Histomorphometric Analysis of Osseointegrated Grade V Titanium Mini Transitional Implants in Edentulous Mandible by Backscattered Scanning Electron Microscopy (BS-SEM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Surgical Procedure
2.2. Histomorphometric Analysis of Samples by BS-SEM
- Bone tissue classification present in the BAFO considering trabecular or cortical bone. The classification was determined considering the predominant form of bone present.
- Bone to implant contact (%BIC): The contact between the bone-calcified tissue and implant surface is expressed as the BIC percentage according to the total implant surface between two threads (Figure 2).
- Bone fill percentage (%Bf): This is the calcified tissue percentage in the BAFO interspersed by vascular and marrow spaces.
- Lamellar bone (LB): This bone tissue type exhibits osteonal organization and is measured according to lamellar apposition (Figure 2).
- Fibroreticular or woven bone (WB): This bone tissue type has a regular bone structure with isolated and polygonal cellular spaces (Figure 2).
- Calcified chondroid tissue (ChT): This bone tissue type presents a characteristic aspect of calcified tissues with large, irregular, and confluent cellular spaces (Figure 2).
- Vascular or medullar spaces: black spaces in the BAFO close to the calcified tissue compatible with spaces occupied by blood vessels or bone marrow (Figure 2).
3. Results
3.1. General Aspects
3.2. Histomorphology Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mundt, T.; Schwahn, C.; Stark, T.; Biffar, R. Clinical response of edentulous people treated with mini dental implants in nine dental practices. Gerodontology 2015, 32, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Veltri, M.; Ferrari, M.; Balleri, P. One-year outcome of narrow diameter blasted implants for rehabilitation of maxillas with knife-edge resorption. Clin. Oral Implants Res. 2008, 19, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.; Saad, M.; Daas, M.; Abdallah, J.; Abdallah, R. Use of narrow-diameter implants in the posterior jaw: A systematic review. Implant Dent. 2015, 24, 294–306. [Google Scholar] [CrossRef]
