Fail-Safe Joints between Copper Alloy (C18150) and Nickel-Based Superalloy (GH4169) Made by Transient Liquid Phase (TLP) Bonding and Using Boron-Nickel (BNi-2) Interlayer
Abstract
:1. Introduction
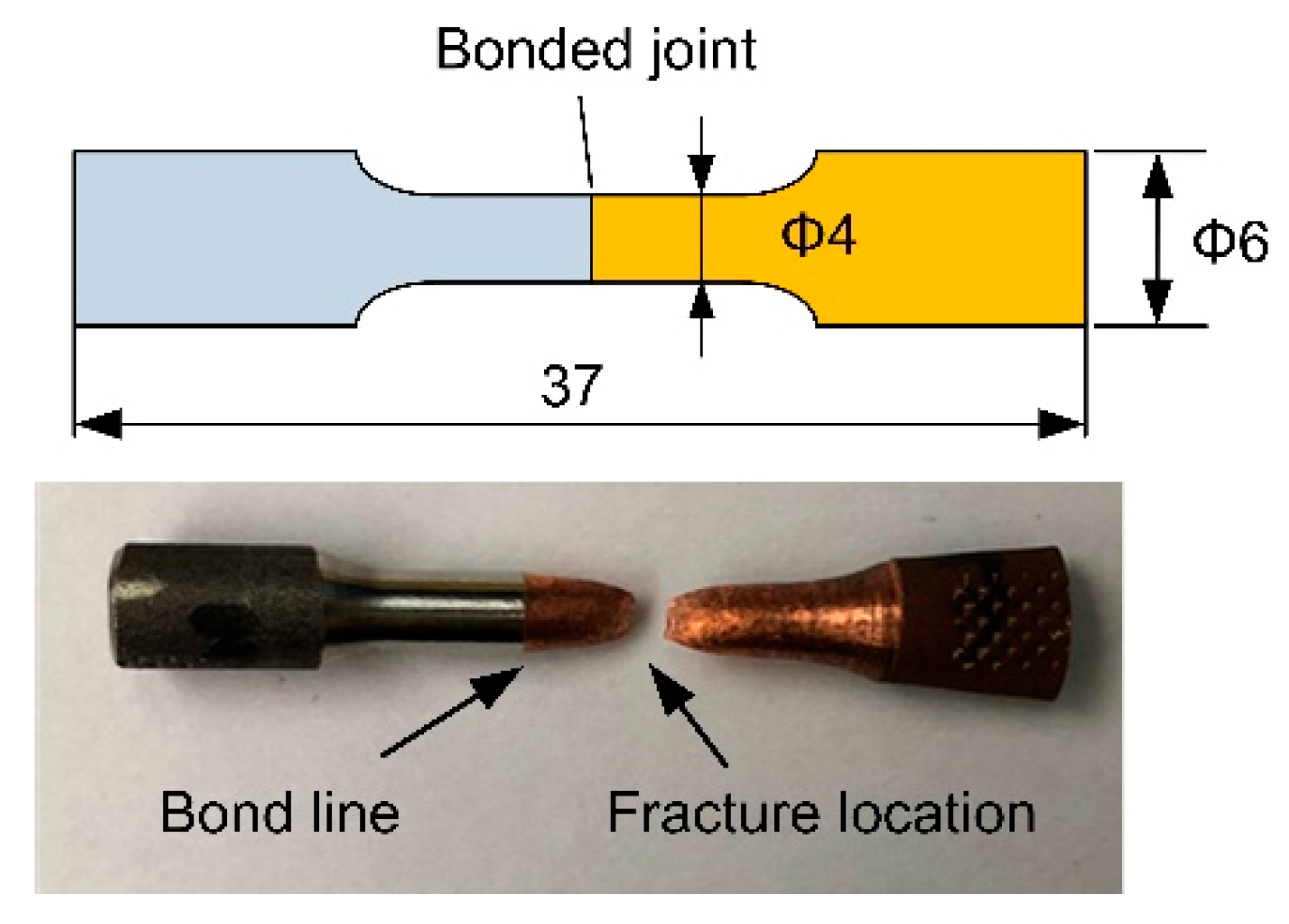
