Abstract
In blast furnaces it is desirable for the burden to hold a lumpy packed structure at as high a temperature as possible. The computational thermodynamic software FactSage (version 7.2, Thermfact/CRCT, Montreal, Canada and GTT-Technologies, Aachen, Germany) was used here to study the softening behavior of blast furnace pellets. The effects of the main slag-forming components (SiO2, MgO, CaO and Al2O3) on liquid formation were estimated by altering the chemical composition of a commercial acid pellet. The phase equilibria for five-component FeO-SiO2-CaO-MgO-Al2O3 systems with constant contents for three slag-forming components were computed case by case and the results were used to estimate the formation of liquid phases. The main findings of this work suggested several practical means for the postponement of liquid formation at higher temperatures: (1) reducing the SiO2 content; (2) increasing the MgO content; (3) reducing the Al2O3 content; and (4) choosing suitable CaO contents for the pellets. Additionally, the olivine phase (mainly the fayalitic type) and its dissolution into the slag determined the amount of the first-formed slag, which formed quickly after the onset of softening. This had an important effect on the acid pellets, in which the amount of the first-formed slag varied between 10 and 40 wt.%, depending on the pellets’ SiO2 content.
1. Introduction
Blast furnaces are still the dominant process for making iron in the world. Traditionally, pig iron is first produced in a blast furnace (BF) from iron ores and further refined into crude steel in a basic oxygen furnace. The world’s total crude steel production was 1878 million tonnes in 2020, of which 73.2% was produced via the blast furnace–oxygen steelmaking route. An alternative route is electric arc steelmaking, in which electric energy is used to melt scrap, direct reduced iron (DRI) or hot briquetted or compacted iron (HBI/HCI). Electric furnace steelmaking constitutes 26.3% of the world’s crude steel production. The rest, which corresponds to 0.5%, was produced via old-fashioned open-hearth furnaces or other technologies [1]. A future megatrend will be the reduction of CO2 emissions in steelmaking, which might speed up the shift from traditional BF-based ironmaking to new, breakthrough technology-based, fossil-free steelmaking [2].
A blast furnace is a large-sized shaft furnace, in which iron oxides are reduced to metallic molten iron. The reduction of iron burden materials in a blast furnace takes place under conditions where the upper charge layers exert a load upon the lower layers. The pressure of the burden causes the deformation of the solid phases, which is increased at elevated temperatures by the onset of melting and leads to a loss of permeability in the cohesive zone. The cohesive zone is an area where ore starts to soften and melt; it has the highest resistance to gas flow, which leads to a high pressure drop over the cohesive zone. A narrow cohesive zone enables a smaller pressure drop and is favourable for BF operation [3].
The formation of molten phases starts with local chemical compositions, which have the lowest melting temperatures. The first melts that are formed in the blast furnace are derived from acid slag components mixed with iron oxides. The melting of the iron burden materials leads to the collapse of the ore bed and the permeability of the gas decreases dramatically. It is estimated that the gas permeability disappears more or less completely between 1200 and 1350 °C, depending on the characteristics of the ore burden. In shaft furnaces, such as blast furnaces, it is desirable for the burden to hold a lumpy packed structure at as high a temperature as possible [4,5,6].
Borinder and Torssell [7], as well as Borinder and Bi [8], have listed several factors that influence the softening and melting properties of iron burden materials. These include: the iron content; the amount of slag-forming components; the melting point of gangue and slag-forming additives; basicity; the SiO2, FeO and MgO content; reducibility; and the particle size of the iron ore. Geerdes et al. [4] highlight that the three most critical factors are the basicity, the SiO2 content and the presence of remaining FeO. According to studies on blast furnaces’ cohesive zones, the softening and melting temperatures are directly related to the ternary basicity (B3) [8]. The lower the SiO2 content in the iron burden material and the lower the remaining FeO content, the higher the melting temperature. Additionally, the behavior of the burden materials depends on operational factors of the BF, including the reducing gas atmosphere (CO, CO2, H2, sulphur, alkalis), the temperature profile, the load value, and their contact with other burden materials [7,8].
The softening–melting behavior of industrial pellets (such as acid or fluxed pellets) has been studied experimentally and reported in a couple of papers by our research unit [9,10,11,12,13,14,15]. The evaluation of the effects of individual components (i.e., SiO2, MgO, CaO and Al2O3) on the softening–melting behavior requires a huge amount of experiments, as well as the very time-consuming pre-treatment of sample materials, in order to prepare samples with different chemical compositions for the experiments. Computational thermodynamics provides a faster and easier method for the evaluation of the effect of individual components, as long as the modelling results for the default compositions (e.g., compositions of either acid or fluxed pellets) are validated with experiments beforehand.