- De Almeida, F.D.; Carvalho, A.C.; Fontes, M.; Pedrosa, A.; Costa, R.; Noleto, J.W.; Mourao, C.F. Radiographic evaluation of marginal bone level around internal-hex implants with switched platform: A clinical case report series. Int. J. Oral Maxillofac. Implants 2011, 26, 587–592. [Google Scholar] [PubMed]
- Calvo-Guirado, J.L.; Gómez-Moreno, G.; Aguilar-Salvatierra, A.; Guardia, J.; Delgado-Ruiz, R.A.; Romanos, G.E. Marginal bone loss evaluation around immediate non-occlusal microthreaded implants placed in fresh extraction sockets in the maxilla: A 3-year study. Clin. Oral Implants Res. 2015, 26, 761–767. [Google Scholar] [CrossRef]
- Diaz-Sanchez, R.-M.; de-Paz-Carrion, A.; Serrera-Figallo, M.-A.; Torres-Lagares, D.; Barranco, A.; León-Ramos, J.-R.; Gutierrez-Perez, J.-L. In Vitro and In Vivo Study of Titanium Grade IV and Titanium Grade V Implants with Different Surface Treatments. Metals 2020, 10, 449. [Google Scholar] [CrossRef]
- Imam, M.; Fraker, A. Titanium Alloys as Implant Materials. In Medical Applications of Titanium and Its Alloys: The Material and Biological Issues; Lemons, J., Brown, S., Eds.; ASTM International: West Conshohocken, PA, USA, 1996; pp. 3–16. [Google Scholar]
- Sharan, J.; Lale, S.V.; Koul, V.; Mishra, M.; Kharbanda, O.P. An Overview of Surface Modifications of Titanium and its Alloys for Biomedical Applications. Trends Biomater. Artif. Organs 2015, 29, 176–187. [Google Scholar]