2. Materials and Methods
3. Results and Discussion
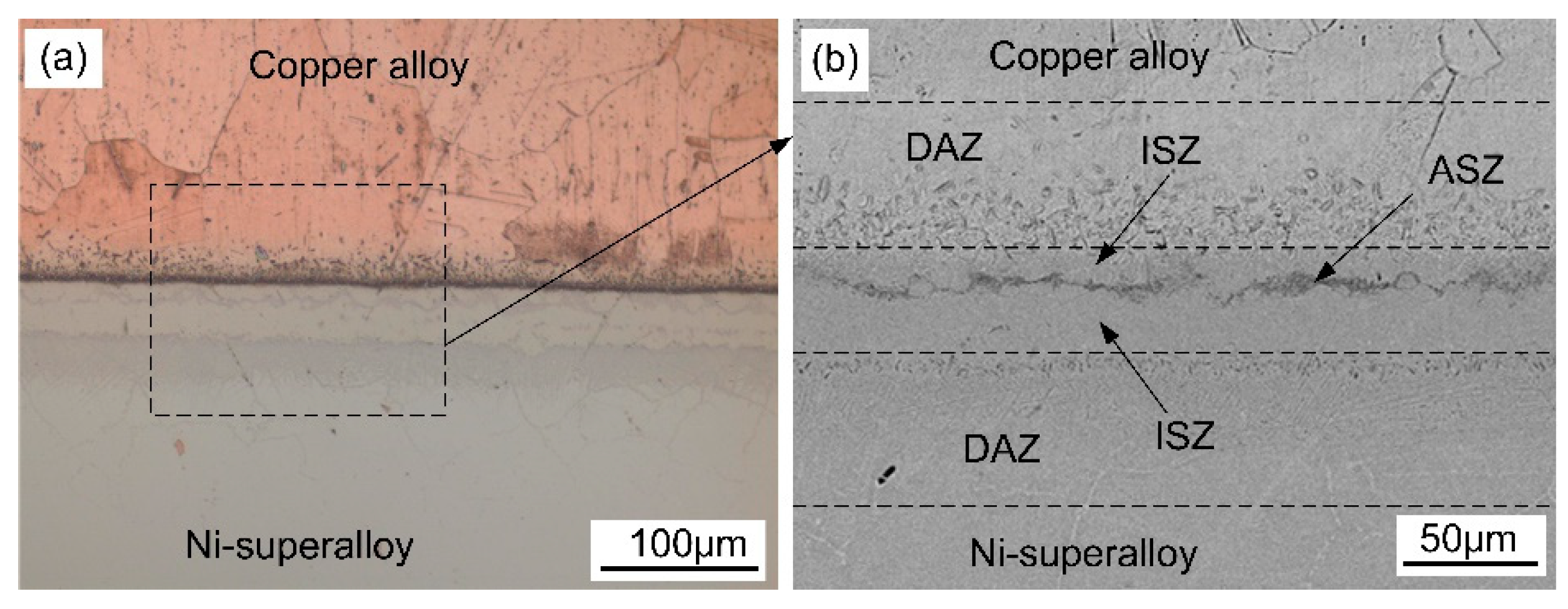
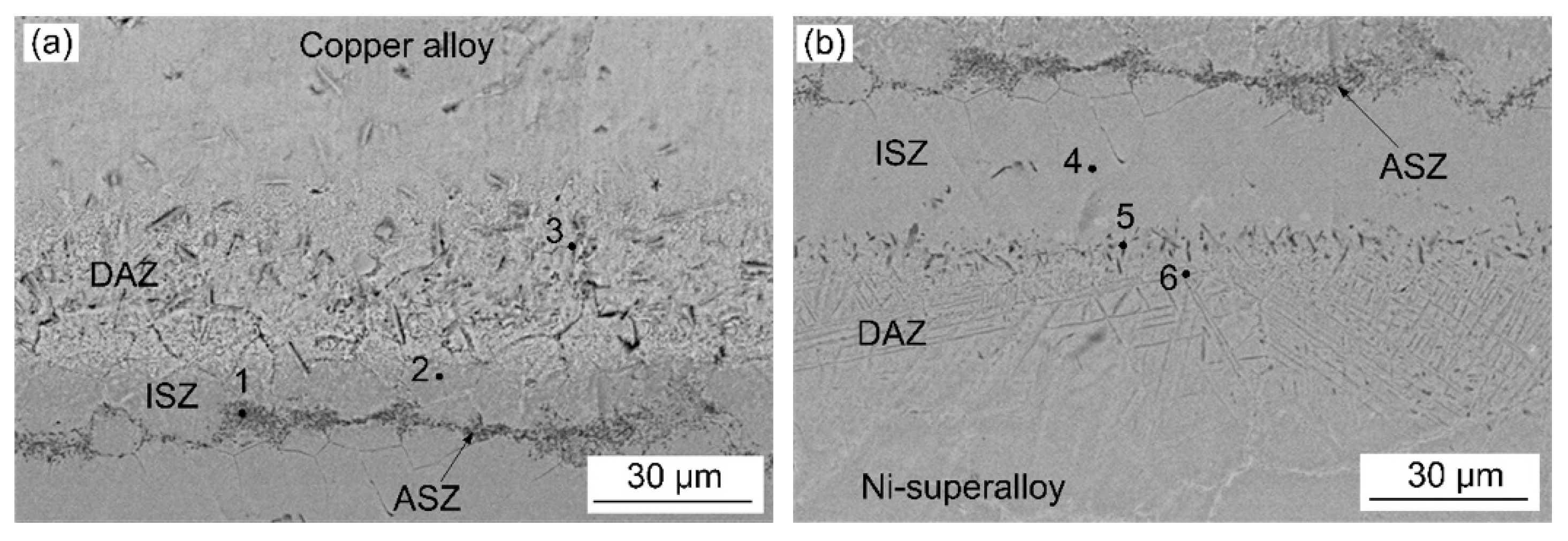
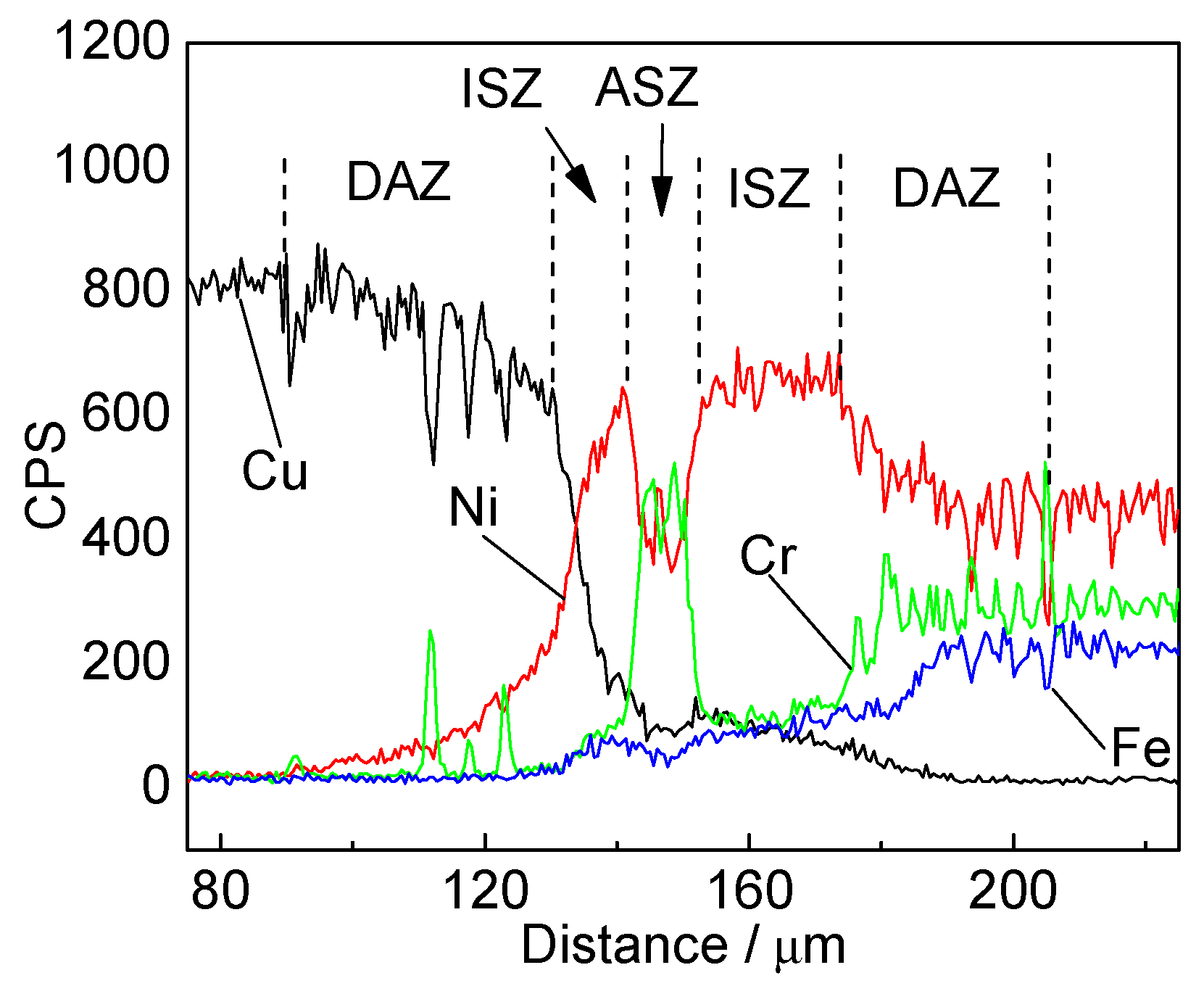
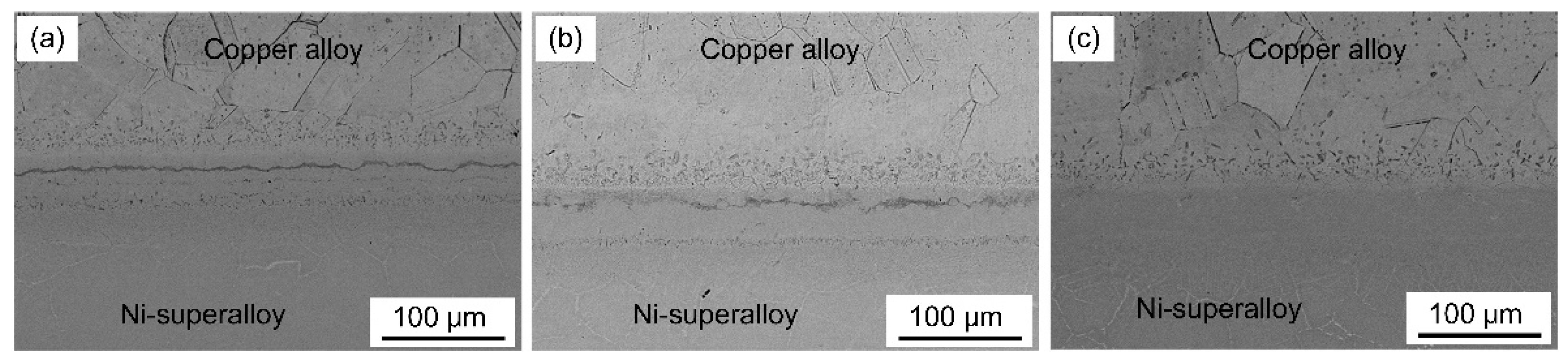
