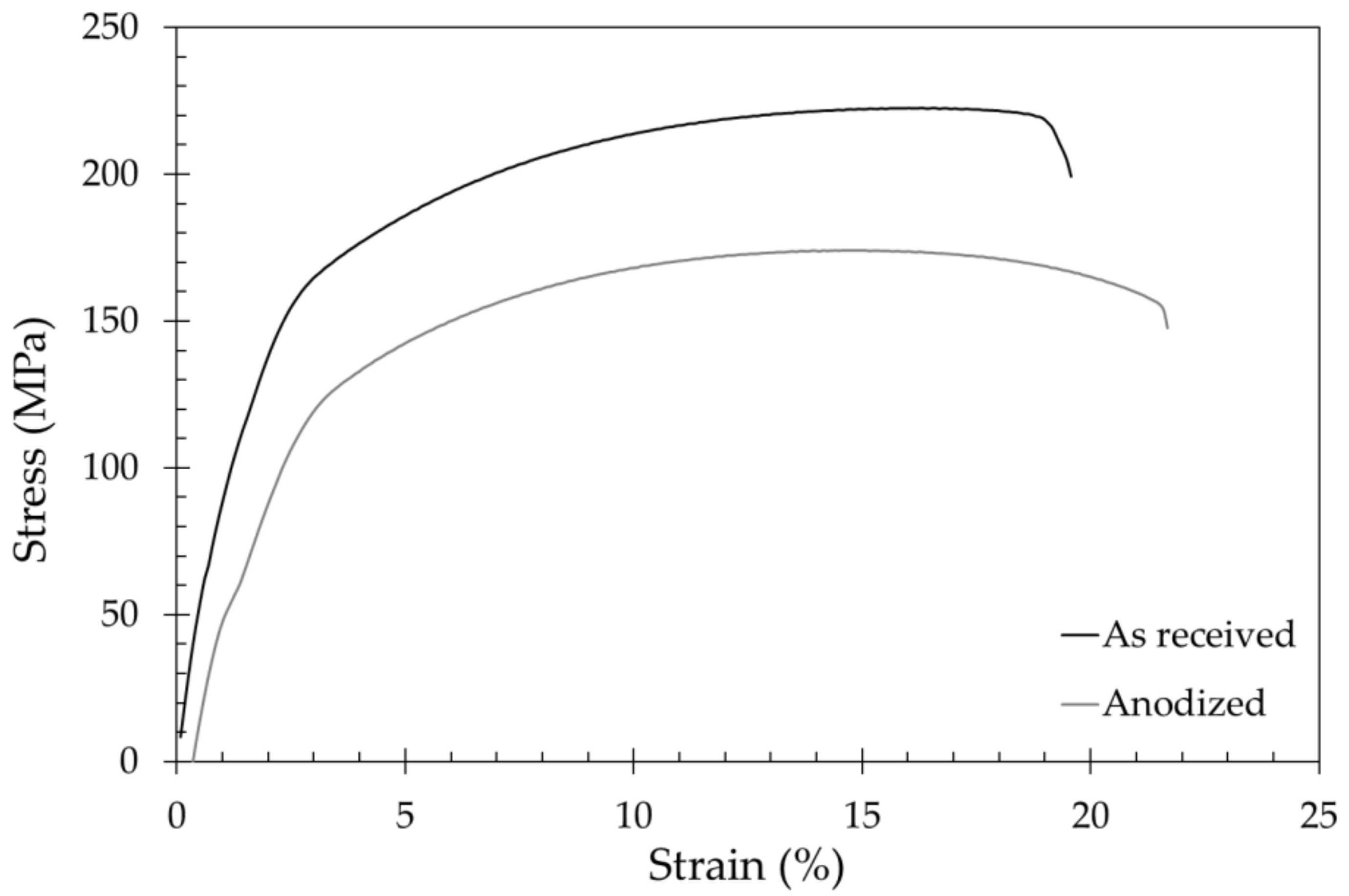
3.2. Tensile Properties
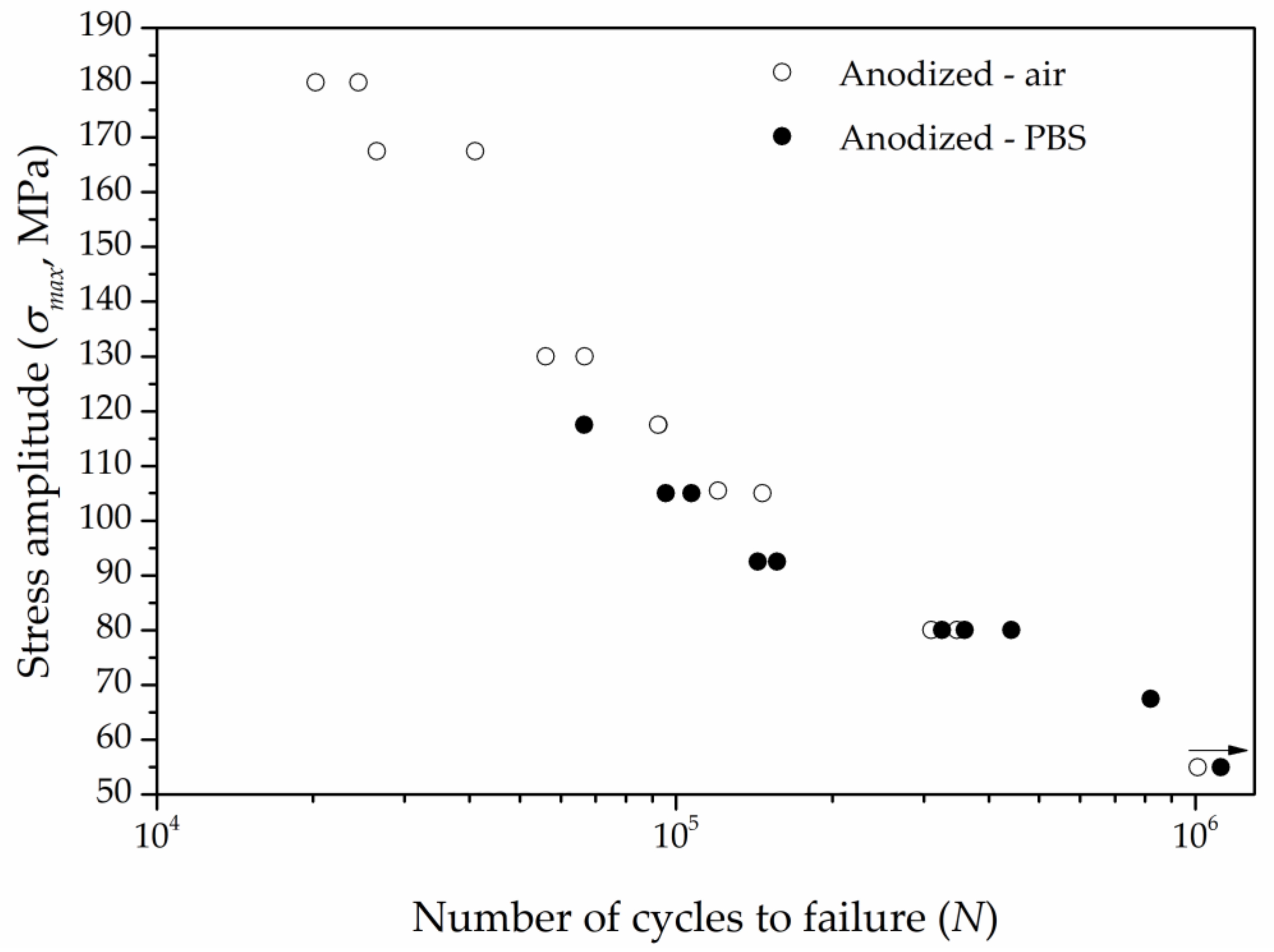
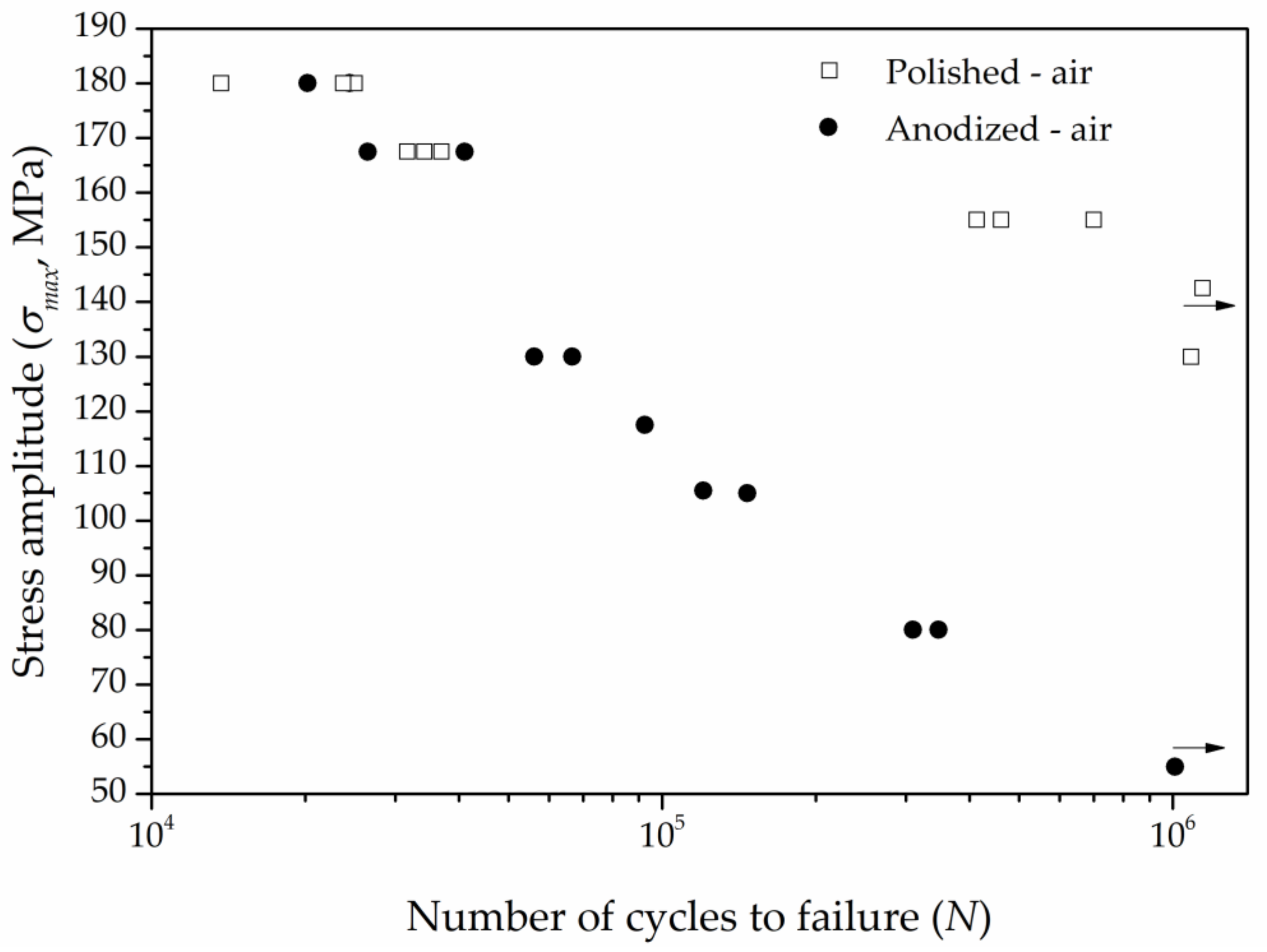
3.3. Fatigue and Corrosion Fatigue Behavior
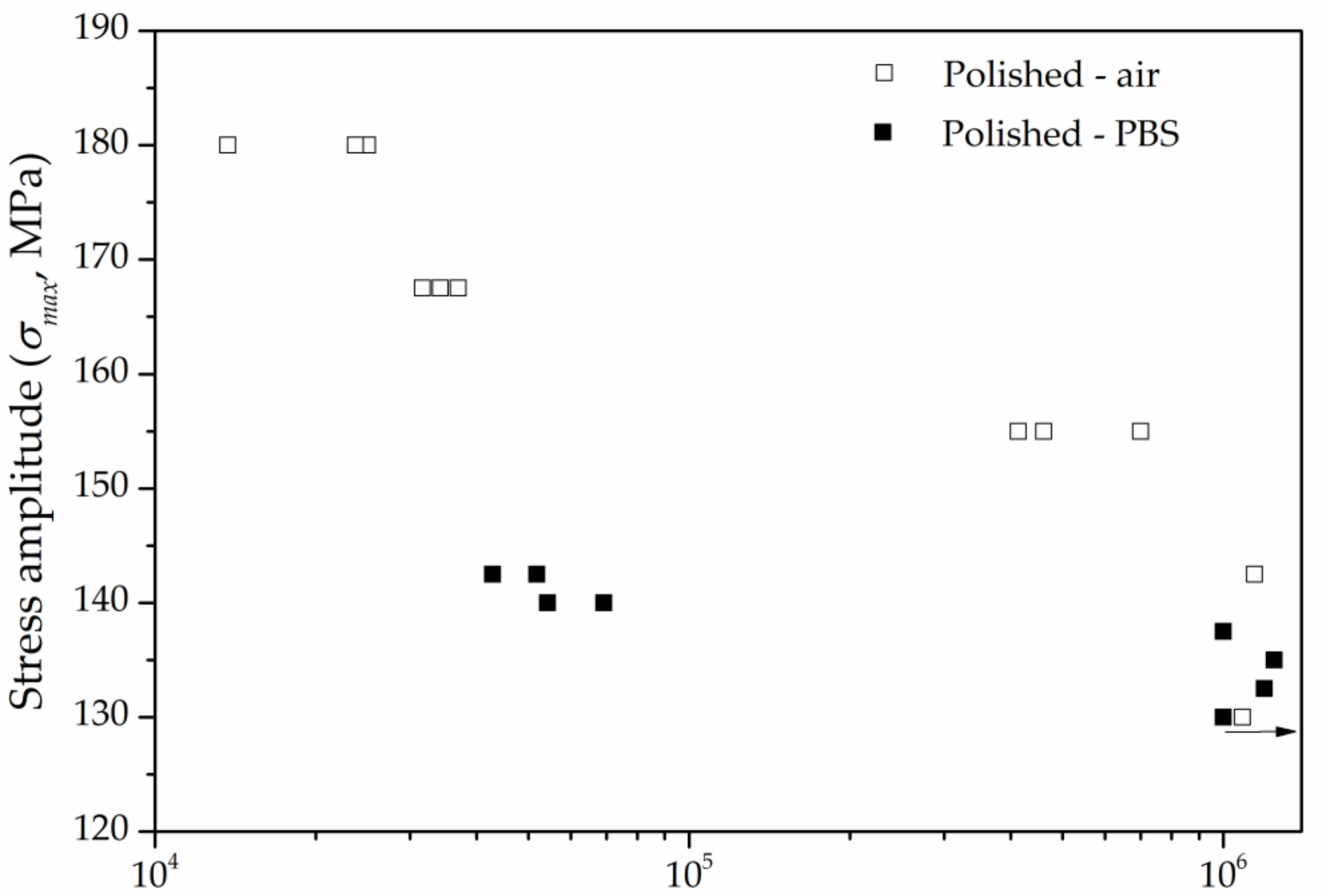
3.3.1. Influence of the Corrosive Environment
3.3.2. Influence of Anodization
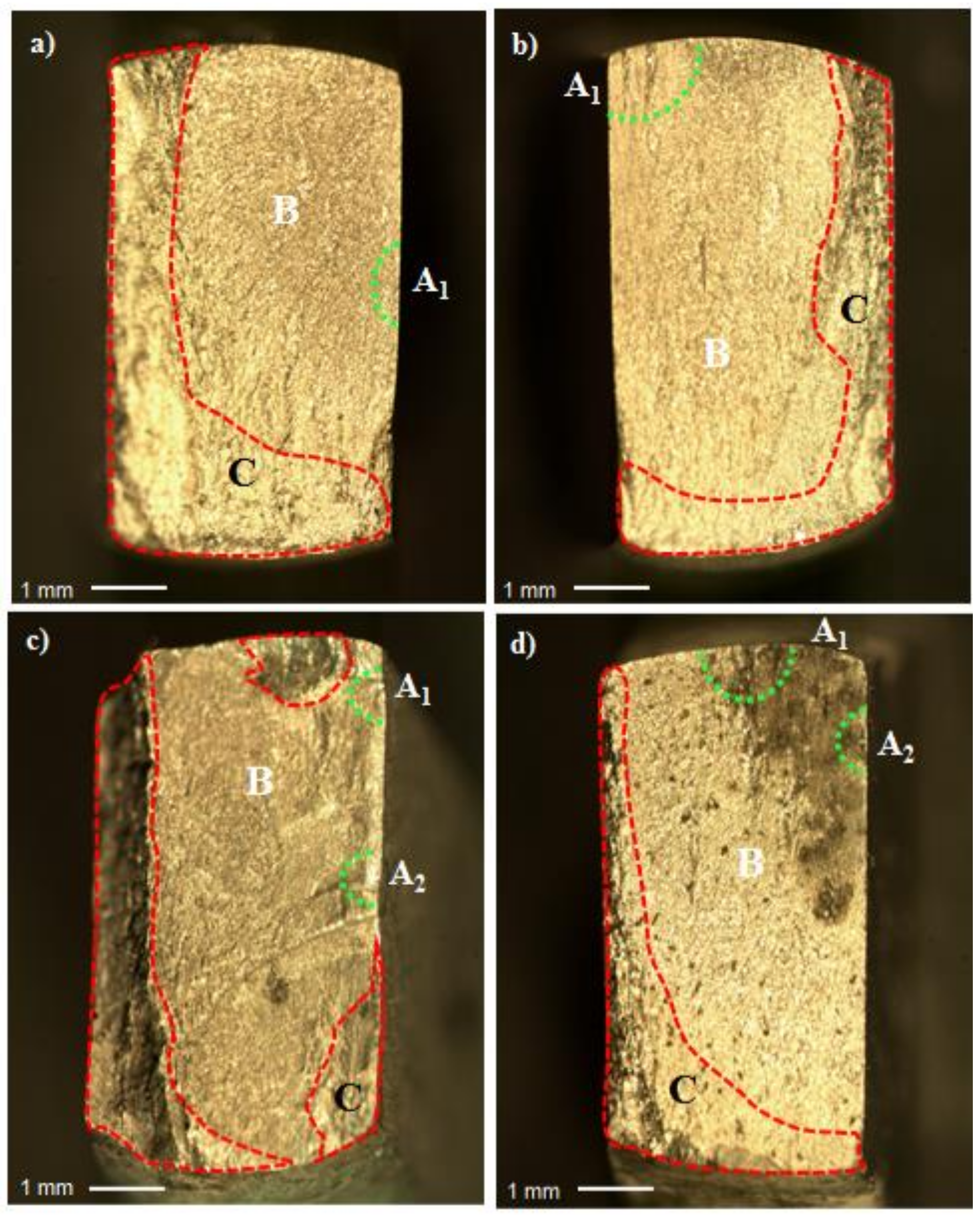
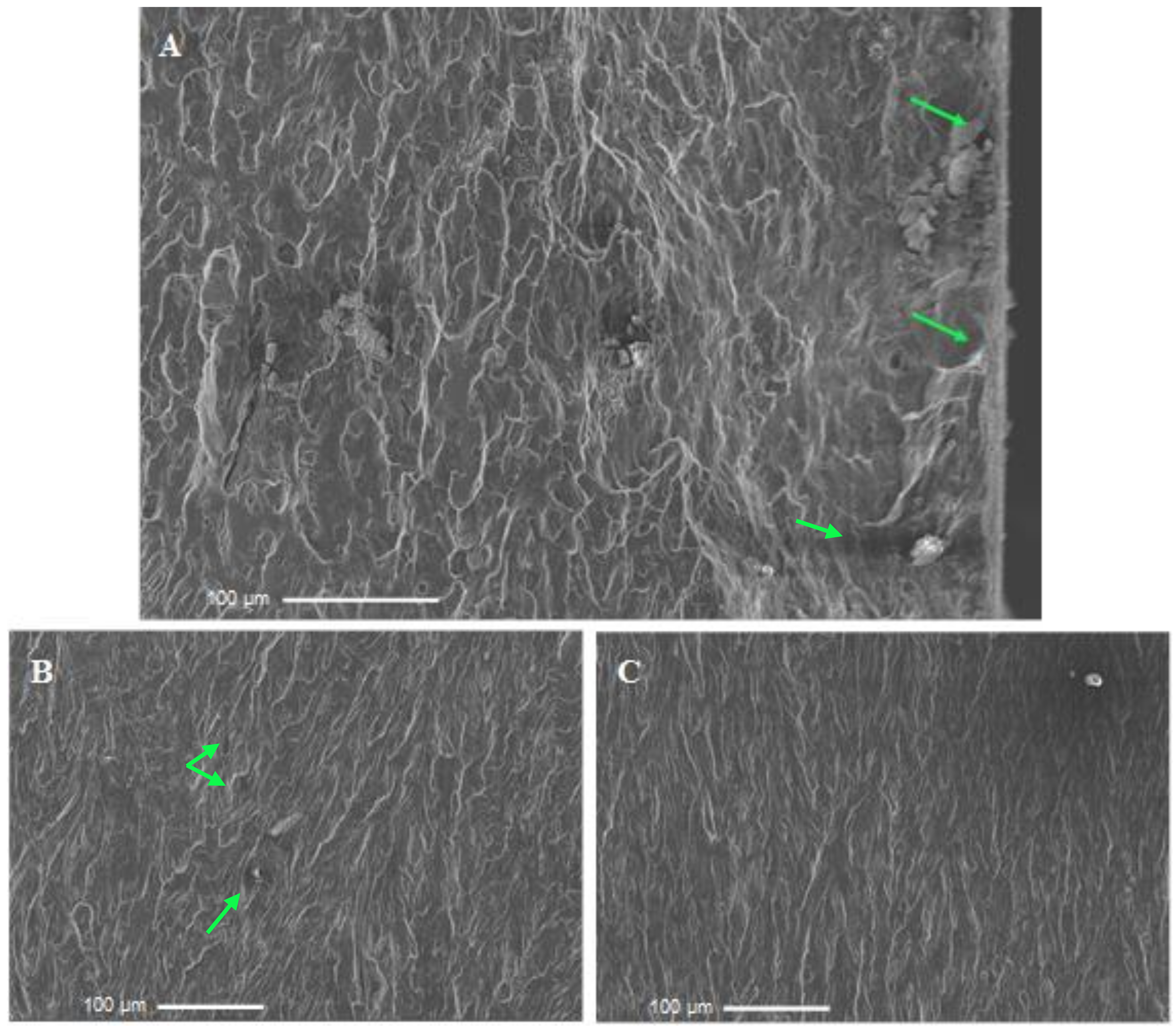
3.4. Fractographic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Si, Y.; Xiong, Z.; Zheng, X.; Li, M.; Yang, Q. Improving the anti-corrosion ability of anodization film of AZ31B magnesium alloy by addition of NH4VO3 in the electrolyte. Int. J. Electrochem. Sci. 2016, 11, 3261–3268. [Google Scholar] [CrossRef]
- Song, G.L.; Shi, Z. Corrosion mechanism and evaluation of anodized magnesium alloys. Corros. Sci. 2014, 85, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Liu, Z.; Hu, P.; Shao, J.; Li, X.; Du, C.; Jiang, B. The corrosion behavior of AZ91D magnesium alloy in simulated haze aqueous solution. Materials 2018, 11, 970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, H.M.; Hussein, K.H.; Woo, H.M.; Park, C.H.; Kim, C.S. One-step anodization deposition of anticorrosive bioceramic compounds on AZ31B magnesium alloy for biomedical application. Ceram. Int. 2015, 41, 10861–10870. [Google Scholar] [CrossRef]
- Wang, B.J.; Wang, S.D.; Xu, D.K.; Han, E.H. Recent progress in fatigue behavior of Mg alloys in air and aqueous media: A review. J. Mater. Sci. Technol. 2017, 33, 1075–1086. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Nassif, N.; Ghayad, I. Corrosion protection and surface treatment of magnesium alloys used for orthopedic applications. Adv. Mater. Sci. Eng. 2013, 2013, 532896. