Kinetics of Arsenic Surface Segregation in Scrap-Based Silicon Electrical Steel
Abstract
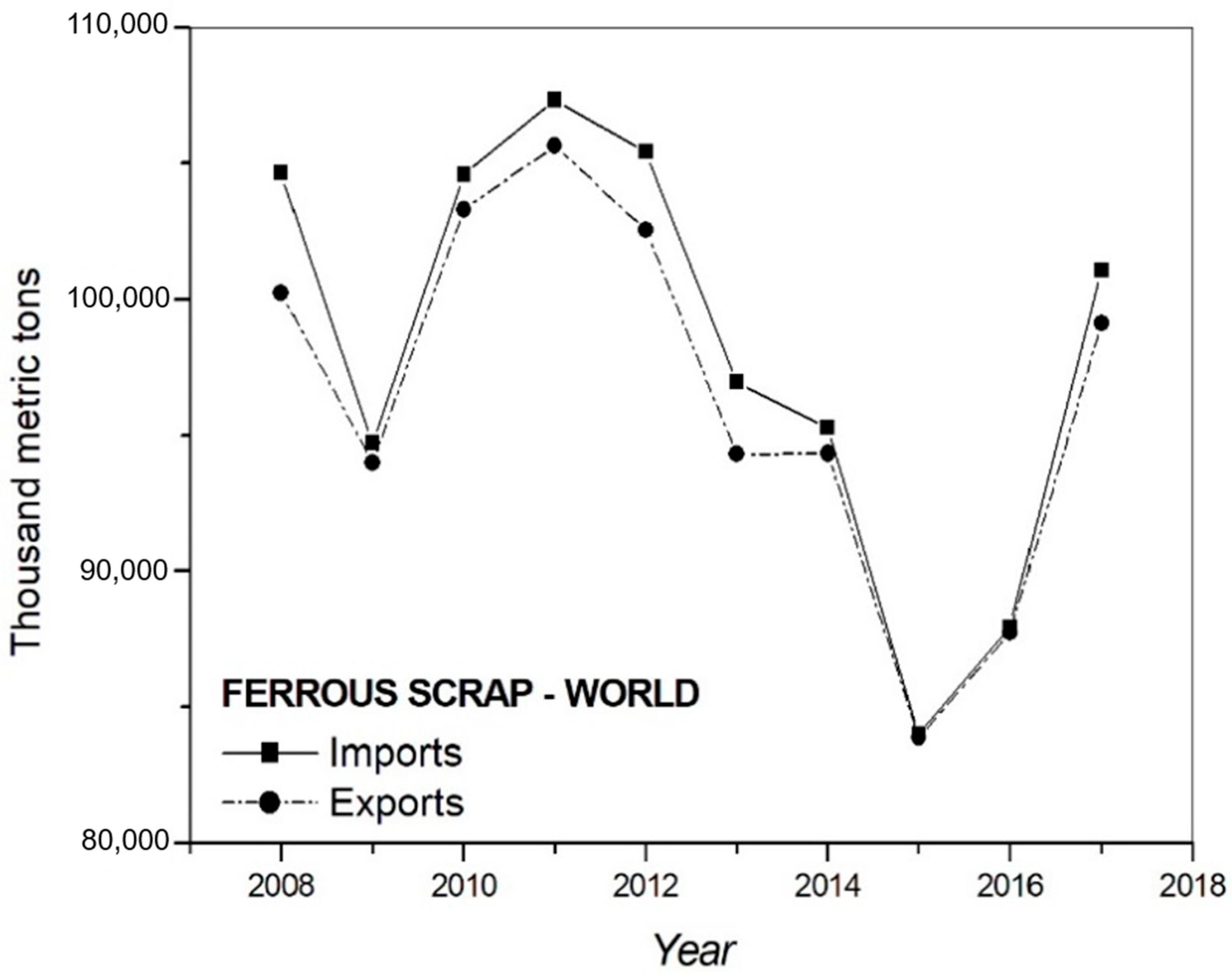
1. Introduction
2. Material and Methodology
2.1. Material
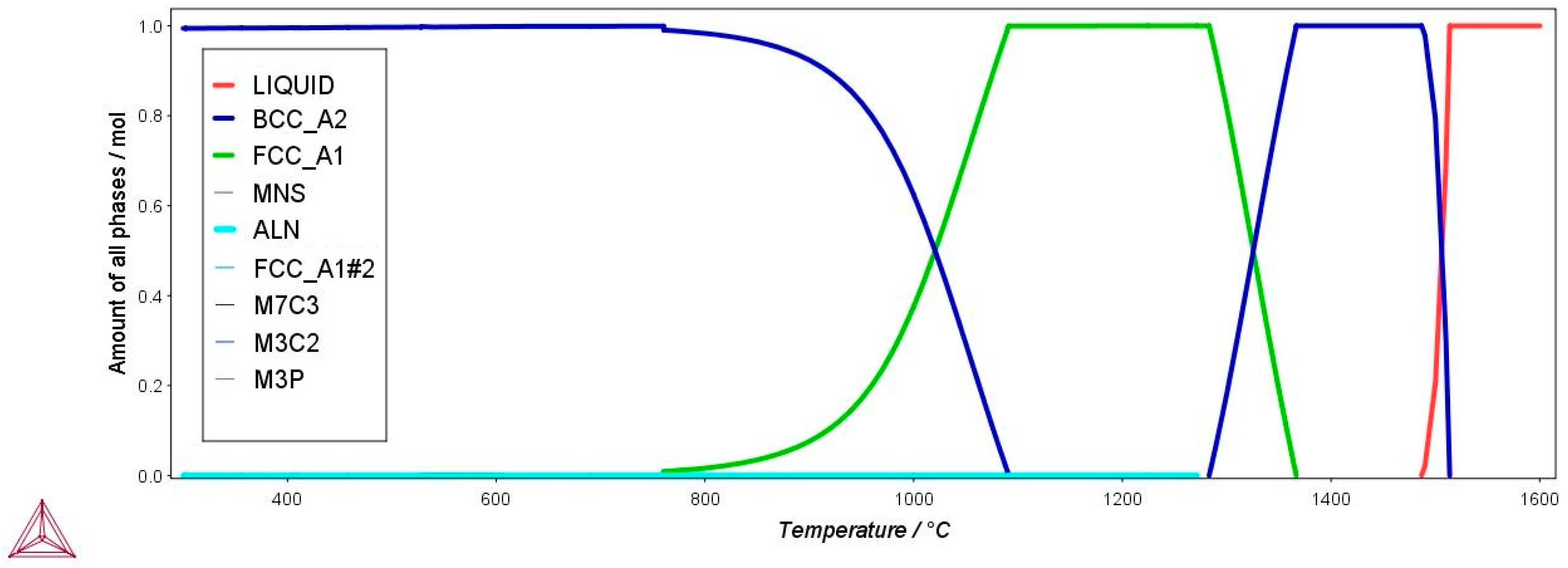
2.2. Thermodynamic Calculations
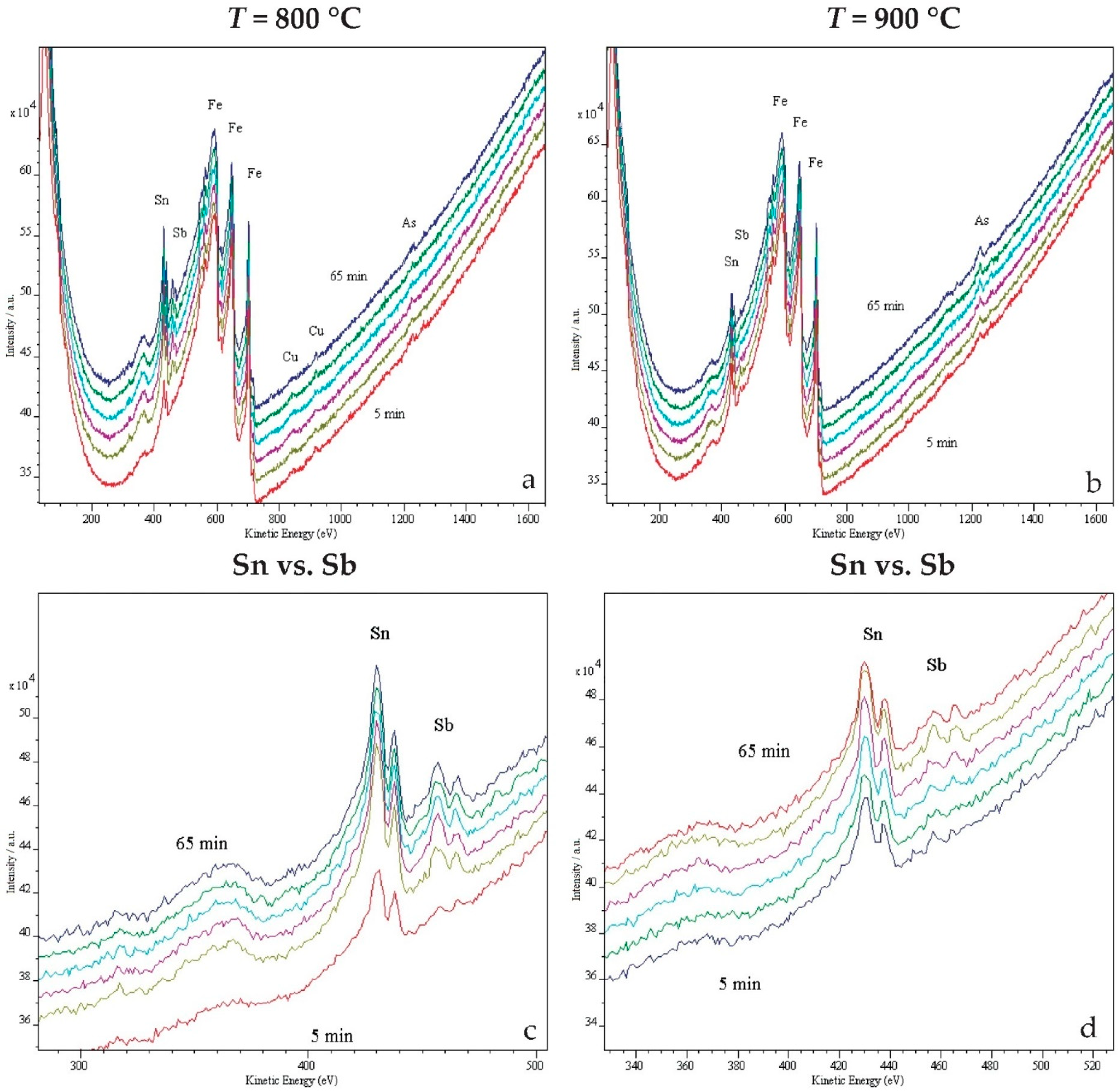
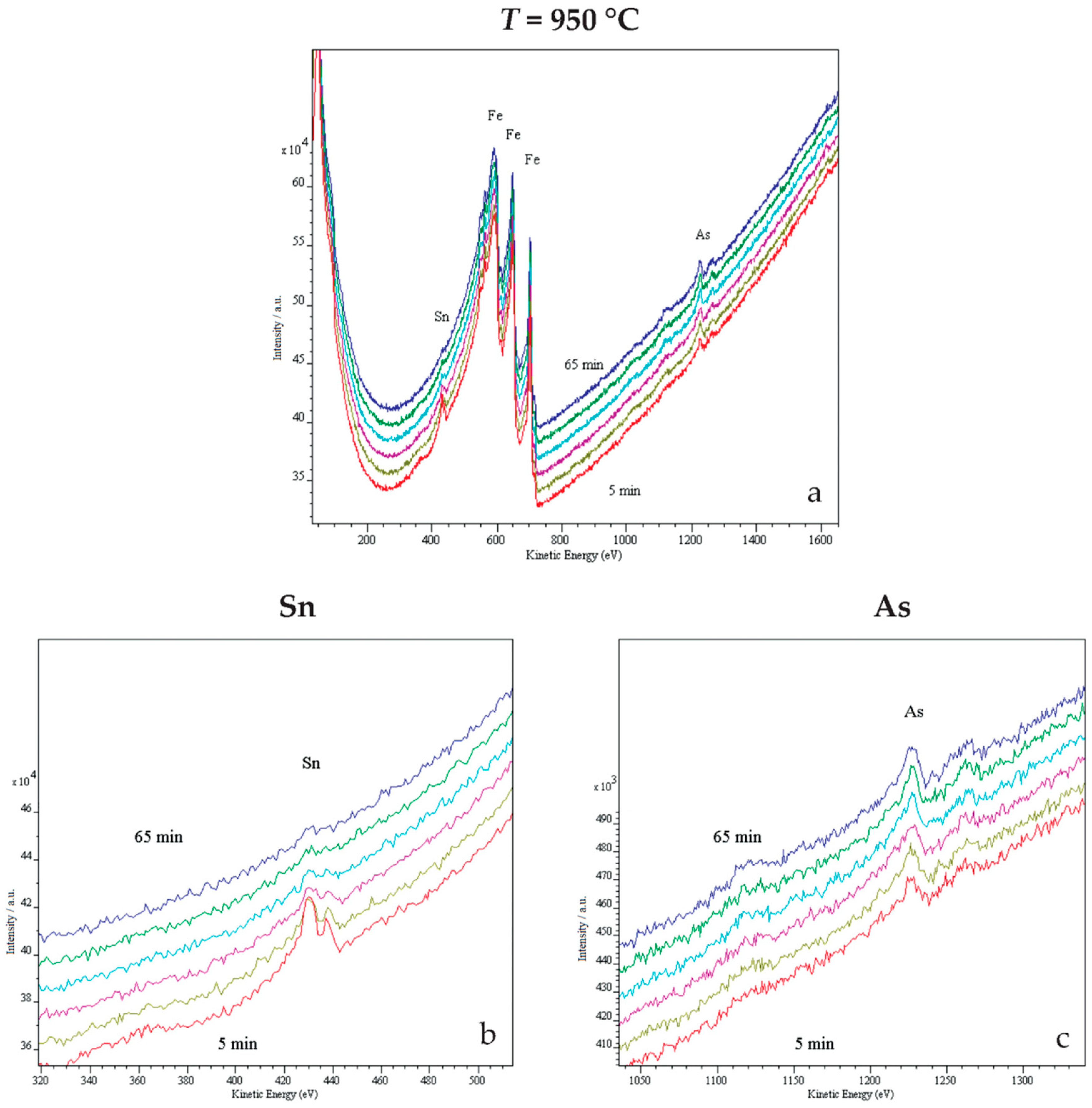
2.3. In Situ Study of Surface Segregations
- the surface concentration of the segregand
- sensitivity factor related to the segregand and the spectrometer (see Table 2)
- the peak height of the segregand.