- Ribeiro da Silva, J.; Castellano, A.; Malta Barbosa, J.P.; Gil, L.F.; Marin, C.; Granato, R.; Bonfante, E.A.; Tovar, N.; Janal, M.N.; Coelho, P.G. Histomorphological and histomorphometric analyses of grade IV commercially pure titanium and grade V Ti-6Al-4V titanium alloy implant substrates: An in vivo study in dogs. Implant Dent. 2016, 25, 650–655. [Google Scholar] [CrossRef]
- Johansson, C.B.; Han, C.H.; Wenneberg, A.; Albrektsson, T. A quantitative comparison of machined commercially pure titanium and titanium-aluminum-vanadium implants in rabbit bone. Int. J. Oral Maxillofac. Implants 1998, 13, 315–321. [Google Scholar]
- Han, C.H.; Johansson, C.B.; Wenneberg, A.; Albrektsson, T. Quantitative and qualitative investigations of surface enlarged titanium and titanium alloy implants. Clin. Oral Implants Res. 1998, 9, 1–10. [Google Scholar] [CrossRef]
- Saulacic, N.; Bosshardt, D.D.; Bornstein, M.M.; Berner, S.; Buser, D. Bone apposition to a titanium-zirconium alloy implant, as compared to two other titanium-containing implants. Eur. Cells Mater. 2012, 23, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Williams, R.L.; Williams, D.F. Conjoint corrosion and wear in titanium alloys. Biomaterials 1999, 20, 765–772. [Google Scholar] [CrossRef]
- Pennekamp, P.H.; Gessmann, J.; Diedrich, O.; Burian, B.; Wimmer, M.A.; Frauchiger, V.M.; Kraft, C.N. Short-term microvascular response of striated muscle to cp-Ti, Ti-6Al-4V, and Ti-6Al-7Nb. J. Orthopaedic Res. Off. Publ. Orthopaedic Res. Soc. 2006, 24, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, M.C.; Franch, J.; Carvalho, P.; Belmonte, A.M.; Tusell, J.; Franch, B.; Fernandez, J.M.; Cleries, L.; Morenza, J.L. BS-SEM evaluation of the tissular interactions between cortical bone and calcium-phosphate covered titanium implants. Bull. Group Int. Rech. Sci. Stomatol. Odontol. 2001, 43, 100–108. [Google Scholar] [PubMed]
- Schnitman, P.A.; Wohrle, P.S.; Rubenstein, J.E. Immediate fixed interim prostheses supported by two-stage threaded implants: Methodology and results. J. Oral Implantol. 1990, 16, 96–105. [Google Scholar] [PubMed]