The results of a previous study by Kemppainen et al. [14] indicate that the softening of iron ore pellets is caused by the softening of the pellet core where wüstite is the dominant phase of iron, and reduction degrees between 50 and 70% do not have a significant effect on the softening behavior. Additionally, in another study, the experimental microscopy of softened samples of ARUL (Advanced Reduction under Load) revealed that all iron takes the form of wüstite or metallic iron and no magnetite or hematite appears when softening occurs [11]. Therefore, this computational study assumed that all the iron takes a divalent (Fe2+) form, simulating the softening occurring in the core regions of the partly reduced materials in which wüstite is the dominant phase of iron. The authors of this paper have used this kind of approach in a couple of previous studies [10,11,12,13,14,15]. The commercial thermochemical software FactSage was chosen as a computational tool because its databases provide thermochemical data for all the compounds relevant in pellet systems. A similar approach involving the application of thermochemical software to study reduction processes was reported by Cheraghi et al. [16], for example, who studied the gaseous reduction of manganese ores by CO and H2.
2. Materials and Methods
2.1. Iron Ore Pellets
Iron ore pellets are commonly used as iron burden materials in blast furnaces. The pellets are small and hard iron ore balls. Their diameter is usually 10–16 mm. The pellets are hot-bonded in order to provide sufficient mechanical strength. A high iron content and a low gangue content is favourable for blast furnace pellets [17]. Additives are often used to meet the blast furnace’s specific requirements. The three main pellet types for iron making purposes are: acid pellets (B2 < 0.5); basic or fluxed pellets (0.9 < B2 < 1.3), which use limestone (CaCO3) or dolomite (Ca,Mg(CO3)2) as an additive; and olivine fluxed pellets, which use olivine (Mg2SiO4) as a fluxing additive. Sometimes, magnesite (MgCO3) is also used as an additive [4,18,19,20]. The typical chemical composition for blast furnace pellets can be seen in Table 1.

Table 1.
Typical chemical composition for commercial blast furnace pellets according to the work by Jonckbloedt in [17].
A commercial acid pellet, whose softening behavior has been previously studied [11] using a tailor-made, high-temperature furnace named ARUL, was chosen for these investigations. The operational description of the ARUL reduction-softening test is also given there. The chemical composition of the acid pellet (“Original”) is shown in Table 2, as well as the imaginary chemical compositions in each computational case, focusing on the effect of SiO2, MgO, CaO and Al2O3 on the softening.

Table 2.
The original and the altered chemical compositions (in wt.%) and basicity values for the pellets. Empty cells indicate no change in the component proportion of the original pellets.
Basicity is a universal ratio for the description of pellet chemistry. Different kinds of basicity values are widely used. B2 is the basicity based on two components (CaO and SiO2), B3 is the basicity based on three components (CaO, SiO2, MgO) and B4 is the basicity based on four components (CaO, SiO2, MgO and Al2O3,) as illustrated in Equations (1)–(3).
2.2. Computational Thermodynamics
The commercial thermochemical software FactSage was chosen as a computational tool because its databases provide thermochemical data for all the compounds relevant in pellet systems. All the equilibrium computations within this study were made with FactSage version 7.2 [21,22] and its FactPS (for pure substances) and FToxid (for oxide systems) databases. All the thermochemical data of the described oxide systems needed in this study were obtained from these databases. Since the purpose of this paper was not to present a comprehensive review on the thermochemical data needed to model and simulate the systems considered in our study, it was considered not to be relevant to repeat the vast list of references, in which all the necessary data was validated. A detailed description of the modelling principles, as well as the restrictions of the models when taking different components into account, are available on the FactSage website [23]. The website also contains the full list of references, in which all the models and sub-models were validated.
This being said, a few aspects of the used data should nevertheless be briefly mentioned. The most crucial data for the purposes of this study were the models used to describe the molten oxide phase and the necessary oxide solid solutions (i.e., monoxide, olivine and melilite type phases). Models available from the FToxid database were used for these purposes. In the FactSage version used in this study, the Modified Quasichemical Model (MQM) was used to describe the molten oxide phase and models considering the mixing of various cations on crystallographically different sublattices were used to model the solid solutions. Concerning the molten oxide phase, the CaO-MgO-SiO2-Al2O3-FeO system was validated for the whole composition range [23]. Additionally, numerous (a total of 88) relevant pure invariant solid phases (i.e., Ca2SiO4 and Al2O3) were considered. Table 3 summarizes the mineralogy of the monoxide, olivine and melilite type phases, which were considered in the FactSage computations.

Table 3.
Mineralogical names and formula for the necessary oxide solid solutions, including monoxide, olivine and melilite type phases, which were considered in the FactSage computations.
The proportions of the different phases, among which the slag phase was under special examination, were calculated with the Equilib module, whereas the phase stability diagrams for the five-component FeO-SiO2-CaO-MgO-Al2O3 systems were computed with the Phase Diagram module in FactSage. Before the FactSage computations, the total iron content (Fetot) was converted to divalent form (FeO), and the contents of SiO2, CaO, MgO, Al2O3 and FeO were normalized to 100%. Components other than SiO2, MgO, CaO, Al2O3 and Fetot were not considered in the FactSage computations. In each computation, three slag-forming components were kept constant while altering the contents for one slag-forming component and FeO, as can be seen in Table 4.

Table 4.
Proportions of the components in the system definition for the FactSage computations. The empty cells indicate no change in the component proportion of the original pellets.