3. Results and Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Moses, A.J. Energy efficient electrical steels: Magnetic performance prediction and optimization. Scr. Mater. 2012, 67, 560–565. [Google Scholar] [CrossRef]
- Petryshynets, I.; Kováč, F.; Petrov, B.; Falat, L.; Puchý, V. Improving the Magnetic Properties of Non-Oriented Electrical Steels by Secondary Recrystallization Using Dynamic Heating Conditions. Materials 2019, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.M.; Santos, J.D.D.A.; Freitas, F.N.C.; Filho, P.P.R.; De Abreu, H.F.G. A novel approach based on pattern recognition techniques to evaluate magnetic properties of a non-grain oriented electrical steel in the secondary recrystallization process. Measurement 2021, 167, 108135. [Google Scholar] [CrossRef]
- El-Refaie, A.; Osama, M. High specific power electrical machines: A system perspective. China Electrotech. Soc. Trans. Electr. Mach. Syst. 2019, 3, 88–93. [Google Scholar] [CrossRef]
- Ahn, Y.-K.; Jeong, Y.-K.; Kim, T.-Y.; Cho, J.-U.; Hwang, N.-M. Texture evolution of non-oriented electrical steel analyzed by EBSD and in-situ XRD during the phase transformation from γ to α. Mater. Today Commun. 2020, 25, 101307. [Google Scholar] [CrossRef]
- Kwon, S.B.; Ahn, Y.K.; Jeong, Y.K.; Kim, T.Y.; Park, J.T.; Han, H.N.; Hwang, N.M. Evolution of cube-on-face texture in Fe-1%Si steel induced by physical contact during the phase transformation from γ to α. Mater. Charact. 2020, 165, 110380. [Google Scholar] [CrossRef]
- Steel Statistical Yearbook 2018. The World Steel Association: Brussels, Belgium, 2019. Available online: https://www.worldsteel.org/en/dam/jcr:e5a8eda5-4b46-4892-856b-00908b5ab492/SSY_2018.pdf (accessed on 30 October 2020).
