Discovery of New Ti-Based Alloys Aimed at Avoiding/Minimizing Formation of α” and ω-Phase Using CALPHAD and Artificial Intelligence
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Stable and Metastable Phases
2.2. Deep Learning Artificial Neural Network (DLANN) Model
2.3. Self Organizing Maps (SOM)
2.4. Computational Infrastructure
2.4.1. CALPHAD-Based Work
2.4.2. Artificial Intelligence-Based Work
3. Results
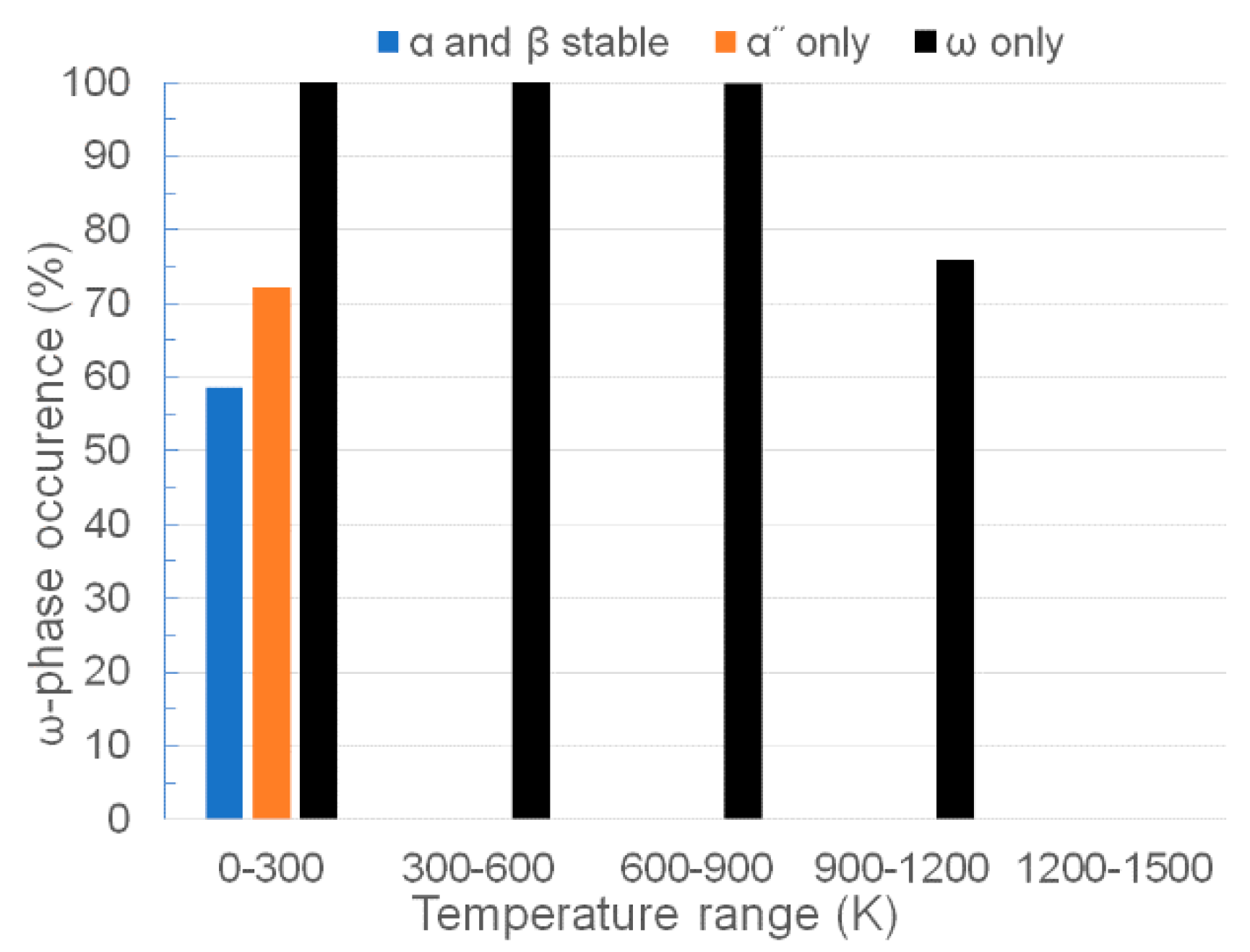
3.1. Stability of Stable and Metastable Phases
3.2. DLANN Model
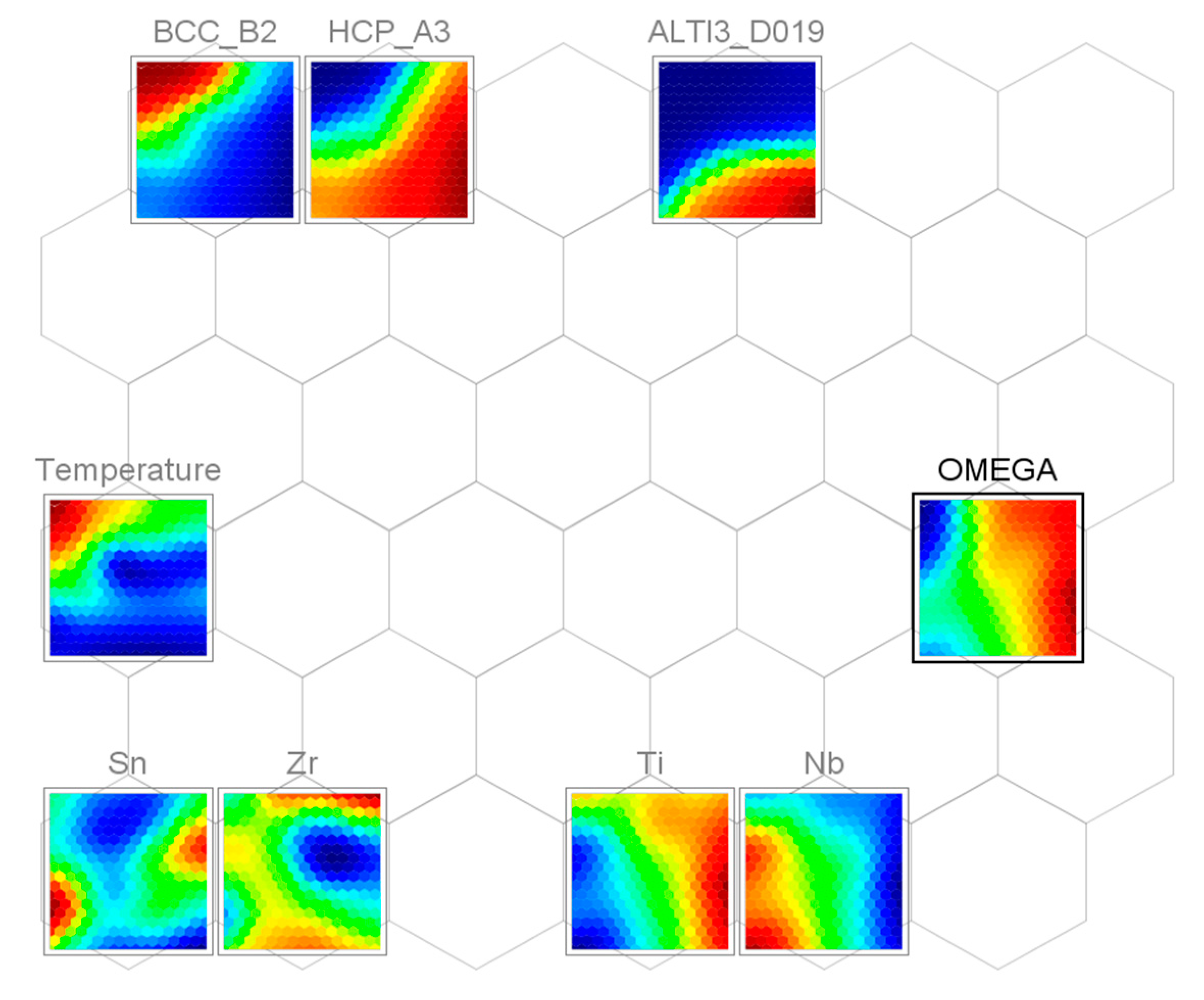
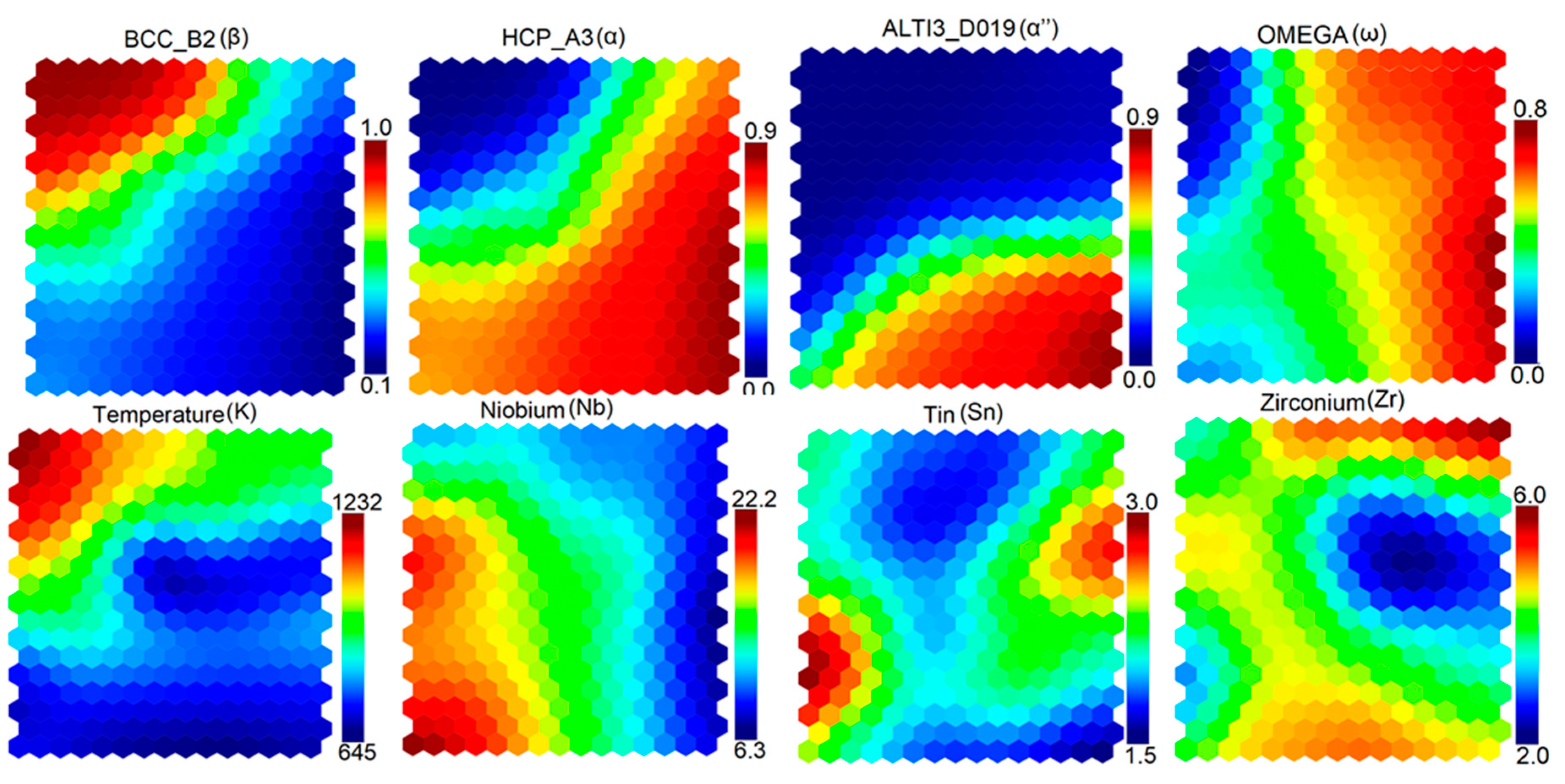
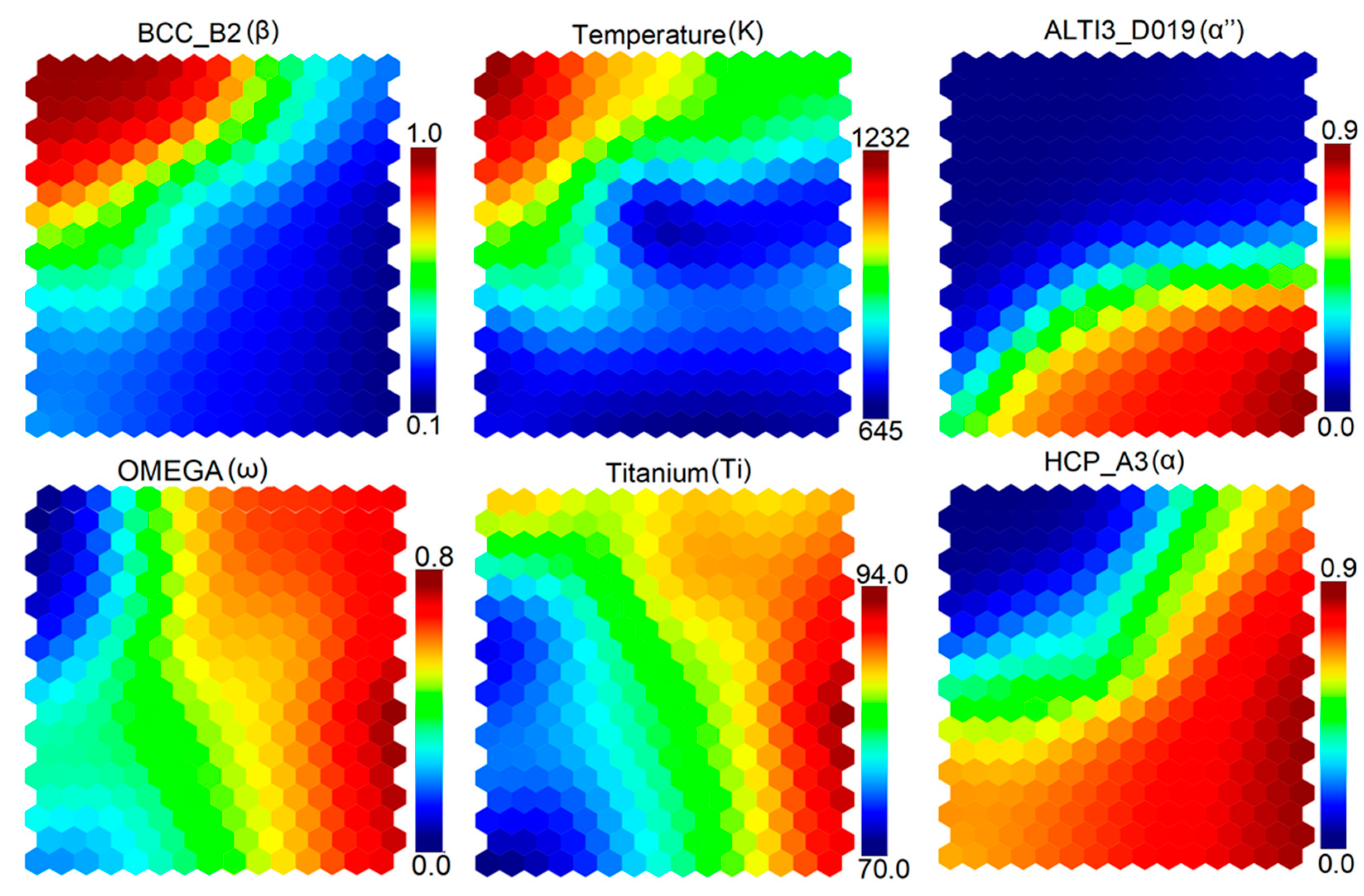
3.3. Self-Organizing Maps (SOM)
4. Discussion
Future Work
- Develop predictive models for Young’s modulus of new proposed alloys through the CALPHAD approach and AI algorithms.