3. Results
The effects of the main slag-forming components on the softening, including SiO2, MgO, CaO and Al2O3, are discussed in the following four sub-sections. The base scenario originated in the original chemical composition of the acid pellet, while in other cases, one component (either SiO2, MgO, CaO or Al2O3) replaced the proportion of FeO. The calculations were carried out between temperatures of 1100 and 1500 °C, because preliminary computations showed that the solidus and liquidus temperatures were within this temperature range in each case. The temperature step in each scenario was 5 °C, which indicated that the accuracy of the solidus and liquidus temperatures was 5 °C as well.
Figure 1 shows the FactSage-computed liquid formation curve for the iron ore pellet with the original chemical composition. The solidus and the liquidus are marked in the figure. The solidus is the temperature at which the first liquid phase was formed and the liquidus is the temperature at which the system was fully melted. For this composition, the solidus and the liquidus temperatures were 1130 °C and 1340 °C, respectively. The area between the solidus and the liquidus was a softening–melting area, which is also known as a cohesive zone in a blast furnace. Between the solidus and liquidus there was a third transition point, which was named the ZPF (Zero Phase Fraction) point of olivine. Up to this temperature, olivine was present in the system; however, it was no longer present at higher temperatures, because at the ZPF point of olivine, all the olivine dissolved in the slag phase. The ZPF point of olivine was 1180 °C for the acid pellets with the original chemical composition. A total of 23 wt.% of the acid pellet melted quickly between 1130 °C (solidus) and 1180 °C (the ZPF point of olivine) and after that, the formation of liquid slowed down until the remaining solid phases melted quickly, just before they reached the liquidus temperature.
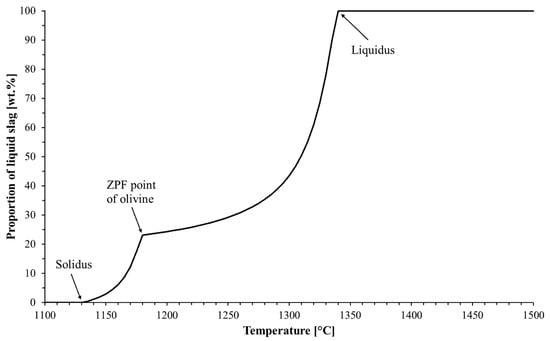
Figure 1.
Liquid formation curve for the iron ore pellet with the original chemical composition.
3.1. Effect of SiO2
The typical SiO2 content in commercial blast furnace pellets is between 1.6 and 10 wt.% [17]; therefore, the focus in this study was on this composition area. The relation between the SiO2 content and the evolution of the liquid oxide phase during softening was computed with FactSage to estimate the effect of SiO2 on the pellets’ softening (see Figure 2). It can be clearly seen that the decrease in the SiO2 content in the pellet lead to a slower formation of the liquid oxide phase, preventing the material from softening. The decrease in the SiO2 content in the material also increased the liquidus’ temperature. The most significant finding might be that the pellets’ SiO2 content greatly affected the proportion of the liquid slag at a given temperature. At a temperature of 1190 °C, for example, the proportion of slag varied between 10 and 40 wt.%, while the pellets’ SiO2 contents varied from 1.82 to 7.82 wt.%.
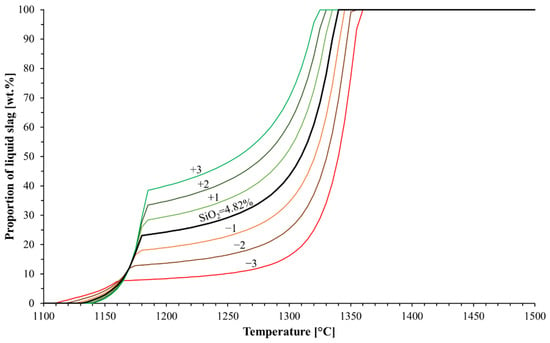
Figure 2.
Effect of SiO2 content on liquid formation. The SiO2 contents considered were 1.82, 2.82, 3.82, 4.82 (original composition), 5.82, 6.82 and 7.82 wt.%.
The phase diagram in Figure 3 was computed with FactSage in relation to the proportion of SiO2 in the system and the temperature, in order to perform a deeper analysis. Within the typical composition area of SiO2 (which is marked with a blue box in the figure), a lower SiO2 content in the pellets indicated a broader softening area (or cohesive zone in a BF), with a lower solidus and a higher liquidus temperature; however, the proportion of the first-formed slag before the ZPF point of olivine was very low, with low SiO2 contents in the pellets, as can be seen in Figure 2. The softening behavior of the pellets with very low SiO2 contents (below ~1 wt.%) was different, but this low SiO2 content is not feasible in blast furnace pellets. The pellets’ SiO2 content in the typical composition area did not affect the existing phases. The predominant phases were wüstite and fayalite-type olivine before the onset of softening. During softening, wüstite, fayalite-type olivine and liquid slag were present, from which the fayalite-type olivine dissolved into the slag phase as the temperature increased. Finally, as the system fully melted, only liquid slag was present. These FactSage computations suggest that lowering the SiO2 content is one feasible method to improve the softening properties of ferrous materials.