- Miranda, A.M.; Assis, P.S.; Brooks, G.A.; Rhamdhani, M.A.; Fontana, A.; King, A.; Sanders, G.; Moreira, G.P.D.C. Monitoring of less-common residual elements in scrap feeds for EAF steelmaking. Ironmak. Steelmak. 2019, 46, 598–608. [Google Scholar] [CrossRef]
- Xin, W.; Song, B.; Yang, Z.; Yang, Y.; Li, L. Effect of Arsenic and Copper+Arsenic on High Temperature Oxidation and Hot Shortness Behavior of C–Mn Steel. ISIJ Int. 2016, 56, 1232–1240. [Google Scholar] [CrossRef]
- Sipola, T.; Alatarvas, T.; Fabritius, T.; Perämäki, P. Determination of Alloying and Impurity Elements from Matrix and Inclusions from a Process Sample of a Double Stabilized Stainless Steel. ISIJ Int. 2016, 56, 1445–1451. [Google Scholar] [CrossRef]
- Xin, W.-B.; Song, B.; Huang, C.-G.; Song, M.-M.; Song, G.-Y. Effect of arsenic content and quenching temperature on solidification microstructure and arsenic distribution in iron-arsenic alloys. Int. J. Miner. Met. Mater. 2015, 22, 704–713. [Google Scholar] [CrossRef]
- Yin, L.; Sridhar, S. Effects of Residual Elements Arsenic, Antimony, and Tin on Surface Hot Shortness. Met. Mater. Trans. A 2011, 42, 1031–1043. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhou, H.; Wang, T. Competitive mechanism between cementite and grain boundaries of arsenic segregation. Mater. Lett. 2015, 138, 151–153. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Jiang, Y.; Deng, Y.; Meng, Q.; Song, B. Effect of rare earth Ce on the isothermal oxidation behavior in air of arsenic bearing steels. Met. Res. Technol. 2019, 116, 415. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Yu, P.; Bai, B.; Sun, L.-F.; Wang, Y. Distribution of Arsenic Inclusions in Rare Earth Steel Ingots. Metals 2020, 10, 146. [Google Scholar] [CrossRef]
- Wang, H.; Yu, P.; Jiang, S.; Bai, B.; Sun, L.-F.; Wang, Y. Evolution of Inclusions in Steelmaking Process of Rare Earth Steels Containing Arsenic with Alumina Crucibles. Metals 2020, 10, 275. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Yu, P.; Sun, L.; Wang, Y. Effect of Steel-refractory Reactions on Removal of Arsenic from Molten Steel with Lanthanum Additions. ISIJ Int. 2020, 60, 2316–2324. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, B.; Liu, P. Effect of Annealing and Hot Rolling on Grain Boundary Segregation of Arsenic in an Mn-Steel Microalloyed by Ti, Cr and Nb. J. Iron Steel Res. Int. 2013, 20, 67–72. [Google Scholar] [CrossRef]
- Zhu, Y.-Z.; Zhu, Z.; Xu, J.-P. Grain boundary segregation of minor arsenic and nitrogen at elevated temperatures in a microalloyed steel. Int. J. Miner. Met. Mater. 2012, 19, 399–403. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Xu, J. Macroscopic Distribution of Residual Elements As, S, and P in Steel Strips Produced by Compact Strip Production (CSP) Process. Met. Mater. Trans. A 2012, 43, 2509–2513. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Liang, D.; Liu, P. Distribution of arsenic on micro-interfaces in a kind of Cr, Nb and Ti microalloyed low carbon steel produced by a compact strip production process. Mater. Chem. Phys. 2011, 130, 524–530. [Google Scholar] [CrossRef]
- Costa, D.; Carraretto, A.; Godowski, P.J.; Marcus, P. Evidence of arsenic segregation in iron. J. Mater. Sci. Lett. 1993, 12, 135–137. [Google Scholar] [CrossRef]
- Godowski, P.J.; Costa, D.; Marcus, P. Surface segregation of arsenic in iron. J. Mater. Sci. 1995, 30, 5166–5172. [Google Scholar] [CrossRef]
- Busch, B.W.; Gustafsson, T.; Viefhaus, H.; Uebing, C. Medium-energy ion scattering study of arsenic and sulphur segregation to the Fe-9%W(100) surface. Surf. Sci. 2000, 463, 145–155. [Google Scholar] [CrossRef]
- Steiner Petrovič, D.; Jenko, M.; Jeram, M.; Marinšek, F.; Prešern, V. The surface segregation of impurity elements in non-oriented electrical steels. Strojarstvo 2006, 48, 45–49. [Google Scholar]
- ThermoCalc Software. Available online: https://thermocalc.com/ (accessed on 1 October 2020).