- Study kinetics of precipitation of various stable and metastable phases within the framework of the CALPHAD approach and work with solidification simulation to have a better understanding of precipitation of various stable and metastable phases for different cooling rates. Thereafter, study precipitation kinetics of nucleation and growth of various phases.
- ○
- This study will be helpful for understanding micro-segregation, especially for cast prosthetics [13,44,45,46]. Studies have shown that during solidification, it is difficult to avoid composition variation in the inter-dendritic region due to solute entrapment, which thus makes the casting composition non-homogeneous [44]. Micro-segregation can be controlled by properly choosing the cooling rate [13,44]. Thus, solidification simulation will be helpful in understanding the temperature regimes where a certain desired or undesired phase is stable [44]. This way, one should be able to design a cooling rate that is fast enough to avoid ageing in the temperature regimes where undesired phases are unstable.
- ○
- Heat-treatment simulations are equally important [46]. Some of these alloys are subjected to ageing at a defined temperature for a prolonged time (several hours). Through heat treatment simulations, one can obtain an estimate of the grain size and volume fractions of a desired phase and observe its growth over time. Grain size and volume fraction affect the Young’s modulus of an alloy, so this study is important.
- Simulate microstructure evolution, micro-segregation, composition variation in the inter-dendritic regions [47,48,49] under the framework of the CALPHAD and phase field approach [47,48,49].
- ○
- The phase field approach is a popular approach for simulating microstructure evolution. A user can get insights required for the understanding of the solidification process and can study the growth of dendrites and composition variation in inter-dendritic regions, which is important for addressing micro-segregation [47,48,49]. The CALPHAD approach will be used for providing vital information on thermodynamics and kinetics to the phase-field models especially regarding the sequence of precipitation of a phase as well as stability of various phases [49]. The CALPHAD approach also provides the grain size, and this information can be used to calibrate the phase field model [49].