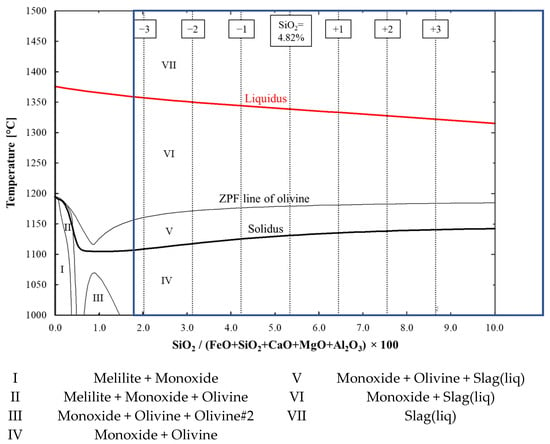
Figure 3.
Effect of SiO2 on solidus and liquidus temperatures and existing phases. Solidus and liquidus are highlighted with bold-type black- and red-coloured curves, respectively. The SiO2 contents of 1.82, 2.82, 3.82, 4.82 (original composition), 5.82, 6.82 and 7.82 wt.% are marked with dashed vertical lines. The typical composition area for SiO2 between 1.6 and 10 wt.% is marked with a blue box.
3.2. Effect of MgO
The typical MgO content in commercial blast furnace pellets is between 0 and 1.4 wt.% [17], but the computations were broadened to allow higher MgO contents due to the high SiO2 content in the studied pellets. The effects of MgO on pellet softening were studied with FactSage by adding MgO and simultaneously decreasing the FeO content in the system. Figure 4 shows the effect of increasing the MgO in the acid pellets on the pellets’ softening. The Figure shows that the increase of MgO increased both the solidus and the liquidus temperatures of the system and lead to a slower formation of the liquid oxide phase, thus preventing the material from softening. The MgO content in the pellets did not seem to affect the volume of the first-formed slag phase. This conclusion is congruent with the Fe2SiO4-Mg2SiO4 phase diagram [24] (p. 142), which suggests that the higher the MgO content in the iron burden material, the higher the solidus and the liquidus temperatures. This is because MgO partly dissolves into the first-formed slag, as verified in Iljana et al. [11].
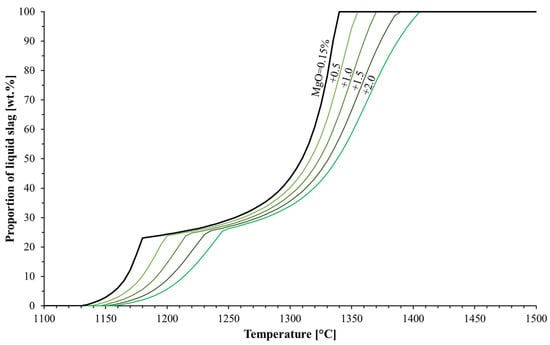
Figure 4.
Effect of MgO content on liquid formation. The MgO contents considered were 0.15 (original composition), 0.65, 1.15, 1.65 and 2.15 wt.%.
The FactSage-computed phase diagram in Figure 5 shows that the increase in the MgO content did not significantly affect the existing phases in the typical composition area of the blast furnace pellets. However, when the amount of MgO in the system was increased, an increased amount of MgO dissolved into the monoxide phase, altering its composition so much that the monoxide phase could be named magnesiowüstite, instead of wüstite. The magnesiowüstite played an important role in the preservation of the high melting point of the pellets, raising the temperatures of the liquidus when it contained a significant amount of MgO, as can be seen in Figure 5. The predominant phases were monoxide (wüstite or magnesiowüstite) and olivine before the onset of softening. Monoxide, olivine and liquid slag were present during softening, through which the olivine dissolved into the slag phase as the temperature increased. Finally, the system fully melted, only liquid slag was present. These FactSage examinations suggest that increasing the MgO content is another feasible method to improve the softening properties of ferrous materials.
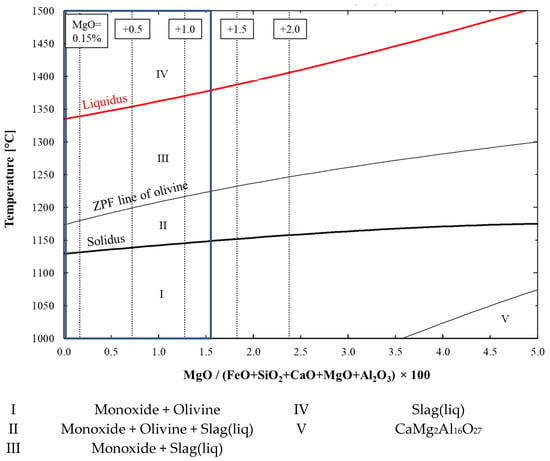
Figure 5.
Effect of MgO on solidus and liquidus temperatures and existing phases. Solidus and liquidus are highlighted with bold-type black- and red-coloured curves, respectively. The MgO contents of 0.15 (original composition), 0.65, 1.15, 1.65 and 2.15 wt.% are marked with dashed vertical lines. The typical composition area for MgO between 0 and 1.4 wt.% is marked with a blue box.