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Hall, C.; Murphy, P. Surface treatments for controlling corrosion rate of biodegradable Mg and Mg-based alloy implants. Sci. Technol. Adv. Mater. 2015, 16, 53501. [Google Scholar] [CrossRef] [Green Version]
- Cheng, I.C.; Fu, E.G.; Liu, L.D.; Lee, C.Y.; Lin, C.S. Effect of Fluorine Anions on Anodizing Behavior of AZ91 Magnesium Alloy in Alkaline Solutions. J. Electrochem. Soc. 2008, 155, C219–C225. [Google Scholar] [CrossRef]
- Chai, L.; Yu, X.; Yang, Z.; Wang, Y.; Okido, M. Anodizing of magnesium alloy AZ31 in alkaline solutions with silicate under continuous sparking. Corros. Sci. 2008, 50, 3274–3279. [Google Scholar] [CrossRef]
- Li, L.L.; Cheng, Y.L.; Wang, H.M.; Zhang, Z. Anodization of AZ91 magnesium alloy in alkaline solution containing silicate and corrosion properties of anodized films. Trans. Nonferrous Met. Soc. China 2008, 18, 722–727. [Google Scholar] [CrossRef]
- Girón, L.; Aperador, W.; Tirado, L.; Franco, F.; Caicedo, J.C. Electrochemical Performance Estimation of Anodized AZ31B Magnesium Alloy as Function of Change in the Current Density. J. Mater. Eng. Perform. 2017, 26, 3710–3718. [Google Scholar] [CrossRef]
- Salami, B.; Afshar, A.; Mazaheri, A. The effect of sodium silicate concentration on microstructure and corrosion properties of MAO-coated magnesium alloy AZ31 in simulated body fluid. J. Magnes. Alloy. 2014, 2, 72–77. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, L.A.; Silva, R.M.P.; Antunes, R.A. Scanning Electrochemical Microscopy (SECM) Study of the Electrochemical Behavior of Anodized AZ31B Magnesium Alloy in Simulated Body Fluid. Mater. Res. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue characteristics of metallic biomaterials. Int. J. Fatigue 2007, 29, 992–1000. [Google Scholar] [CrossRef]
