Effects of Ti6Al4V Surfaces Manufactured through Precision Centrifugal Casting and Modified by Calcium and Phosphorus Ion Implantation on Human Osteoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Human Osteoblasts
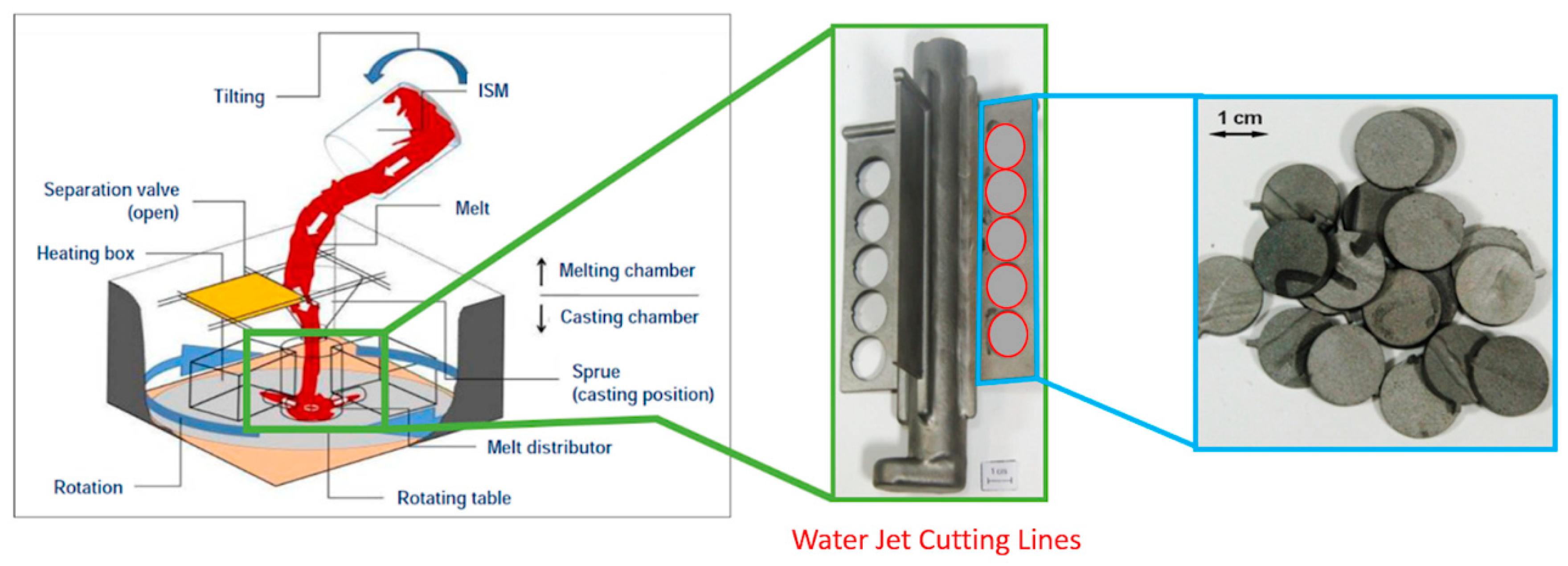
2.2. Test Specimen
2.3. SEM Analysis of Cell Adhesion
2.4. Molecular Biological Methods
2.5. Elisa
2.6. MTT Assay
2.7. Alizarin Red S Staining
2.8. Statistical Testing
3. Results
3.1. Surface Characteristics and SEM Analysis
3.2. Cell Adhesion and Proliferation
3.3. Optimized Centrifugal Casting Did Not Impair Osteogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ha, S.-W.; Wintermantel, E. Biokompatible Metalle. In Medizintechnik, 5th ed.; Wintermantel, E., Ha, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 191–217. [Google Scholar] [CrossRef]
- Nastac, L.; Gungor, M.N.; Ucok, I.; Klug, K.L.; Tack, W.T. Advances in investment casting of Ti–6Al–4V alloy: A review. Int. J. Cast Met. Res. 2013, 19, 73–93. [Google Scholar] [CrossRef]
- Billhofer, H.; Hauptmann, T. Feingusssystem für Titan und Titanlegierungen. Lightweight Des. 2010, 3, 47–51. [Google Scholar] [CrossRef]
- Wolfle-Roos, J.V.; Katmer Amet, B.; Fiedler, J.; Michels, H.; Kappelt, G.; Ignatius, A.; Durselen, L.; Reichel, H.; Brenner, R.E. Optimizing Manufacturing and Osseointegration of Ti6Al4V Implants through Precision Casting and Calcium and Phosphorus Ion Implantation? In Vivo Results of a Large-Scale Animal Trial. Materials 2020, 13, 1670. [Google Scholar] [CrossRef]
- Kienapfel, H.; Sprey, C.; Wilke, A.; Griss, P. Implant fixation by bone ingrowth. J. Arthroplast. 1999, 14, 355–368. [Google Scholar] [CrossRef]
- Wolfle, J.V.; Fiedler, J.; Durselen, L.; Reichert, J.; Scharnweber, D.; Forster, A.; Schwenzer, B.; Reichel, H.; Ignatius, A.; Brenner, R.E. Improved anchorage of Ti6Al4V orthopaedic bone implants through oligonucleotide mediated immobilization of BMP-2 in osteoporotic rats. PLoS ONE 2014, 9, e86151. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Bilinski, A.; Lewandowska-Szumiel, M.; Rajchel, B. Effect of dual ion implantation of calcium and phosphorus on the properties of titanium. Biomaterials 2005, 26, 2847–2856. [Google Scholar] [CrossRef]
- Lazarinis, S.; Makela, K.T.; Eskelinen, A.; Havelin, L.; Hallan, G.; Overgaard, S.; Pedersen, A.B.; Karrholm, J.; Hailer, N.P. Does hydroxyapatite coating of uncemented cups improve long-term survival? An analysis of 28,605 primary total hip arthroplasty procedures from the Nordic Arthroplasty Register Association (NARA). Osteoarthr. Cartil. 2017, 25, 1980–1987. [Google Scholar] [CrossRef][Green Version]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Pham, M.T.; Reuther, H.; Matz, W.; Mueller, R.; Steiner, G.; Oswald, S.; Zyganov, I. Surface induced reactivity for titanium by ion implantation. J. Mater. Sci. Mater. Med. 2000, 11, 383–391. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kwon, T.Y.; Kim, K.H. Surface modification of titanium and titanium alloys by ion implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 581–591. [Google Scholar] [CrossRef]