3.3. Effect of CaO
The typical CaO content in commercial blast furnace pellets is between 0 and 3.7 wt.% [17], but the computations were broadened to include higher CaO contents due to the high SiO2 content in the studied pellets. The effects of the CaO content on the pellets’ softening was estimated with FactSage by altering the CaO and FeO contents in the system. Figure 6 shows the liquid formation curves with the original and altered CaO contents in the system. At first glance, one can see from the Figure that CaO lowered the liquidus temperature, but either decreased or increased the solidus temperature, which is more important when considering the softening in a blast furnace. At first, CaO decreased the solidus temperature, but with elevated CaO contents the solidus temperature increased compared to that of the original acid pellets. Because of this two-way effect, a five-component FeO-SiO2-CaO-MgO-Al2O3 phase stability diagram, shown in Figure 7, was computed with FactSage. The computed phase diagram shows the relation of the CaO proportion in the system and the temperature of the existing phases. The original pellet composition is on the left side and the compositions altered by the addition of corresponding levels of CaO are marked with vertical dashed lines. The solidus and liquidus are highlighted with bold-type black- and red-coloured curves, respectively. Additionally, from Figure 6, one can see that the amount of CaO in the pellets did not significantly affect the amount of first-formed slag until the ZPF point of olivine, which was roughly 20 wt.% in each computational case.
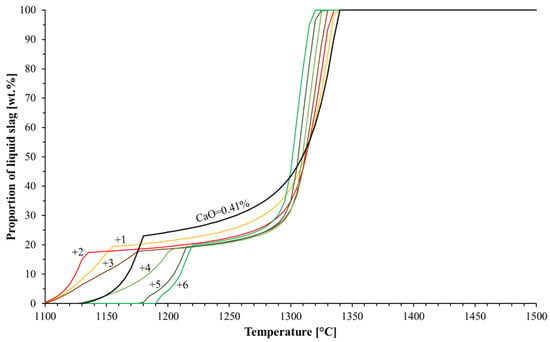
Figure 6.
Effect of CaO content on liquid formation. The CaO contents considered were 0.41 (original composition), 1.41, 2.41, 3.41, 4.41, 5.41 and 6.41 wt.%.
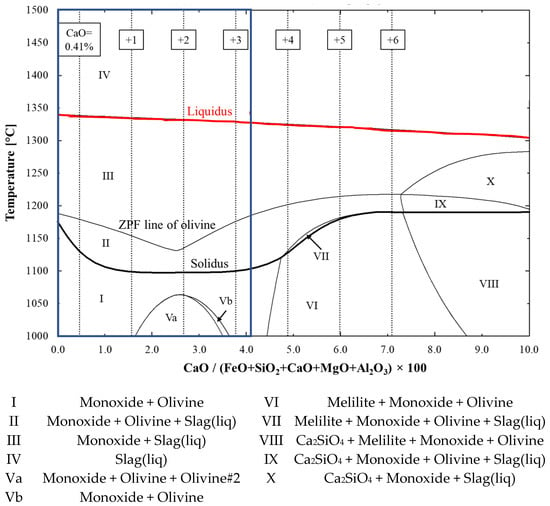
Figure 7.
Effect of CaO on solidus and liquidus temperatures and existing phases. Solidus and liquidus are highlighted with bold-type black- and red-coloured curves, respectively. The CaO contents of 0.41 (original composition), 1.41, 2.41, 3.41, 4.41, 5.41 and 6.41 wt.% are marked with dashed vertical lines. The typical composition area for CaO between 0 and 3.7 wt.% is marked with a blue box.
From Figure 7, it can be deduced that if an iron burden material was fluxed with CaO, then the proportion of CaO should reach a sufficient level. Figure 6 and Figure 7 both show that the B2 basicity needed to exceed 0.9 in order to achieve a higher solidus temperature compared to the acid pellet, without the addition of CaO. Otherwise, this would have meant that the fluxing iron ore pellets with CaO-containing additives were better suited to the low-SiO2 pellets, because the amount of additive needed to be very high in high-SiO2 pellets in order to attain effective softening properties. The predominant phases were wüstite and olivine before the onset of softening. Wüstite, olivine and liquid slag were present during softening, through which the olivine dissolved into the slag phase as the temperature increased. Finally, as the system fully melted, only liquid slag was present. From Figure 7, one can see that when B2 basicity exceeded roughly 1.4, solid dicalciumsilicate (Ca2SiO4) with a very high melting point was formed, which was verified previously in a sinter sample with a B2 basicity of 2.1 [11].
3.4. Effect of Al2O3
The typical Al2O3 content in commercial blast furnace pellets is between 0.2 and 3 wt.% [17], but the computations were not carried out for the highest Al2O3 contents, because we assumed that such high Al2O3 contents in blast furnace pellets are relative rare. The effect of the Al2O3 content on the pellets’ softening was estimated with FactSage by altering the Al2O3 and FeO contents in the system; Figure 8 was drawn based on the FactSage computations. Figure 8 indicates that the proportion of the first-formed slag was very similar (approximately 20–25 wt.%), despite the Al2O3 content in the pellets. Additionally, the Al2O3 seemed to affect the pellets’ softening so that the lower the proportion of Al2O3 in the iron ore pellets, the higher the solidus temperature, leaving more time for the ore for gaseous reduction. Therefore, the separation of Al2O3 from iron ore concentrate is a fourth feasible way to enhance the softening properties of ferrous materials.