- Manzanares, M.C.; Calero, M.I.; Franch, J.; Jiménez, M.P.; Serra, I. Optimisation of a scheduled study for undecalcified samples. Microsc. Anal. 1997, 50, 17–19. [Google Scholar]
- Franch, J.; Pastor, J.; Franch, B.; Durall, I.; Manzanares, M.C. Back-scattered electron imaging of a non-vertebral case of hypervitaminosis A in a cat. J. Feline Med. Surg. 2000, 2, 49–56. [Google Scholar] [CrossRef]
- Castellano, A.; Gil, L.F.; Bonfante, E.A.; Tovar, N.; Neiva, R.; Janal, M.N.; Coelho, P.G. Early healing evaluation of commercially pure titanium and Ti-6Al-4V presenting similar surface texture: An in vivo study. Implant Dent. 2017, 26, 338–344. [Google Scholar] [CrossRef]
- Granato, R.; Bonfante, E.; Castellano, A.; Khan, R.; Jimbo, R.; Marin, C.; Morsi, S.; Witek, L.; Coelho, P.G. Osteointegrative and microgeometric comparison between micro-blasted and alumina blasting/acid etching on grade III and V titanium alloys (Ti-6Al-4V). J. Mech. Behav. Biomed. Mater. 2019, 97, 288–295. [Google Scholar] [CrossRef]
- Zhang, H.; Lewis, C.G.; Aronow, M.S.; Gronowicz, G.A. The effects of patient age on human osteoblasts’ response to Ti-6Al-4V implants in vitro. J. Orthop. Res. 2004, 22, 30–38. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Effects of occlusal forces on the peri-implant-bone interface stability. Periodontology 2000 2019, 81, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Romero, D.M.; Huang, S.; Rahman, J.; Currey, J.A.; Nanci, A.; Brunski, J.B.; Helms, J.A. Molecular analysis of healing at a bone-implant interface. J. Dent. Res. 2007, 86, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.S.; Araujo, M.A.; Benfatti, C.A.; Araujo Cdos, R.; Piattelli, A.; Perrotti, V.; Lezzi, G. Comparative histological and histomorphometrical evaluation of marginal bone resorption around external