- Jafari, S.; Singh Raman, R.K.; Davies, C.H.J. Corrosion fatigue of a magnesium alloy in modified simulated body fluid. Eng. Fract. Mech. 2015, 137, 2–11. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Zhu, S.; Zhang, Y.; Sun, Y.; Wang, L.; Guan, S. Corrosion fatigue of the extruded Mg–Zn–Y–Nd alloy in simulated body fluid. J. Magnes. Alloy. 2020, 8, 231–240. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion fatigue behaviors of two biomedical Mg alloys—AZ91D and WE43—In simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef]
- Bian, D.; Zhou, W.; Liu, Y.; Li, N.; Zheng, Y.; Sun, Z. Fatigue behaviors of HP-Mg, Mg–Ca and Mg–Zn–Ca biodegradable metals in air and simulated body fluid. Acta Biomater. 2016, 41, 351–360. [Google Scholar] [CrossRef]
- Eifert, A.J.; Thomas, J.P.; Rateick, R.G. Influence of anodization on the fatigue life of WE43A-T6 magnesium. Scr. Mater. 1999, 40, 929–935. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y. Corrosion fatigue behavior of AM60 magnesium alloy with anodizing layer and chemical-conversion-coating layer. Mater. Corros. 2015, 66, 940–948. [Google Scholar] [CrossRef]
- Němcová, A.; Skeldon, P.; Thompson, G.E.; Morse, S.; Čížek, J.; Pacal, B. Influence of plasma electrolytic oxidation on fatigue performance of AZ61 magnesium alloy. Corros. Sci. 2014, 82, 58–66. [Google Scholar] [CrossRef]
- ASTM E8M-16a. Standard test methods for tension testing of metallic materials 1. ASTM Stand. 2010, 4, 1–27. [Google Scholar] [CrossRef]