3.1. Microstructural Examinations
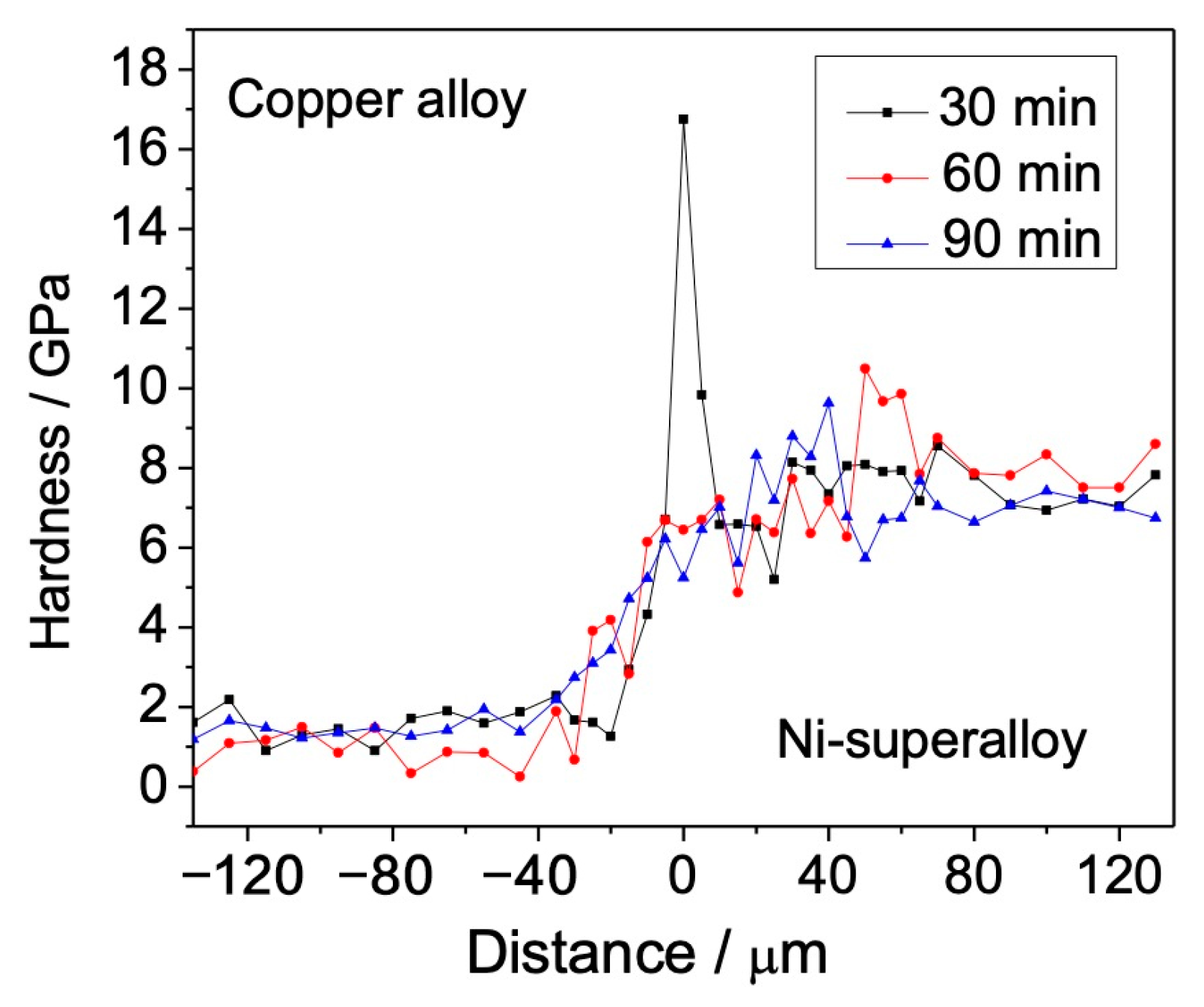
3.2. Tensile Strength and Microhardness Distribution
4. Conclusions
- Three different zones were formed in a joint made at 1030 °C for 60 min: an isothermal solidification zone (ISZ) containing Cu-Ni solid solutions on both sides; an athermal solidification zone (ASZ), which was identified by the presence of eutectic phase; and a diffusion affected zone (DAZ) located further away from the joint.
- Full isothermal solidification was achieved when bonding was performed at 1030 °C for 90 min.
- All of the tensile-tested samples failed in the copper alloy and away from the joints regardless of bonding times of 30, 60, and 90 min.