- Design new manufacturing protocols with special emphasis on additive manufacturing [50,51,52,53,54,55,56].
- ○
- ○
- Several modes of designing new parts through additive manufacturing exist, such as selective laser beam, electron beam, etc. [50,51,52,56]. All of these methods have advantages and limitations [50,51,52,56]. Optimization of operation parameters plays a vital role in achieving targeted properties of a prosthetic/implant manufactured by additive manufacturing [50,53].
- ○
- CALPHAD, and the phase field approach have been used for studying microstructure evolution for additively manufactured parts [47,48,49]. AI algorithms have been used to study data and develop inexpensive predictive models within the framework of additive manufacturing [57]. We plan to work on these topics.
- Finally, the most important characteristic of an implant is its biocompatibility, and osteointegration [55,58,59,60,61,62]. Several coatings have been developed and there is always room for improvement [55,58,59,60,61,62]. We plan to use AI-based tools to understand these coatings and possibly design new coatings with enhanced biocompatibility and osteointegration.
5. Conclusions
- Data for various stable and metastable phases were generated for about 3000 composition and temperature values of a Ti-Nb-Zr-Sn system through the commercial software Thermo-Calc and TCTI database for titanium alloys.
- Phase stability data were used for developing deep learning artificial neural network (DLANN) models for various phases as a function of alloy composition and temperature. DLANN models were used to predict the concentrations of phases for new compositions and temperatures. DLANN models can be used on a personal computer and even on an Android phone.
- The SOM algorithm was used to determine correlations among alloying elements, temperature, and various stable and metastable phases.
- Finally, we predicted compositions of five select alloys that are expected to meet our expectations regarding the phase stability of β phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Long, M.; Rack, H. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Marker, C. Development of a Knowledge Base of Ti-Alloys from First-Principles and Thermodynamic Modeling. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, August 2017. Available online: https://www.proquest.com/docview/1988756108 (accessed on 30 October 2019).
- Jung, H.D.; Jang, T.S.; Wang, L.; Kim, H.E.; Koh, Y.H.; Song, J. Novel strategy for mechanically tunable and bioactive metal implants. Biomaterials 2015, 37, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, T.S.; Song, J.; Kim, H.E.; Jung, H.D. Multi-scale porous Ti6Al4V scaffolds with enhanced strength and biocompatibility formed via dynamic freeze-casting coupled with micro-arc oxidation. Mater. Lett. 2016, 185, 21–24. [Google Scholar] [CrossRef]
- Jang, T.S.; Kim, D.; Han, G.; Yoon, C.B.; Jung, H.D. Powder based additive manufacturing for biomedical application of titanium and its alloys: A review. Biomed. Eng. Lett. 2020, 10, 505–516. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Beta titanium alloys: The lowest elastic modulus for biomedical applications: A review. Int. J. Chem. Nucl. Metall. Mater. Eng. 2014, 8, 726–731. [Google Scholar]
- Soundararajan, S.R.; Vishnu, J.; Manivasagam, G.; Muktinutalapati, N.R. Processing of Beta Titanium Alloys for Aerospace and Biomedical Applications. In Titanium Alloys—Novel Aspects of Their Processing; IntechOpen: London, UK, 2018. [Google Scholar]
- Magdalen, H.C.; Baghi, A.D.; Ghomashchi, R.; Xiao, W.; Oskouei, R.H. Effect of niobium content on the microstructure and Young’s modulus of Ti-xNb-7Zr alloys for medical implants. J. Mech. Behav. Biomed. 2019, 99, 78–85. [Google Scholar]
- Banerjee, S.; Tewari, R.; Dey, G.K. Omega phase transformation—Morphologies and mechanisms. Int. J. Mater. Res. 2006, 97, 963–977. [Google Scholar] [CrossRef]