- ASTM E466-15. Standard Practice for Conducting Force Controlled Constant Amplitude Axial Fatigue Tests of Metallic Materials. ASTM Stand. 2002, 3, 4–8. [Google Scholar] [CrossRef]
- De Oliveira, L.A.; Silva, R.M.P.; Rodas, A.C.D.; Souto, R.M.; Antunes, R.A. Surface chemistry, film morphology, local electrochemical behavior and cytotoxic response of anodized AZ31B magnesium alloy. J. Mater. Res. Technol. 2020, 9, 14754–14770. [Google Scholar] [CrossRef]
- De Oliveira, M.C.L.; Pereira, V.S.M.; Correa, O.V.; Antunes, R.A. Corrosion performance of anodized AZ91D magnesium alloy: Effect of the anodizing potential on the film structure and corrosion behavior. J. Mater. Eng. Perform. 2014, 23, 593–603. [Google Scholar] [CrossRef]
- ASTM F1801-97. Standard Practice for Corrosion Fatigue Testing of Metallic Implant Materials 1. ASTM Stand. 2014, 3, 5–10. [Google Scholar] [CrossRef]
- ASTM G1-90. Standard Practice for Preparing, Cleaning, and Evaluation Corrosion Test Specimens. ASTM Stand. 2003, 8, 1–9. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y.; Koike, T. Effect of anodized layer thickness on fatigue behavior of magnesium alloy. Mater. Sci. Eng. A 2008, 474, 261–269. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Deen, K.M.; Haider, W. Controlling corrosiion kinetics of magnesium alloys by electrochemical anodization and investigation of film mechanical properties. Appl. Surf. Sci. 2019, 484, 906–916. [Google Scholar] [CrossRef]
- Barjaktarevic, D.; Medjo, B.; Gubeljak, N.; Cvijovic-Alagic, I.; Stefane, P.; Djokic, V.; Rakin, M. Experimental and numerical analysis of tensile properties of Ti-13Nb-13Zr alloy and determination of influence of anodization process. Proc. Struct. Integr. 2020, 28, 2187–2194. [Google Scholar]