- Excluding some sharp peaks in the hardness profile due to the formation of intermetallics, the hardness gradually increases from the copper alloy to the nickel-based superalloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goods, S.H.; Puskar, J.D. Solid state bonding of CuCrZr to 316L stainless steel for ITER applications. Fusion Eng. Des. 2011, 86, 1634–1638. [Google Scholar] [CrossRef]
- Lee, H.S. Diffusion Bonding of Metal Alloys in Aerospace and Other Applications. In Welding and Joining of Aerospace Materials; Woodhead Publishing Limited: Sawston, UK, 2012; pp. 320–344. [Google Scholar]
- Sabetghadam, H.; Hanzaki, A.Z.; Araee, A. Diffusion bonding of 410 stainless steel to copper using a nickel interlayer. Mater. Charact. 2010, 61, 626–634. [Google Scholar] [CrossRef]
- Batra, I.S.; Kale, G.B.; Saha, T.K.; Ray, A.K.; Derose, J.; Krishnan, J. Diffusion bonding of a Cu-Cr-Zr alloy to stainless steel and tungsten using nickel as an interlayer. Mater. Sci. Eng. A 2004, 369, 119–123. [Google Scholar] [CrossRef]
- Singh, K.P.; Patel, A.; Bhope, K.; Khirwadkar, S.S.; Mehta, M. Optimization of the diffusion bonding parameters for SS316L/CuCrZr with and without Nickel interlayer. Fusion Eng. Des. 2016, 112, 274–282. [Google Scholar] [CrossRef]
- Zhang, C.; Shirzadi, A.A. Diffusion bonding of copper alloy to nickel-based superalloy: Effect of heat treatment on the microstructure and mechanical properties of the joints. Sci. Technol. Weld. Join. 2021, 26, 213–219. [Google Scholar] [CrossRef]
- Jamaloei, A.D.; Khorram, A.; Jafari, A. Characterization of microstructure and mechanical properties of dissimilar TLP bonding between IN718/IN600 with BNi-2 interlayer. J. Manuf. Process. 2017, 29, 447–457. [Google Scholar] [CrossRef]
- Liu, M.c.; Sheng, G.m.; He, H.j.; Jiao, Y.j. Microstructural evolution and mechanical properties of TLP bonded joints of Mar-M247 superalloys with Ni-Cr-Co-W-Ta-B interlayer. J. Mater. Process. Technol. 2017, 246, 245–251. [Google Scholar] [CrossRef]
- Hadibeyk, S.; Beidokhti, B.; Sajjadi, S.A. The effect of interlayer thickness, bonding temperature and atmosphere on transient liquid phase bonding of GTD-111 to FSX-414. J. Mater. Process. Technol. 2018, 255, 673–678. [Google Scholar] [CrossRef]
- Alhazaa, A.; Haneklaus, N. Diffusion bonding and transient liquid phase (TLP) bonding of type 304 and 316 austenitic stainless steel—A review of similar and dissimilar material joints. Metals 2020, 10, 613. [Google Scholar] [CrossRef]
- Jing, Y.; Zheng, Z.; Liu, E.; Guo, Y. Microstructural Evolution of a Ni-base Alloy DZ468 Joint Bonded with a New Co-base Filler. J. Mater. Sci. Technol. 2014, 30, 480–486. [Google Scholar] [CrossRef]
- Abbasi-Khazaei, B.; Jahanbakhsh, A.; Bakhtiari, R. TLP bonding of dissimilar FSX-414/IN-738 system with MBF-80 interlayer: The effect of homogenizing treatment on microstructure and mechanical properties. Mater. Sci. Eng. A 2016, 651, 93–101. [Google Scholar] [CrossRef]
- Binesh, B.; Gharehbagh, A.J. Transient Liquid Phase Bonding of IN738LC/MBF-15/IN738LC: Solidification Behavior and Mechanical Properties. J. Mater. Sci. Technol. 2016, 32, 1137–1151. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, H.J.; Shim, D.N.; Kim, D.J. Effect of bonding parameters on microstructural characteristics during TLP bonding of directionally solidified Ni-based superalloy. J. Manuf. Process. 2017, 30, 208–216. [Google Scholar] [CrossRef]
- Hadibeyk, S.; Beidokhti, B.; Sajjadi, S.A. Effect of bonding time and homogenization heat treatment on the microstructure and mechanical properties of the transient liquid phase bonded dissimilar GTD-111/FSX-414 TLP superalloys. J. Alloys Compd. 2018, 731, 929–935. [Google Scholar] [CrossRef]