- Mantri, S.; Choudhuri, D.; Behera, A.; Hendrickson, M.; Alam, T.; Banerjee, R. Role of isothermal omega phase precipitation on the mechanical behavior of a Ti-Mo-Al-Nb alloy. Mater. Sci. Eng. A 2019, 767, 138397. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Manivasagam, G.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Aleixo, G.T.; Afonso, C.; Coelho, A.; Caram, R. Effects of Omega Phase on Elastic Modulus of Ti-Nb Alloys as a Function of Composition and Cooling Rate. Solid State Phenom. 2008, 138, 393–398. [Google Scholar] [CrossRef]
- De Fontaine, D.; Paton, N.; Williams, J. The omega phase transformation in titanium alloys as an example of displacement controlled reactions. Acta Met. 1971, 19, 1153–1162. [Google Scholar] [CrossRef]
- Ferrari, A.; Paulsen, A.; Langenkämper, D.; Piorunek, D.; Somsen, C.; Frenzel, J.; Rogal, J.; Eggeler, G.; Drautz, R. Discovery of ω-free high-temperature Ti-Ta-X shape memory alloys from first-principles calculations. Phys. Rev. Mater. 2019, 3, 103605. [Google Scholar] [CrossRef]
- Ito, A.; Okazaki, Y.; Tateishi, T.; Ito, Y. In vitro biocompatibility, mechanical properties, and corrosion resistance of Ti-Zr-Nb-Ta-Pd and Ti-Sn-Nb-Ta-Pd alloys. J. Biomed. Mater. Res. 1995, 29, 893–899. [Google Scholar] [CrossRef]
- Boyce, B.F.; McWilliams, S.; Mocan, M.Z.; Elder, H.Y.; Boyle, I.T.; Junor, B.J.R. Histological and electron microprobe studies of mineralization in aluminum related osteomalacia. J. Clin. Pathol. 1992, 45, 502–508. [Google Scholar] [CrossRef]
- Domingo, J.L. Vanadium and Tungsten Derivatives as Antidiabetic Agents. Biol. Trace Elem. Res. 2002, 88, 97–112. [Google Scholar] [CrossRef]
- Tane, M.; Akita, S.; Nakano, T.; Hagihara, K.; Umakoshi, Y.; Niinomi, M.; Nakajima, H. Peculiar elastic behavior of Ti–Nb–Ta–Zr single crystals. Acta Mater. 2008, 56, 2856–2863. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Aleixo, G.T.; Lopes, E.; Contieri, R.; Cremasco, A.; Afonso, C.R.; Caram, R. Effects of Cooling Rate and Sn Addition on the Microstructure of Ti-Nb-Sn Alloys. Solid State Phenom. 2011, 172, 190–195. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kohonen, T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- TensorFlow. Available online: https://www.tensorflow.org/ (accessed on 30 May 2020).
- Keras: The Python Deep Learning Library. Available online: https://keras.io/ (accessed on 30 May 2020).
- Jha, R.; Dulikravich, G.S. Design of High Temperature Ti–Al–Cr–V Alloys for Maximum Thermodynamic Stability Using Self-Organizing Maps. Metals 2019, 9, 537. [Google Scholar] [CrossRef]
- Jha, R.; Dulikravich, G.S. Solidification and heat treatment simulation for aluminum alloys with scandium addition through CALPHAD approach. Comput. Mater. Sci. 2020, 182, 109749. [Google Scholar] [CrossRef]
- Jha, R.; Dulikravich, G.S. Determination of Composition and Temperature Regimes for Stabilizing Precipitation Hardening Phases in Aluminum Alloys with Scandium Addition: Combined CALPHAD—Deep Learning Approach. Calphad 2021. [Google Scholar]
- Jha, R.; Dulikravich, G.S.; Chakraborti, N.; Fan, M.; Schwartz, J.; Koch, C.C.; Colaco, M.J.; Poloni, C.; Egorov, I.N. Self-organizing maps for pattern recognition in design of alloys. Mater. Manuf. Process. 2017, 32, 1067–1074. [Google Scholar] [CrossRef]
- Jha, R.; Dulikravich, G.S.; Colaco, M.J.; Fan, M.; Schwartz, J.; Koch, C.C. Magnetic Alloys Design Using Multi-Objective Optimization. In Advanced Structured Materials; Oechsner, A., Da Silva, L.M., Altenbac, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 33, pp. 261–284, 978–981. [Google Scholar]
- Jha, R.; Chakraborti, N.; Diercks, D.R.; Stebner, A.P.; Ciobanu, C.V. Combined machine learning and CALPHAD approach for discovering processing-structure relationships in soft magnetic alloys. Comput. Mater. Sci. 2018, 150, 202–211. [Google Scholar] [CrossRef]
- Jha, R.; Diercks, D.R.; Chakraborti, N.; Stebner, A.P.; Ciobanu, C.V. Interfacial energy of copper clusters in Fe-Si-B-Nb-Cu alloys. Scr. Mater. 2019, 162, 331–334. [Google Scholar] [CrossRef]
- Jha, R.; Pettersson, F.; Dulikravich, G.S.; Saxen, H.; Chakraboti, N. Evolutionary Design of Nickel-Based Superalloys Using Data-Driven Genetic Algorithms and Related Strategies. Mater. Manuf. Process. 2015, 30, 488–510. [Google Scholar] [CrossRef]
- Thermo-Calc Software: Thermo-Calc Version 2019b. Available online: https://thermocalc.com/release-news/ (accessed on 30 October 2019).