hexagon and Morse cone implants: An experimental study in dogs. Implant Dent. 2014, 23, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.S.; Ren, L.F.; Wang, L.Z.; Lin, H.S.; Wang, S.B.; Tong, Y.Q. H2O2/HCl and heat-treated Ti-6Al-4V stimulates pre-osteoblast proliferation and differentiation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, P.; Zhang, H.; Guo, Z.; Zheng, Y.; Han, Y. Osteoimmunomodulation, osseointegration, and in vivo mechanical integrity of pure Mg coated with HA nanorod/pore-sealed MgO bilayer. Biomater. Sci. 2018, 6, 3202–3218. [Google Scholar] [CrossRef]
- Shi, R.; Hayashi, K.; Ishikawa, K. Rapid Osseointegration Bestowed by Carbonate Apatite Coating of Rough Titanium. Adv. Mater. Interfaces 2020, 7, 2000636. [Google Scholar] [CrossRef]
- Vannozzi, L.; Gouveia, P.; Pingue, P.; Canale, C.; Ricotti, L. Novel Ultrathin Films Based on a Blend of PEG-b-PCL and PLLA and Doped with ZnO Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21398–21410. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible im- plant surfaces Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Fourmousis, I.; Karoussis, I.K.; Bamia, C. A systematic review and meta-analysis on the effect of implant length on the survival of rough-surface dental implants. J. Periodontol. 2009, 80, 1700–1718. [Google Scholar] [CrossRef]
- McCracken, M. Dental Implant Materials: Commercially Pure Titanium and Titanium Alloys. J. Prosthodontics 1999, 8, 40–43. [Google Scholar] [CrossRef]
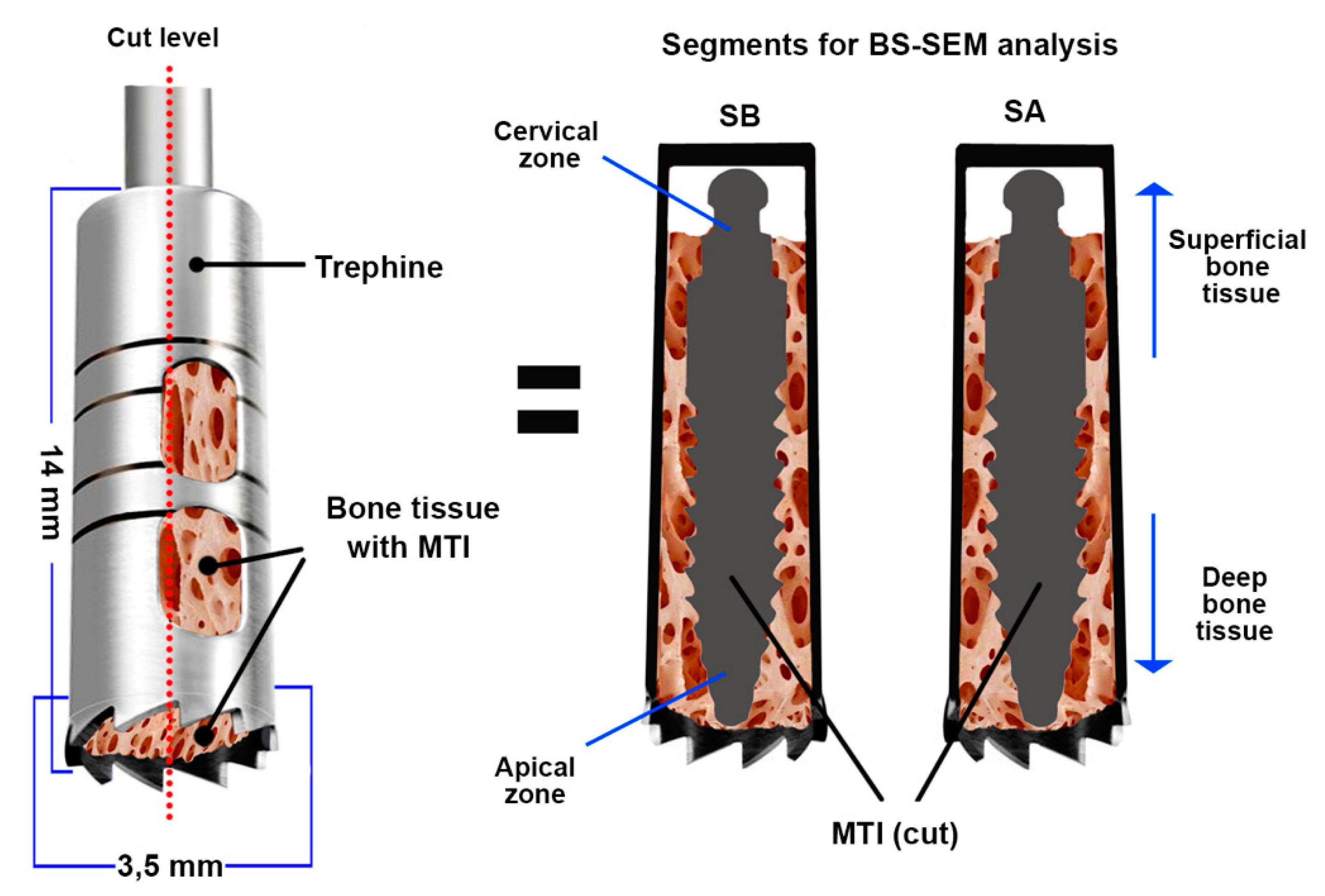

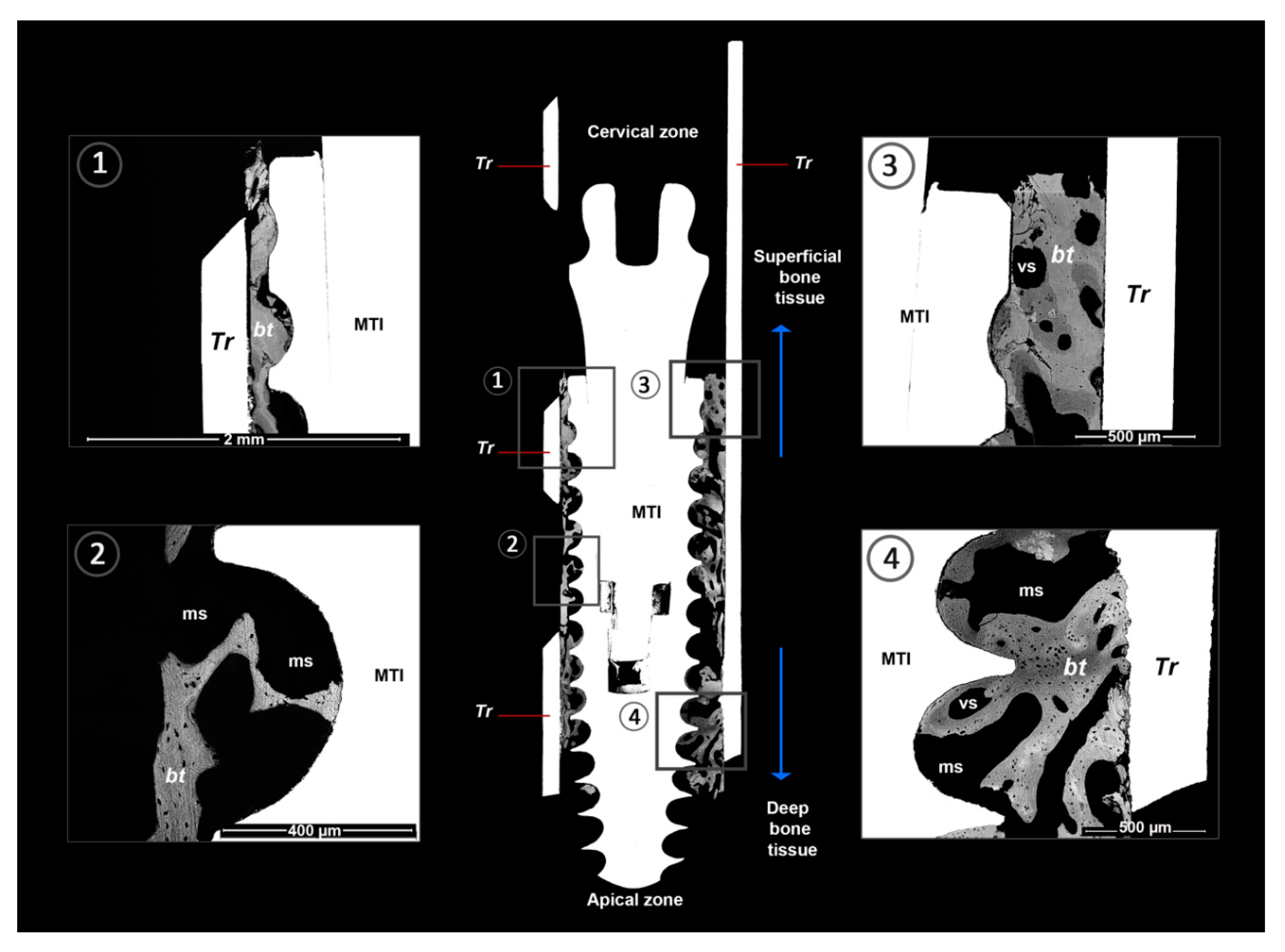
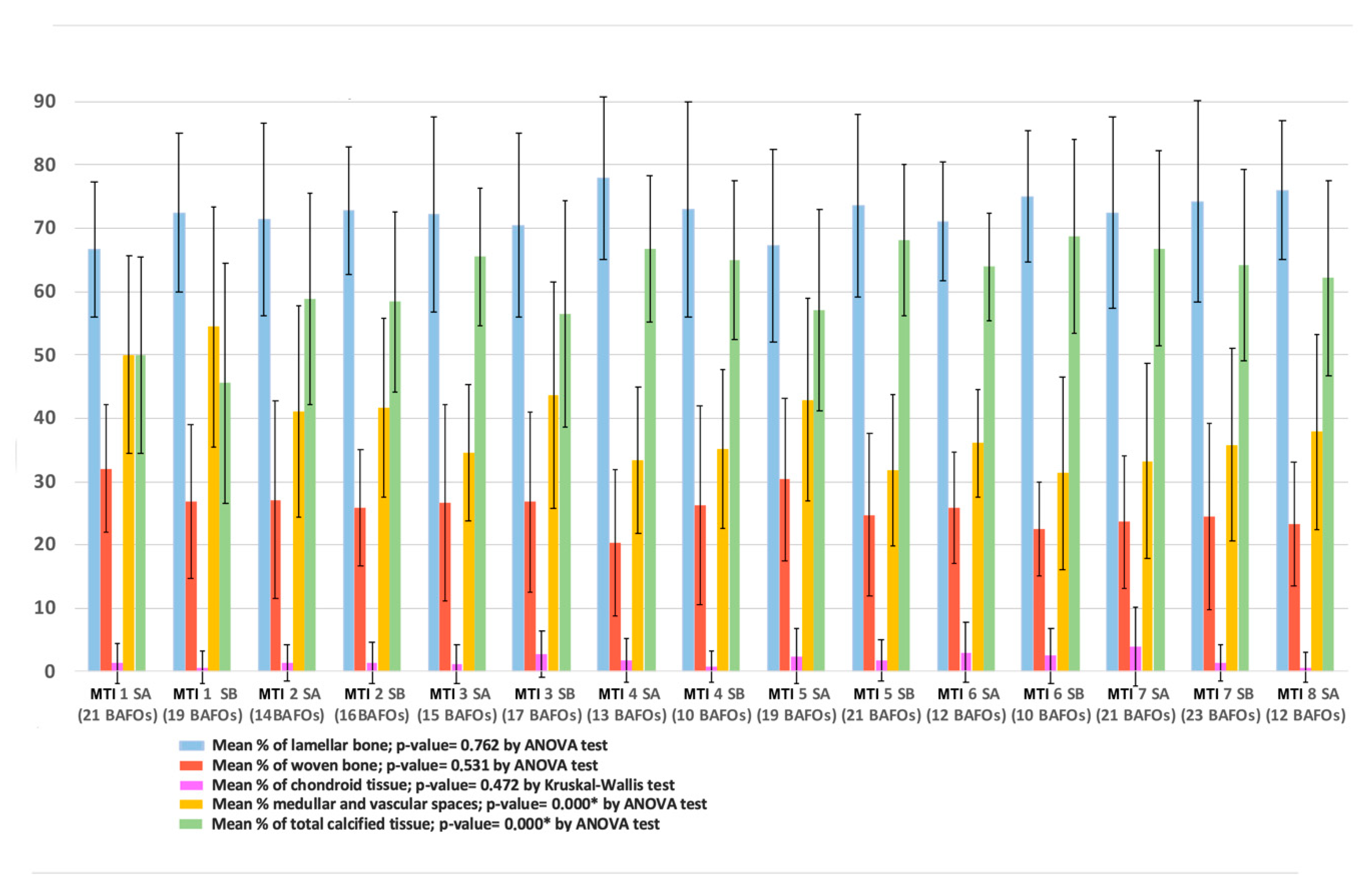
| Bone Tissue | Number of BAFOs Analyzed (Total: 306) | %Bf in BAFO | %BIC (Total) |
|---|---|---|---|
| Mainly filled with cortical bone | 94 | 37% to 100% (mean 62% SD 15.65) | 63% |
| Mainly filled with trabecular bone | 149 | 24% to 73% (mean 44% SD 14.01) | 37% |