- Biggs, M.J.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.; Dalby, M.J. The effects of nanoscale pits on primary human osteoblast adhesion formation and cellular spreading. J. Mater. Sci. Mater. Med. 2007, 18, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Yarwood, S.J.; Riehle, M.O.; Johnstone, H.J.; Affrossman, S.; Curtis, A.S. Increasing fibroblast response to materials using nanotopography: Morphological and genetic measurements of cell response to 13-nm-high polymer demixed islands. Exp. Cell Res. 2002, 276, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.D.; Wen, J.H.; del Alamo, J.C.; Engler, A.J. Dynamic and reversible surface topography influences cell morphology. J. Biomed. Mater. Res. A 2013, 101, 2313–2321. [Google Scholar] [CrossRef]
- Fiedler, J.; Kolitsch, A.; Kleffner, B.; Henke, D.; Stenger, S.; Brenner, R.E. Copper and silver ion implantation of aluminium oxide-blasted titanium surfaces: Proliferative response of osteoblasts and antibacterial effects. Int. J. Artif. Organs 2011, 34, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Ozdemir, B.; Bartholoma, J.; Plettl, A.; Brenner, R.E.; Ziemann, P. The effect of substrate surface nanotopography on the behavior of multipotnent mesenchymal stromal cells and osteoblasts. Biomaterials 2013, 34, 8851–8859. [Google Scholar] [CrossRef]
- Mane, V.P.; Heuer, M.A.; Hillyer, P.; Navarro, M.B.; Rabin, R.L. Systematic method for determining an ideal housekeeping gene for real-time PCR analysis. J. Biomol. Tech. 2008, 19, 342–347. [Google Scholar]
- Katmer Amet, B. Bewertung von Ti-6Al-4V Oberflächen Hergestellt Mittels neu Entwickelter Schleudergusstechnologie im In Vitro- und In Vivo-Modell. Ph.D. Thesis, University of Ulm, Ulm, Germany, 2019. [Google Scholar] [CrossRef]
- Mohammadi, S.; Esposito, M.; Wictorin, L.; Aronsson, B.O.; Thomsen, P. Bone response to machined cast titanium implants. J. Mater. Sci. 2001, 36, 1987–1993. [Google Scholar] [CrossRef]
- Sommer, U.; Laurich, S.; de Azevedo, L.; Viehoff, K.; Wenisch, S.; Thormann, U.; Alt, V.; Heiss, C.; Schnettler, R. In Vitro and In Vivo Biocompatibility Studies of a Cast and Coated Titanium Alloy. Molecules 2020, 25, 3399. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. A 2014, 102, 2636–2643. [Google Scholar] [CrossRef]
- Mitra, J.; Tripathi, G.; Sharma, A.; Basu, B. Scaffolds for bone tissue engineering: Role of surface patterning on osteoblast response. RSC Adv. 2013, 3, 11073–11094. [Google Scholar] [CrossRef]
- Yokose, S.; Klokkevold, P.R.; Takei, H.H.; Kadokura, H.; Kikui, T.; Hibino, Y.; Shigeta, H.; Nakajima, H.; Kawazu, H. Effects of surface microtopography of titanium disks on cell proliferation and differentiation of osteoblast-like cells isolated from rat calvariae. Dent. Mater. J. 2018, 37, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Inoue, H.; Kojo, T.; Matsunaga, T.; Tsujisawa, T.; Uchiyama, C.; Uchida, Y. Effect of surface roughness on proliferation and alkaline phosphatase expression of rat calvarial cells cultured on polystyrene. Bone 1999, 25, 439–445. [Google Scholar] [CrossRef]