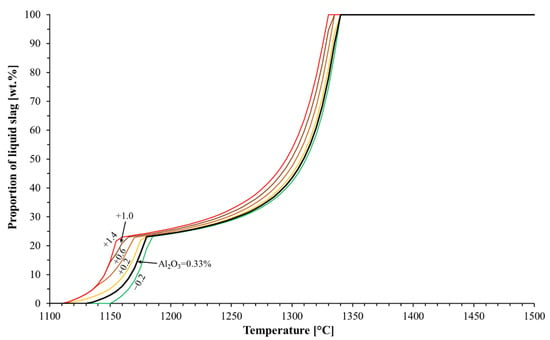
Figure 8.
Effect of Al2O3 content on liquid formation. The Al2O3 contents considered were 0.13, 0.33 (original composition), 0.53, 0.93, 1.33, and 1.73 wt.%.
The FactSage-computed phase diagram in Figure 9 shows that different solid phases were present in the pellets, depending on the pellets’ Al2O3 content. The predominant phases were wüstite and olivine before the onset of softening. Wüstite, olivine and liquid slag were present during softening, through which the olivine dissolved into the slag phase as the temperature increased. Finally, as the system fully melted, only liquid slag was present. In the high-Al2O3 pellets, calcium aluminosilicate (CaAl2Si2O8) and even aluminum oxide (Al2O3) could be present in pure phases before the first liquids formed, together with wüstite and olivine.
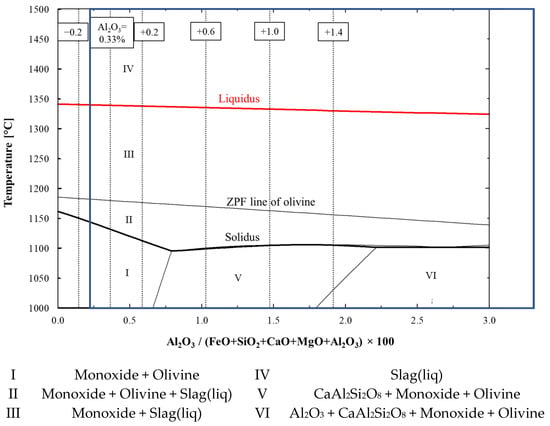
Figure 9.
Effect of Al2O3 on solidus and liquidus temperatures and existing phases. Solidus and liquidus are highlighted with bold-type black- and red-coloured curves, respectively. The Al2O3 contents of 0.13, 0.33 (original composition), 0.53, 0.93, 1.33, and 1.73 wt.% are marked with dashed vertical lines. The typical composition area for Al2O3 between 0.2 and 3.7 wt.% is marked with a blue box.
4. Discussion
The predominant phases in each computational case are summarized in Table 5. Below solidus temperature the predominant phases were monoxide and olivine. The monoxide phase was wüstite, except for the systems with a high MgO content, in which magnesiowüstite was present. Furthermore, the composition of the olivine phase differed depending on the pellets’ chemical composition. Between the solidus temperature and the ZPF point of olivine, wüstite, olivine and slag phases were present and, within that temperature range, the amount of olivine decreased as the temperature increased, while the olivine dissolved into the slag phase. At the ZPF point of olivine, all the olivine dissolved to slag and wüstite and slag became the predominant phases. At liquidus temperature, the pellet fully melted, and only liquid slag was present. In the solid state, there was a solubility gap between two olivine phases, which was seen when studying the effect of CaO; alternatively, other solid phases than olivine, such as calcium aluminosilicate (CaAl2Si2O8) and aluminum oxide (Al2O3) in pure phases, were seen in the pellets with a high Al2O3 content.

Table 5.
Predominant phases when softening occurred.
In our previous studies [10,11,12,13,14,15] the reduction-softening behaviors of acid pellets, olivine fluxed pellets and basic sinter were investigated using the ARUL test with a compression force of 200 kPa and a dynamic gas composition–temperature program from ambient temperature up to elevated temperatures, until the pressure difference over the sample exceeded 7.0 kPa, causing major difficulties for in the reduction of gases penetrating through the material layer. Similar liquid oxide phase fraction curves and phase diagrams for FeO-SiO2-CaO-MgO-Al2O3 systems to those produced in this study were computed with FactSage to estimate the formation of liquid phases using the chemical composition of the test materials. Because the computed phase diagrams in these previous studies offered a good estimate of the liquid formation, which was experimentally verified, we were able to write a computational paper about the effect of the main slag-forming components on pellet softening.
Although we suggest that computational thermodynamic software, such as FactSage, can be utilized to study the effects of pellet chemistry on its softening behavior, some limitations were found in the thermodynamic computations because of the need to define the system to a certain degree of simplicity. Firstly, there was an assumption of global equilibrium conditions in the FactSage-computed phase diagrams, but individual phases appeared in the microstructures of the pellets and the sinter in a study by Iljana et al. [11], for example, and these were not evenly distributed, leading to local equilibrium conditions. Secondly, the system had to be simplified, ignoring components other than SiO2, MgO, CaO, Al2O3 and iron oxides and the reduction reactions of iron (in the computations, the system was defined at the reduction stage, in which the wüstite is the predominant phase, because softening starts in the partly reduced pellet core). For instance, even in small amounts, alkalis can decrease the solidus temperature significantly; however, the fraction of liquid at this point is small [25]. Thirdly, thermodynamics does not take into account reaction kinetics, which determine how quickly the composition of the system changes towards thermodynamic equilibrium.