- Nan, Z.Y.; Ishihara, S.; McEvily, A.J.; Shibata, H.; Komano, K. On the sharp bend of the S-N curve and the crack propagation behavior of extruded magnesium alloy. Scr. Mater. 2007, 56, 649–652. [Google Scholar] [CrossRef]
- Nan, Z.Y.; Ishihara, S.; Goshima, T. Corrosion fatigue behavior of extruded magnesium alloy AZ31 in sodium chloride solution. Int. J. Fatigue 2008, 30, 1181–1188. [Google Scholar] [CrossRef]
- He, X.L.; Wei, Y.H.; Hou, L.F.; Yan, Z.F.; Guo, C.L. High-frequency corrosion fatigue behavior of AZ31 magnesium alloy in different environments. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2013, 228, 1645–1657. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Mutoh, Y.; Murai, T.; Iwakami, S. Corrosion fatigue behavior of extruded magnesium alloy AZ61 under three different corrosive environments. Int. J. Fatigue 2008, 30, 1756–1765. [Google Scholar] [CrossRef]
- Raman, S.R.K.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Hilpert, M.; Wagner, L. Corrosion fatigue behavior of the high-strength magnesium alloy AZ 80. J. Mater. Eng. Perform. 2000, 9, 402–407. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y.; Koike, T. Fatigue behavior of anodized AM60 magnesium alloy under humid environment. Mater. Sci. Eng. A 2008, 498, 377–383. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Shatrov, A.; Samsonov, V.; Shashkov, P.; Leyland, A.; Matthews, A. Fatigue properties of Keronite® coatings on a magnesium alloy. Surf. Coat. Technol. 2004, 182, 78–84. [Google Scholar] [CrossRef]