- Thermo-Calc Software: TCTI2: TCS Ti/TiAl-Based Alloys Database. Available online: https://thermocalc.com/products/databases/titanium-and-titanium-aluminide-based-alloys/ (accessed on 30 October 2019).
- Thermo-Calc Software. Available online: https://thermocalc.com/solutions/solutions-by-material/titanium-and-titanium-aluminide/ (accessed on 30 October 2020).
- Thermo-Calc Software TCAL5: TCS Aluminium-Based Alloys Database v.5. Available online: https://www.thermocalc.com/media/56675/TCAL5-1_extended_info.pdf (accessed on 30 August 2019).
- Assadiki, A.; Esin, V.A.; Bruno, M.; Martinez, R. Stabilizing effect of alloying elements on metastable phases in cast aluminum alloys by CALPHAD calculations. Comput. Mater. Sci. 2018, 145, 1–7. [Google Scholar] [CrossRef]
- TensorBoard: TensorFlow’s Visualization Toolkit. Available online: https://www.tensorflow.org/tensorboard (accessed on 30 May 2020).
- ESTECO-ModeFRONTIER. Available online: https://www.esteco.com/technology/analytics-visualization (accessed on 30 May 2020).
- Liang, S.; Feng, X.; Yin, L.; Liu, X.Y.; Ma, M.; Liu, R. Development of a new β Ti alloy with low modulus and favorable plasticity for implant material. Mater. Sci. Eng. C 2016, 61, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, D.M.; Ruxanda, R. Solidification Structures of Titanium Alloys. In Metallography and Microstructures; ASM International: Geauga, OH, USA, 2004; Volume 9, pp. 116–126. [Google Scholar]
- Raabe, D.; Sander, B.; Friák, M.; Ma, D.; Neugebauer, J. Theory-guided bottom-up design of β-titanium alloys as biomaterials based on first principles calculations: Theory and experiments. Acta Mater. 2007, 55, 4475–4487. [Google Scholar] [CrossRef]
- Kuroda, P.B.; Quadros, F.D.F.; De Araújo, R.O.; Afonso, C.R.; Grandini, C. Effect of Thermomechanical Treatments on the Phases, Microstructure, Microhardness and Young’s Modulus of Ti-25Ta-Zr Alloys. Materials 2019, 12, 3210. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chou, K. Phase-field simulation of microstructure evolution of Ti–6Al–4V in electron beam additive manufacturing process. Addit. Manuf. 2016, 9, 14–24. [Google Scholar] [CrossRef]
- Ding, L.; Sun, Z.; Liang, Z.; Li, F.; Xu, G.; Chang, H. Investigation on Ti-6Al-4V Microstructure Evolution in Selective Laser Melting. Metals 2019, 9, 1270. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Laskowski, R.; Ng, N.; Wong, M.; Quek, S.S.; Wu, D.T. Phase field simulation of α/βmicrostructure in titanium alloy welds. Mater. Res. Express 2020, 7, 046517. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Soro, N.; Kent, D.; Dargusch, M.S. Additive manufacturing of low-cost porous titanium-based composites for biomedical applications: Advantages, challenges and opinion for future development. J. Alloys Compd. 2020, 827, 154263. [Google Scholar] [CrossRef]
- Trevisan, F.; Calignano, F.; Aversa, A.; Marchese, G.; Lombardi, M.; Biamino, S.; Ugues, D.; Manfredi, D. Additive manufacturing of titanium alloys in the biomedical field: Processes, properties and applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 57–67. [Google Scholar] [CrossRef]
- Jakubowicz, J. Special Issue: Ti-Based Biomaterials: Synthesis, Properties and Applications. Materials 2020, 13, 1696. [Google Scholar] [CrossRef]
- Majumdar, T.; Eisenstein, N.; Frith, J.E.; Cox, S.; Birbilis, N. Additive Manufacturing of Titanium Alloys for Orthopedic Applications: A Materials Science Viewpoint. Adv. Eng. Mater. 2018, 20, 1800172. [Google Scholar] [CrossRef]