When compared to studies by other researchers, no major contradictions were seen. Silva et al. [3] studied sixteen pellets containing varying contents of MgO, CaO and SiO2. They concluded that increasing the pellets’ MgO content leads to a higher softening temperature. Pellets fluxed with CaO or MgO exhibit better softening and melting characteristics in relation to acid pellets. In the operation of blast furnaces, fluxed pellets tend to be present in a solid phase within a larger region in relation to acid pellets.
Li et al. [26] studied experimentally the effects of MgO and Al2O3 on the softening-melting properties of high-basicity sinter. They concluded that Al2O3 enters enter into the slag phase rather than MgO and forms a low melting point phase, while MgO remains in a solid state and is mainly present in wüstite in the form of FeO-MgO solid solution (magnesiowüstite). They also suggested that the effect of Al2O3 is of great importance, because Al2O3 is distributed unevenly at the softening stage in the slag phase, leading to local conditions. This finding is in accordance with the FactSage computations in this study, which showed that liquid formation starts at lower temperatures when Al2O3 is added to the system.
5. Conclusions
A computational thermodynamic study with FactSage was carried out to evaluate the effects of the main slag-forming components (SiO2, MgO, CaO and Al2O3) on the liquid formation of iron ore pellets. In previous studies [10,11,12,13,14,15], we verified that FactSage-computed liquid evolution curves and phase diagrams provide good estimates of the softening behavior of blast furnace burden materials by comparing the computational thermodynamic results to experimental reduction-softening test results. This study suggests multiple methods through which to enhance the softening and melting properties of acid blast furnace pellets by altering their chemical composition:
- Decreasing the content of SiO2 and/or Al2O3 and simultaneously increasing the pellets’ total iron content.
- Increasing the MgO content.
- Increasing the CaO content to a sufficient level corresponding to a B2 basicity of ~0.9 or higher. The addition of CaO addition is particularly feasible, with the pellets having a low SiO2 content.
The olivine phase (mainly fayalitic type) and its dissolution into the slag is important because it determines the amount of first-formed slag, which forms quickly after the onset of softening. This was shown to be particularly important in the acid pellets, in which the amount of the first-formed slag varied between 10 and 40 wt.% in the computations, depending on the pellets’ SiO2 content. Instead, MgO play an important role in olivine-fluxed pellets as it partly dissolves to the (magnesio)wüstite phase, increasing the solidus and liquidus temperatures.
Author Contributions
Conceptualization, M.I.; methodology, M.I. and E.-P.H.; software, E.-P.H.; formal analysis, M.I. and E.-P.H.; investigation, M.I. and E.-P.H.; resources, T.F.; writing—original draft preparation, M.I.; writing—review and editing, M.I., E.-P.H. and T.F.; visualization, M.I.; supervision, T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Business Finland as a part of the Towards Fossil-free Steel (FFS) research programme, grant number 45774/31/2020.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Timo Paananen and Olli Mattila from SSAB Europe Oy for fruitful discussions about this computational thermodynamic method to estimate iron ore pellet softening.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Steel in Figures 2021; World Steel Association: Brussels, Belgium, 2021.
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of Hydrogen Direct Reduction for Fossil-Free Steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- Silva, F.R.; Lemos, L.R.; de Freitas Nogueira, P.; Bressan, M. Effect of Ternary Basicity of Iron Ore-Fluxed Pellets on Melting and Softening Properties in a Blast Furnace. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2021, 52, 69–76. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ricketts, J. Modern Blast Furnace Ironmaking, 3rd ed.; IOS Press BV: Amsterdam, The Netherlands, 2015; ISBN 978-1-61499-498-5. [Google Scholar]
- Bakker, T. Softening in the Blast Furnace Process: Local Melt Formation as the Trigger for Softening of Ironbearing Burden Materials. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 1999. [Google Scholar]
- Matsui, Y.; Sato, A.; Oyama, T.; Matsuo, T.; Kitayama, S.; Ono, R. All Pellets Operation in Kobe No. 3 Blast Furnace under Intensive Coal Injection. ISIJ Int. 2003, 43, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Borinder, T.; Torssell, K. High Temperature Behaviour of Blast Furnace Pellets. In Proceedings of the 45th Ironmaking Conference, Washington, DC, USA, 6–9 April 1986; Iron & Steel Society: Warrendale, PA, USA, 1986; pp. 13–18, ISBN 9780932897107. [Google Scholar]