- Ceschini, L.; Morri, A.; Angelini, V.; Messieri, S. Fatigue behavior of the rare earth rich EV31A Mg alloy: Influence of plasma electrolytic oxidation. Metals 2017, 7, 212. [Google Scholar] [CrossRef]
- Lonyuk, B.; Apachitei, I.; Duszczyk, J. The effect of oxide coatings on fatigue properties of 7475-T6 aluminium alloy. Surf. Coat. Technol. 2007, 201, 8688–8694. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Wang, S.D.; Sheng, L.Y.; Zeng, R.C.; Han, E. hou Influence of solution treatment on the corrosion fatigue behavior of an as-forged Mg-Zn-Y-Zr alloy. Int. J. Fatigue 2019, 120, 46–55. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Han, E.H.; Dong, C. Effect of corrosion attack on the fatigue behavior of an as-cast Mg-7%Gd-5%Y-1%Nd-0.5%Zr alloy. Mater. Des. 2015, 84, 185–193. [Google Scholar] [CrossRef]
- Xu, D.K.; Liu, L.; Xu, Y.B.; Han, E.H. The crack initiation mechanism of the forged Mg-Zn-Y-Zr alloy in the super-long fatigue life regime. Scr. Mater. 2007, 56, 1–4. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.B. High cycle fatigue characterization of two die-cast magnesium alloys. Mater. Charact. 2008, 59, 567–570. [Google Scholar] [CrossRef]
- Lv, F.; Yang, F.; Duan, Q.Q.; Yang, Y.S.; Wu, S.D.; Li, S.X.; Zhang, Z.F. Fatigue properties of rolled magnesium alloy (AZ31) sheet: Influence of specimen orientation. Int. J. Fatigue 2011, 33, 672–682. [Google Scholar] [CrossRef]
- Ochi, Y.; Masaki, K.; Hirasawa, T.; Wu, X.; Matsumura, T.; Takigawa, Y.; Higashi, K. High cycle fatigue property and micro crack propagation behavior in extruded AZ31 magnesium alloys. Mater. Trans. 2006, 47, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Tokaji, K.; Kamakura, M.; Ishiizumi, Y.; Hasegawa, N. Fatigue behaviour and fracture mechanism of a rolled AZ31 magnesium alloy. Int. J. Fatigue 2004, 26, 1217–1224. [Google Scholar] [CrossRef]