- Jang, T.S.; Jung, H.D.; Kim, S.; Moon, B.S.; Baek, J.; Park, C.; Song, J.; Kim, H.E. Multiscale porous titanium surfaces via a two-step etching process for improved mechanical and biological performance. Biomed. Mater. 2017, 12, 025008. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Anselme, K.; Noel, B.; Hardouin, P. Human osteoblast adhesion on titanium alloy, stainless steel, glass and plastic substrates with same surface topography. J. Mater. Sci. Mater. Med. 1999, 10, 815–819. [Google Scholar] [CrossRef]
- Lagonegro, P.; Trevisi, G.; Nasi, L.; Parisi, L.; Manfredi, E.; Lumetti, S.; Rossi, F.; Macaluso, G.M.; Salviati, G.; Galli, C. Osteoblasts preferentially adhere to peaks on micro-structured titanium. Dent. Mater. J. 2018, 37, 278–285. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, Y.; Cai, Q.; Li, B.; Yang, H.; Wang, H.; Qi, H.; Zhou, Y.; Meng, W. Effects of the micro-nano surface topography of titanium alloy on the biological responses of osteoblast. J. Biomed. Mater. Res. A 2017, 105, 757–769. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Biliniski, A.; Lewandowska-Szumiel, M.D.; Rajchel, B. Effect of calcium-ion implantation on the corrosion resistance and biocompatibility of titanium. Biomaterials 2001, 22, 2139–2151. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Bilinski, A.; Lewandowska-Szumiel, M.; Rajchel, B. Effect of phosphorus-ion implantation on the corrosion resistance and biocompatibility of titanium. Biomaterials 2002, 23, 3329–3340. [Google Scholar] [CrossRef]
- Nayab, S.N.; Jones, F.H.; Olsen, I. Effects of calcium ion implantation on human bone cell interaction with titanium. Biomaterials 2005, 26, 4717–4727. [Google Scholar] [CrossRef]
- Nayab, S.N.; Jones, F.H.; Olsen, I. Effects of calcium ion-implantation of titanium on bone cell function in vitro. J. Biomed. Mater. Res. A 2007, 83, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Knabe, C.; Berger, G.; Gildenhaar, R.; Klar, F.; Zreiqat, H. The modulation of osteogenesis in vitro by calcium titanium phosphate coatings. Biomaterials 2004, 25, 4911–4919. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Park, J.W. Osteogenic differentiation of mesenchymal stem cells modulated by a chemically modified super-hydrophilic titanium implant surface. J. Biomater. Appl. 2018, 33, 205–215. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Garcia-Giralt, N.; Dosta, S.; Cano, I.G.; Nogues, X.; Guilemany, J.M. Osteoblastic cell response on high-rough titanium coatings by cold spray. J. Mater. Sci. Mater. Med. 2018, 29, 19. [Google Scholar] [CrossRef]
- Cheng, M.; Qiao, Y.; Wang, Q.; Jin, G.; Qin, H.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X. Calcium Plasma Implanted Titanium Surface with Hierarchical Microstructure for Improving the Bone Formation. ACS Appl. Mater. Interfaces 2015, 7, 13053–13061. [Google Scholar] [CrossRef]