- CasaXPS Processing Software. Available online: http://www.casaxps.com/ (accessed on 1 October 2020).
- Microlab 310-F, Operators Manual; VG Scientific Ltd.: East Grinstead, UK, 2004; pp. 1–191.
- Klančnik, G.; Medved, J.; Nagode, A.; Novak, G.; Steiner Petrovič, D. Influence of Mn on the solidification of Fe-Si-Al Alloy for Non-Oriented Electrical Steel. J. Therm. Anal. Calorim. 2014, 116, 295–302. [Google Scholar] [CrossRef]
- Uebing, C. Surface Segregation of Nonmetallic Solutes on Metals and Alloys. Heterog. Chem. Rev. 1996, 3, 351–388. [Google Scholar] [CrossRef]
- Grabke, H.J.; Tauber, G.; Viefhaus, H. Equilibrium surface segregation of carbon on iron (100) faces. Scripta Metall. Mater. 1975, 9, 1181–1184. [Google Scholar] [CrossRef]
- Grabke, H.J. Segregation at Interfaces. In Chemistry and Physics of Fracture; Latanision, R.M., Jones, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1987; Volume 130, pp. 388–415. [Google Scholar]
- Grabke, H. Adsorption, segregation and reactions of non-metal atoms on iron surfaces. Mater. Sci. Eng. 1980, 42, 91–99. [Google Scholar] [CrossRef]
- Reichl, B.; Eisl, M.; Weiß, T.; Störi, H. Investigation of the diffusion properties of impurities in polycrystalline α-iron by means of AES. Surf. Sci. 1995, 331, 243–248. [Google Scholar] [CrossRef]
- Swart, H.C.; Roos, W.D.; Terblans, J.J. Surface segregating kinetics in a ternary system. Surf. Interface Anal. 2004, 36, 285–289. [Google Scholar] [CrossRef]
- Massalski, T.B. Binary Alloy Phase Diagrams, 2nd ed.; ASM: Materials Park, OH, USA, 1990; pp. 1766–1775. [Google Scholar]
- Bakker, H.; Bonzel, H.P.; Bruff, C.M.; Dayananda, M.A.; Gust, W.; Horvath, J.; Kaur, I.; Kidson, G.V.; Le Claire, A.D.; Mehrer, H.; et al. Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology. Group III: Crystal and Solid State Physics; Mehrer, H., Ed.; Springer: Heidelberg, Germany, 1990; Volume 26, pp. 129–130. [Google Scholar]
- Pavlinov, L.V. Impurity Diffusion in Iron and Steels: Antimony Diffusion; Report No. FEI-1411; State Committee on the Utilization of Atomic Energy: Moscow, Russia, 1983; pp. 1–8. [Google Scholar]
- Celmer, R.; Yamamoto, M.; Toguri, J.M. Vapour pressure of Arsenic. Can. Metall. Q. 1984, 23, 169–172. [Google Scholar] [CrossRef]
- CRC Press. Handbook of Chemistry and Physics, 84th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2003; p. 2616. [Google Scholar]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/inchi?ID=C7440360&Mask=4 (accessed on 13 December 2020).