- Rivers, G. Cyclic Fatigue Behaviour of Wrought AZ80 Magnesium Alloy from Forged Automotive Wheel. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2011. [Google Scholar]
- Fouad, Y. Fatigue behavior of a rolled AZ31 magnesium alloy after surface treatment by EP and BB conditions. Alex. Eng. J. 2011, 50, 23–27. [Google Scholar] [CrossRef] [Green Version]





| Condition | Ecorr | jcorr | Rp | baAZ31B | |bc| | P |
|---|---|---|---|---|---|---|
| (V vs. Ag/AgCl/(3 M) KCl) | (μA cm−2) | (kΩ cm2) | (mV dec−1) | (mV dec−1) | (%) | |
| As polished | −1.407 ± 0.004 | 30.4 ± 8.5 | 0.71 | 100 | 130 | - |
| Anodized | −1.470 ± 0.041 | 2.0 ± 0.7 | 51.2 | - | 160 | 0.84 |
| Specimens | Ultimate Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) |
|---|---|---|---|
| As received | 223 ± 5 | 165 ± 5 | 20 ± 3 |
| Anodized | 175 ± 5 | 120 ± 5 | 20 ± 3 |
| Specimen Conditions and Environment | Equations |
|---|---|
| Polished–air | log(σmax) = −7894lnN + 254.61 |
| Polished – PBS solution | log(σmax) = −1336lnN + 155.86 |
| Anodized–air | log(σmax) = −32,96lnN + 501.26 |
| Anodized–PBS solution | log(σmax) = −20,39lnN + 340.47 |
| Conditions | σair (MPa) | σPBS (MPa) | RσN (%) |
|---|---|---|---|
| Polished | 144.6 | 137.4 | 5.6 |
| Anodized | 45.9 | 58.8 | −28.0 |
| Corrosion Fatigue Results | Polished | Anodized | ||
|---|---|---|---|---|
| Air | PBS Solution | Air | PBS Solution | |
| Applied stress (MPa) | 167.5 | 140 | 105 | 92.5 |
| Number of cycles to failure | 34,200 | 54,355 | 146,754 | 143,681 |