- Borinder, T.; Bi, X. Softening-Melting Properties of Pellets under Simulated Blast Furnace Conditions. Scand. J. Metall. 1989, 18, 280–287. [Google Scholar]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Pisilä, E.; Kondrakov, M.; Fabritius, T. Effect of Adding Limestone on the Metallurgical Properties of Iron Ore Pellets. Int. J. Miner. Process. 2015, 141, 34–43. [Google Scholar] [CrossRef]
- Iljana, M.; Kemppainen, A.; Heikkinen, E.-P.; Fabritius, T.; Paananen, T.; Mattila, O. A New Sophisticated Method for Evaluating the Reduction-Softening Properties of Iron Burden Materials. In Proceedings of the METEC & 2nd European Steel Technology and Application Days (ESTAD), Düsseldorf, Germany, 15–19 June 2015; Steel Institute VDEh: Düsseldorf, Germany, 2015; pp. 1–10. [Google Scholar]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Heikkinen, E.-P.; Fabritius, T. Evaluating the Reduction-Softening Behaviour of Blast Furnace Burden with an Advanced Test. ISIJ Int. 2016, 56, 1705–1714. [Google Scholar] [CrossRef] [Green Version]
- Iljana, M. Iron Ore Pellet Properties under Simulated Blast Furnace Conditions. Investigation on Reducibility, Swelling and Softening. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2017. [Google Scholar]
- Kemppainen, A.; Iljana, M.; Heikkinen, E.-P.; Fabritius, T.; Ohno, K.-I.; Maeda, T.; Kunitomo, K.; Mattila, O.; Paananen, T. Softening Behavior of Iron Ore Pellets in the Cohesive Zone of a Blast Furnace. In Proceedings of the METEC & 2nd European Steel Technology and Application Days (ESTAD), Düsseldorf, Germany, 15–19 June 2015; Steel Institute VDEh: Düsseldorf, Germany, 2015; pp. 1–10. [Google Scholar]
- Kemppainen, A.; Ohno, K.-I.; Iljana, M.; Mattila, O.; Paananen, T.; Heikkinen, E.-P.; Maeda, T.; Kunitomo, K.; Fabritius, T. Softening Behaviors of Acid and Olivine Fluxed Iron Ore Pellets in the Cohesive Zone of a Blast Furnace. ISIJ Int. 2015, 55, 2039–2046. [Google Scholar] [CrossRef] [Green Version]
- Kemppainen, A. Limiting Phenomena Related to the Use of Iron Ore Pellets in a Blast Furnace. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2015. [Google Scholar]
- Cheraghi, A.; Yoozbashizadeh, H.; Safarian, J. Gaseous Reduction of Manganese Ores: A Review and Theoretical Insight. Miner. Process. Extract. Metall. Rev. 2020, 41, 198–215. [Google Scholar] [CrossRef]
- Jonckbloedt, R. Pelletizing of Iron Ores. In Proceedings of the International Online Seminar Iron Ores, 20–21 April 2021; Steel Institute VDEh: Düsseldorf, Germany, 2021; pp. 1–46. [Google Scholar]
- Dwarapudi, S.; Ghosh, T.K.; Tathavadkar, V.; Denys, M.B.; Bhattacharjee, D.; Venugopal, R. Effect of MgO in the Form of Magnesite on the Quality and Microstructure of Hematite Pellets. Int. J. Miner. Process. 2012, 112–113, 55–62. [Google Scholar] [CrossRef]
- Nogueira, P.F.; Fruehan, R.J. Blast Furnace Burden Softening and Melting Phenomena—Melting Onset in Acid and Basic Fluxed Pellets. Ironmak. Steelmak. 2003, 30, 29–35. [Google Scholar]
- Umadevi, T.; Kumar, P.; Lobo, N.F.; Prabhu, M.; Mahapatra, P.C.; Ranjan, M. Influence of Pellet Basicity (CaO/SiO2) on Iron Ore Pellet Properties and Microstructure. ISIJ Int. 2011, 51, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Bale, C.W.; Pelton, A.D.; Thompson, W.T.; Eriksson, G.; Hack, K.; Chartrand, P.; Decterov, S.A.; Jung, I.-H.; Melançon, J.; Petersen, S. FactSage v. 7.2; Thermfact/CRCT: Montreal, QC, Canada; GTT-Technologies: Aachen, Germany, 2018. [Google Scholar]
- Jung, I.-H.; Van Ende, M.-A. Computational Thermodynamic Calculations: FactSage from CALPHAD Thermodynamic Database to Virtual Process Simulation. Metall. Mater. Trans. B 2020, 51, 1851–1874. [Google Scholar] [CrossRef]
- FactSage Database Documentation. Available online: https://www.crct.polymtl.ca/fact/documentation/ (accessed on 13 April 2021).
- Verein Deutscher Eisenhüttenleute (VDEh) (Ed.) Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995; ISBN 3-514-00457-9. [Google Scholar]
- Nogueira, P.F.; Fruehan, R.J. Blast Furnace Burden Softening and Melting Phenomena: Part III. Melt Onset and Initial Microstructural Transformations in Pellets. Metall. Mater. Trans. B 2006, 37, 551–558. [Google Scholar] [CrossRef]
- Li, T.; Sun, C.; Liu, X.; Song, S.; Wang, Q. The Effects of MgO and Al2O3 Behaviours on Softening–Melting Properties of High Basicity Sinter. Ironmak. Steelmak. 2018, 45, 755–763. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).