- Godec, M.; Jenko, M.; Mast, R.; Grabke, H.J. Texture measurements on electrical steels alloyed with tin. Vaccum 2001, 61, 151–155. [Google Scholar] [CrossRef]
- Steiner Petrovič, D. Non-oriented electrical steel sheets. Mater. Tehnol. 2010, 44, 317–325. [Google Scholar]
- Park, J.T.; Kim, J.K.; Szpunar, J.A. Recrystallisation, Grain Growth and Texture Evolution in Nonoriented Electrical Steels. Mater. Sci. Forum 2007, 2007, 657–664. [Google Scholar] [CrossRef]
- Sidor, J.J.; Verbeken, K.; Gomes, E.; Schneider, J.; Calvillo, P.R.; Kestens, L. Through process texture evolution and magnetic properties of high Si non-oriented electrical steels. Mater. Charact. 2012, 71, 49–57. [Google Scholar] [CrossRef]
- Huňady, J.; Černik, M.; Hilinski, E.; Predmerský, M.; Magurová, A. Influence of chemistry and hot rolling conditions on high permeability non-grain oriented silicon steel. J. Magn. Magn. Mater. 2006, 304, e620–e623. [Google Scholar] [CrossRef]
- Fischer, O.; Schneider, J. Influence of deformation process on the improvement of non-oriented electrical steel. J. Magn. Magn. Mater. 2003, 254, 302–306. [Google Scholar] [CrossRef]
- Chang, S.K. Magnetic Anisotropies and Textures in High-alloyed Non-oriented Electrical Steels. ISIJ Int. 2007, 47, 466–471. [Google Scholar] [CrossRef]
- Rodrigues, M.F.; Da Cunha, M.A.; Paolinelli, S.D.C.; Cota, A.B. Texture and magnetic propetries improvement of a 3% Si non-oriented electrical steel by Sb addition. J. Magn. Magn. Mater. 2013, 331, 24–27. [Google Scholar] [CrossRef][Green Version]
- Sahoo, G.; Singh, C.D.; Deepa, M.; Dhua, S.K.; Saxena, A. Recrystallization behavior and texture of non-oriented electrical steels. Mater. Sci. Eng. A 2018, 734, 229–243. [Google Scholar] [CrossRef]


| Fe | C | Si | Al | Mn | P | S | N | O | Cu | Sb | Cr | Ni | Sn | As |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bal. | 0.023 | 1.68 | 0.24 | 0.24 | 0.010 | 0.002 | 0.003 | <0.01 | 0.33 | 0.005 | 0.13 | 0.15 | 0.019 | 0.010 |
| Segregand | Al | As | C | Cu | Fe | N | O | P | S | Sb | Si | Sn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ek | 1396 | 1229 | 270 | 918 | 654 | 389 | 510 | 123 | 153 | 458 | 1621 | 432 |
| ki | 0.263 | 0.334 | 0.224 | 0.675 | 0.363 | 0.066 | 0.413 | 0.65 | 0.71 | 0.831 | 0.193 | 0.502 |
| Temperature | Equilibrium Phases/mol | |
|---|---|---|
| T/°C | α-Ferrite | γ-Austenite |
| 1000 | 0.624 | 0.375 |
| 950 | 0.829 | 0.170 |
| 900 | 0.923 | 0.076 |
| 850 | 0.964 | 0.035 |
| 800 | 0.983 | 0.016 |
| 760.6 | 0.990 | 0.009 |
| Segregand/Temperature | Solubility | Diffusion Coefficient D0/10−4·m2·s−1 | |||
|---|---|---|---|---|---|
| /wt. % | |||||
| - | α-Fe | γ-Fe | α-f-Fe | α-p-Fe | γ-Fe |
| As | 12.4 | 2.0 | 4.3 * | n.a. | 0.58 |
| T/°C | 845 | 1130 | 950–1380 | - | 1050–1300 |
| Sn | 17.7 | 1.7 | 5.4 | 2.4 | 9 × 10−4–0.845 |
| T/°C | 910 | 1130 | 700–760 | 800–910 | 1027–1380 |
| Sb | 10.3 | 2.37 | 80 | 440 | 2.25 × 10−11–7.43 × 10−9 |
| T/°C | 996 | 1150 | 500–600 | 767–900 | 950–1350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner Petrovič, D. Kinetics of Arsenic Surface Segregation in Scrap-Based Silicon Electrical Steel. Metals 2021, 11, 1. https://doi.org/10.3390/met11010001
Steiner Petrovič D. Kinetics of Arsenic Surface Segregation in Scrap-Based Silicon Electrical Steel. Metals. 2021; 11(1):1. https://doi.org/10.3390/met11010001
Chicago/Turabian StyleSteiner Petrovič, Darja. 2021. "Kinetics of Arsenic Surface Segregation in Scrap-Based Silicon Electrical Steel" Metals 11, no. 1: 1. https://doi.org/10.3390/met11010001
APA StyleSteiner Petrovič, D. (2021). Kinetics of Arsenic Surface Segregation in Scrap-Based Silicon Electrical Steel. Metals, 11(1), 1. https://doi.org/10.3390/met11010001