- Park, J.W.; Hanawa, T.; Chung, J.H. The relative effects of Ca and Mg ions on MSC osteogenesis in the surface modification of microrough Ti implants. Int. J. Nanomed. 2019, 14, 5697–5711. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglic, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglic, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar] [CrossRef]
- Miura, M.; Chen, X.D.; Allen, M.R.; Bi, Y.; Gronthos, S.; Seo, B.M.; Lakhani, S.; Flavell, R.A.; Feng, X.H.; Robey, P.G.; et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J. Clin. Investig. 2004, 114, 1704–1713. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]









| Gene | Gene Accession No. | TaqMan Assay ID | Common Name |
|---|---|---|---|
| ALP | NM_000478.5 | Hs01029144_m1 | Alkaline phosphatase |
| BGLAP | NM_199173.5 | Hs01587814_g1 | Bone gamma-carboxyglutamate protein |
| CASP3 | NM_004346.3 | Hs00234387_m1 | Caspase 3 |
| COL1a1 | NM_000088.3 | Hs00164004_m1 | Collagen type I alpha 1 |
| HPRT1 | NM_000194.2 | Hs02800695_m1 | Hypoxanthin-Phosphoribosyl-Transferase 1 |
| OPG | NM_002546.3 | Hs00900358_m1 | Osteoprotegerin |
| RUNX2 | NM_001015051.3 | Hs00231692_m1 | Runt-related transcription factor 2 |
| TNF | NM_000594.3 | Hs01113624_g1 | Tumor necrosis factor |
| RANKL | NM_003701.3 | Hs00243522_m1 | Receptor activator of nuclear factor kappa-Β ligand |
| SP7 | NM_001173467.2 | HS001866874_s1 | Transcription factor Sp7, Osterix |
| Element | Ti6Al4V ELI Specification | wt.% before Processing | wt.% after Melting, Casting & HIP |
|---|---|---|---|
| C | 0.1 (max) | 0.011 | 0.053 |
| V | 3.5–4.5 | 4.53 | 4.43 |
| Al | 5.5–6.75 | 6.18 | 6.03 |
| O | 0.2 (max) | 0.176 | 0.131 |
| N | 0.05 (max) | 0.005 | 0.001 |
| Fe | 0.3 (max) | 0.215 | 0.223 |
| H | 0.015 (max) | 0.005 | 0.012 |
| Y | - | <0.001 | <0.002 |
| Ti | Balance | Balance | Balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jörg, F.; Betül, K.A.; Heiner, M.; Gerhard, K.; Erwin, B.R. Effects of Ti6Al4V Surfaces Manufactured through Precision Centrifugal Casting and Modified by Calcium and Phosphorus Ion Implantation on Human Osteoblasts. Metals 2020, 10, 1681. https://doi.org/10.3390/met10121681
Jörg F, Betül KA, Heiner M, Gerhard K, Erwin BR. Effects of Ti6Al4V Surfaces Manufactured through Precision Centrifugal Casting and Modified by Calcium and Phosphorus Ion Implantation on Human Osteoblasts. Metals. 2020; 10(12):1681. https://doi.org/10.3390/met10121681
Chicago/Turabian StyleJörg, Fiedler, Katmer Amet Betül, Michels Heiner, Kappelt Gerhard, and Brenner Rolf Erwin. 2020. "Effects of Ti6Al4V Surfaces Manufactured through Precision Centrifugal Casting and Modified by Calcium and Phosphorus Ion Implantation on Human Osteoblasts" Metals 10, no. 12: 1681. https://doi.org/10.3390/met10121681
APA StyleJörg, F., Betül, K. A., Heiner, M., Gerhard, K., & Erwin, B. R. (2020). Effects of Ti6Al4V Surfaces Manufactured through Precision Centrifugal Casting and Modified by Calcium and Phosphorus Ion Implantation on Human Osteoblasts. Metals, 10(12), 1681. https://doi.org/10.3390/met10121681