| Conditions | Crack Status | Possible Causes | Fracture Appearance |
|---|---|---|---|
| Polished—Air Figure 8 | Single crack nucleation | Surface and subsurface discontinuities | Brittle with radial pattern |
| Anodized—Air Figure 8 | Single crack nucleation | Rough or uneven anodized layer | Brittle with radial pattern |
| Polished—PBS solution Figure 8 | Multiple crack nucleation | Surface and subsurface discontinuities combined with local corrosion | Brittle and hackly types |
| Anodized—PBS solution Figure 8 | Multiple crack nucleation | Rough or uneven anodized layer | Brittle and quasi-cleavage types |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, L.A.; dos Santos, S.L.; de Oliveira, V.A.; Antunes, R.A. Influence of Anodization on the Fatigue and Corrosion-Fatigue Behaviors of the AZ31B Magnesium Alloy. Metals 2021, 11, 1573. https://doi.org/10.3390/met11101573
de Oliveira LA, dos Santos SL, de Oliveira VA, Antunes RA. Influence of Anodization on the Fatigue and Corrosion-Fatigue Behaviors of the AZ31B Magnesium Alloy. Metals. 2021; 11(10):1573. https://doi.org/10.3390/met11101573
Chicago/Turabian Stylede Oliveira, Leandro Antonio, Silvano Leal dos Santos, Vinicius Antonio de Oliveira, and Renato Altobelli Antunes. 2021. "Influence of Anodization on the Fatigue and Corrosion-Fatigue Behaviors of the AZ31B Magnesium Alloy" Metals 11, no. 10: 1573. https://doi.org/10.3390/met11101573
APA Stylede Oliveira, L. A., dos Santos, S. L., de Oliveira, V. A., & Antunes, R. A. (2021). Influence of Anodization on the Fatigue and Corrosion-Fatigue Behaviors of the AZ31B Magnesium Alloy. Metals, 11(10), 1573. https://doi.org/10.3390/met11101573