- Azqadan, E.; Ekrami, A. Transient liquid phase bonding of dual phase steels using Fe-based, Ni-based, and pure Cu interlayers. J. Manuf. Process. 2017, 30, 106–115. [Google Scholar] [CrossRef]
- Esmaeili, H.; Mirsalehi, S.E.; Farzadi, A. Vacuum TLP bonding of Inconel 617 superalloy using Ni-Cr-Si-Fe-B filler metal: Metallurgical structure and mechanical properties. Vacuum 2018, 152, 305–311. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Transient liquid phase bonding of wrought IN718 nickel based superalloy using standard heat treatment cycles: Microstructure and mechanical properties. Mater. Des. 2013, 50, 694–701. [Google Scholar] [CrossRef]
- Bakhtiari, R. Effect of configuration and composition of interlayer on TLP joints of FSX-414 superalloy. J. Mater. Process. Technol. 2016, 231, 8–17. [Google Scholar] [CrossRef]
- Shamsabadi, A.Y.; Bakhtiari, R.; Eisaabadi, B.G. TLP bonding of IN738/MBF20/IN718 system. J. Alloys Compd. 2016, 685, 896–904. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Solidification and solid state phenomena during TLP bonding of IN718 superalloy using Ni-Si-B ternary filler alloy. J. Alloys Compd. 2013, 563, 143–149. [Google Scholar] [CrossRef]
- Shirzadi, A.A.; Saindrenan, G. New method for flux free diffusion brazing of aluminium alloys using liquid gallium (UK patent application 0128623.6). Sci. Technol. Weld. Join. 2003, 8, 149–153. [Google Scholar] [CrossRef]
- Zhang, L.X.; Sun, Z.; Xue, Q.; Lei, M.; Tian, X.Y. Transient liquid phase bonding of IC10 single crystal with GH3039 superalloy using BNi2 interlayer: Microstructure and mechanical properties. Mater. Des. 2016, 90, 949–957. [Google Scholar] [CrossRef]


| Materials | Cu | Ni | Cr | Fe | Ti | Nb | Mo | Zr | Si | B |
|---|---|---|---|---|---|---|---|---|---|---|
| C18150 | Bal. | - | 0.80 | 0.50 | - | - | - | 0.10 | - | - |
| GH4169 | - | Bal. | 18.19 | 17.34 | 1.22 | 4.29 | 2.44 | - | - | - |
| BNi-2 | - | Bal. | 7 | 3 | - | - | - | - | 4.5 | 3.2 |
| Positions | Cu | Ni | Cr | Fe | Ti | Nb | Mo | Si | B |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.3 | 32.7 | 33.1 | 2.6 | - | - | - | 1.5 | 20.8 |
| 2 | 22.1 | 63.5 | 6.1 | 4.6 | - | - | - | 3.8 | - |
| 3 | 77.6 | 8.7 | 2.4 | - | - | - | - | - | 11.3 |
| 4 | 8.8 | 72.8 | 7.4 | 7.3 | - | - | - | 3.7 | - |
| 5 | 3.0 | 39 | 17.6 | 6.3 | 0.3 | 1.6 | 0.8 | 30.8 | |
| 6 | - | 20 | 7.3 | 7.1 | 0.4 | 1.1 | 0.7 | - | 63.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Shirzadi, A. Fail-Safe Joints between Copper Alloy (C18150) and Nickel-Based Superalloy (GH4169) Made by Transient Liquid Phase (TLP) Bonding and Using Boron-Nickel (BNi-2) Interlayer. Metals 2021, 11, 1504. https://doi.org/10.3390/met11101504
Zhang C, Shirzadi A. Fail-Safe Joints between Copper Alloy (C18150) and Nickel-Based Superalloy (GH4169) Made by Transient Liquid Phase (TLP) Bonding and Using Boron-Nickel (BNi-2) Interlayer. Metals. 2021; 11(10):1504. https://doi.org/10.3390/met11101504
Chicago/Turabian StyleZhang, Chengcong, and Amir Shirzadi. 2021. "Fail-Safe Joints between Copper Alloy (C18150) and Nickel-Based Superalloy (GH4169) Made by Transient Liquid Phase (TLP) Bonding and Using Boron-Nickel (BNi-2) Interlayer" Metals 11, no. 10: 1504. https://doi.org/10.3390/met11101504
APA StyleZhang, C., & Shirzadi, A. (2021). Fail-Safe Joints between Copper Alloy (C18150) and Nickel-Based Superalloy (GH4169) Made by Transient Liquid Phase (TLP) Bonding and Using Boron-Nickel (BNi-2) Interlayer. Metals, 11(10), 1504. https://doi.org/10.3390/met11101504