- Wei, J.; Sun, H.; Zhang, D.; Gong, L.; Lin, J.; Wen, C. Influence of Heat Treatments on Microstructure and Mechanical Properties of Ti–26Nb Alloy Elaborated In Situ by Laser Additive Manufacturing with Ti and Nb Mixed Powder. Materials 2018, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, N.; Inoue, K.; Matsumoto, K.; Ochi, A.; Omori, M.; Yamamoto, K.; Nakajima, Y.; Kato-Kogoe, N.; Nakano, H.; Matsushita, T.; et al. Mechanical, Histological, and Scanning Electron Microscopy Study of the Effect of Mixed-Acid and Heat Treatment on Additive-Manufactured Titanium Plates on Bonding to the Bone Surface. Materials 2020, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- Calignano, F.; Galati, M.; Iuliano, L.; Minetola, P. Design of Additively Manufactured Structures for Biomedical Applications: A Review of the Additive Manufacturing Processes Applied to the Biomedical Sector. J. Health Eng. 2019, 2019, 9748212. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Xiao, Y.; Cui, X.; Hu, Z.; Chen, L. A Data-Driven Approach for Process Optimization of Metallic Additive Manufacturing Under Uncertainty. J. Manuf. Sci. Eng. 2019, 141, 1–51. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Kuroda, K.; Okido, M. Hydroxyapatite Coating of Titanium Implants Using Hydroprocessing and Evaluation of Their Osteoconductivity. Bioinorg. Chem. Appl. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Lu, R.-J.; Wang, X.; He, H.-X.; Ling-Ling, E.; Li, Y.; Zhang, G.-L.; Li, C.-J.; Ning, C.-Y.; Liu, H. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
| Nb | Zr | Sn | Ti | Temp. (K) | |
|---|---|---|---|---|---|
| Minimum | 0.03 | 0.02 | 0.01 | 58.9 | 50.0 |
| Maximum | 31.6 | 9.95 | 4.95 | 97.7 | 1526.0 |
| Phase | DLANN Architecture | Error Metrics (Validation Set) | |
|---|---|---|---|
| Mean Absolute Error (MAE) | Mean Squared Error (MSE) | ||
| ALTI3_D019(α’’) | 50-100-100-100 | 0.03286 | 0.00549 |
| BCC_B2 (β) | 80-160-160-160 | 0.03182 | 0.00608 |
| BCC_B2#2 | 90-180-180-180 | 0.03574 | 0.00916 |
| HCP_A3(α) | 90-180-180-180 | 0.01783 | 0.00135 |
| OMEGA (ω) | 70-140-140-140 | 0.01922 | 0.00248 |
| Quantization Error | Topological Error |
|---|---|
| 0.064 | 0.028 |
| Alloy No. | Ti (Mole %) | Nb (Mole %) | Zr (Mole %) | Sn (Mole %) | Temp. (K) |
|---|---|---|---|---|---|
| 1 | 63.11244 | 29.85438 | 5.11341 | 1.91977 | 641.7 |
| 2 | 65.56413 | 27.26226 | 6.32562 | 0.84798 | 807.825 |
| 3 | 65.44534 | 27.15556 | 6.62609 | 0.77301 | 955.644 |
| 4 | 64.15603 | 26.81113 | 7.31843 | 1.71441 | 989.739 |
| 5 | 73.28298 | 23.13284 | 1.39528 | 2.1889 | 1024.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, R.; Dulikravich, G.S. Discovery of New Ti-Based Alloys Aimed at Avoiding/Minimizing Formation of α” and ω-Phase Using CALPHAD and Artificial Intelligence. Metals 2021, 11, 15. https://doi.org/10.3390/met11010015
Jha R, Dulikravich GS. Discovery of New Ti-Based Alloys Aimed at Avoiding/Minimizing Formation of α” and ω-Phase Using CALPHAD and Artificial Intelligence. Metals. 2021; 11(1):15. https://doi.org/10.3390/met11010015
Chicago/Turabian StyleJha, Rajesh, and George S. Dulikravich. 2021. "Discovery of New Ti-Based Alloys Aimed at Avoiding/Minimizing Formation of α” and ω-Phase Using CALPHAD and Artificial Intelligence" Metals 11, no. 1: 15. https://doi.org/10.3390/met11010015
APA StyleJha, R., & Dulikravich, G. S. (2021). Discovery of New Ti-Based Alloys Aimed at Avoiding/Minimizing Formation of α” and ω-Phase Using CALPHAD and Artificial Intelligence. Metals, 11(1), 15. https://doi.org/10.3390/met11010015