| Without calcified tissue | 63 | --- | --- |
| Bone Tissue Types | Mean % (SD) |
|---|---|
| Lamellar bone (% of calcified tissues in BAFOs) | 72.13% (13.58) |
| Woven bone (% of calcified tissues in BAFOs) | 26.04% (12.39) |
| Chondroid bone (% of calcified tissues in BAFOs) | 1.82% (3.69) |
| Medullar and vascular spaces (considering all BAFOs area) | 39.41% (15.98) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán, V.; Weber, B.; Lillo, R.; Manzanares, M.-C.; Sanzana, C.; Fuentes, N.; Acuña-Mardones, P.; Valdivia-Gandur, I. Histomorphometric Analysis of Osseointegrated Grade V Titanium Mini Transitional Implants in Edentulous Mandible by Backscattered Scanning Electron Microscopy (BS-SEM). Metals 2021, 11, 2. https://doi.org/10.3390/met11010002
Beltrán V, Weber B, Lillo R, Manzanares M-C, Sanzana C, Fuentes N, Acuña-Mardones P, Valdivia-Gandur I. Histomorphometric Analysis of Osseointegrated Grade V Titanium Mini Transitional Implants in Edentulous Mandible by Backscattered Scanning Electron Microscopy (BS-SEM). Metals. 2021; 11(1):2. https://doi.org/10.3390/met11010002
Chicago/Turabian StyleBeltrán, Víctor, Benjamín Weber, Ricardo Lillo, María-Cristina Manzanares, Cristina Sanzana, Nicolás Fuentes, Pablo Acuña-Mardones, and Ivan Valdivia-Gandur. 2021. "Histomorphometric Analysis of Osseointegrated Grade V Titanium Mini Transitional Implants in Edentulous Mandible by Backscattered Scanning Electron Microscopy (BS-SEM)" Metals 11, no. 1: 2. https://doi.org/10.3390/met11010002
APA StyleBeltrán, V., Weber, B., Lillo, R., Manzanares, M.-C., Sanzana, C., Fuentes, N., Acuña-Mardones, P., & Valdivia-Gandur, I. (2021). Histomorphometric Analysis of Osseointegrated Grade V Titanium Mini Transitional Implants in Edentulous Mandible by Backscattered Scanning Electron Microscopy (BS-SEM). Metals, 11(1), 2. https://doi.org/10.3390/met11010002







