The Structural and Phase State of the TiAl System Alloyed with Rare-Earth Metals of the Controlled Composition Synthesized by the “Hydride Technology”
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Alloys
2.2. Research Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clemens, H.; Chladil, H.; Wallgram, W.; Block, B.; Kremmer, S. Structural Aluminides for Elevated Temperature Applications; Kim, Y.W., Morris, D., Yang, R., Leyens, C., Eds.; The Minerals, Metals and Materials Society (TMS): Warrendale, MI, USA, 2008; pp. 217–228. [Google Scholar]
- Clemens, H.; Smarsly, W. Light-Weight Intermetallic Titanium Aluminides—Status of Research and Development. Adv. Mater. Res. 2011, 278, 551–556. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.; Oehring, M. Science and Technology, 1st ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Appel, F.; Clemens, H.; Fischer, F.D. Modeling concepts for intermetallic titanium aluminides. Prog. Mater. Sci. 2016, 81, 55–124. [Google Scholar] [CrossRef]
- Kurzina, I.A.; Kozlov, E.V.; Popova, N.A.; Kalinnikov, M.P.; Nikonenko, E.L.; Savkin, K.P.; Oks, E.M.; Sharkeev, Y.P. Modifying the Structural Phase State of Fine Grained Titanium under Conditions of Ion Irradiation. Bull. Russ. Acad. Sci. Phys. 2012, 76, 1238–1245. [Google Scholar] [CrossRef]
- Kurzina, I.A. Physical Base of the Metallic Gradient Surface Layers of Titanium Alloys Formed under Ion Implantation. Adv. Mater. Res. 2014, 872, 184–190. [Google Scholar] [CrossRef]
- Zykova, A.P.; Kazantseva, L.A.; Kurzina, I.A.; Dammer, V.K.; Chumaevaskii, A.V. Influence of the Modifying Ability of Various Compositions on the Microstructure and Properties of the AK7ch Alloy. Russ. J. Non-Ferr. Met. 2015, 56, 593–598. [Google Scholar] [CrossRef]
- Kurzina, I.; Nikonenko, A.; Popova, N.; Nikonenko, E.; Kalashnikov, M. Fine structure and phase composition of Fe-14Mn-1.2C steel: Influe, nce of the modified mixture based on refractory metals. Int. J. Miner. 2017, 24, 523–529. [Google Scholar] [CrossRef]
- Kazantseva, L.A.; Kurzina, I.A.; Kosova, N.I.; Pichugina, A.A.; Sachkov, V.I.; Vladimirov, A.A.; Sachkova, A.S. Synthesis of titanium hydrides and obtaining alloys based on them. Bull. Tomsk. State Univ. Chem. 2015, 2, 69–75. [Google Scholar]
- Dolukhanyan, S.K.; Aleksanyan, A.G.; Ter-Galstyan, O.P.; Mailyan, D.G.; Shekhtman, V.S.; Sakharov, M.K. Carbon Nanomaterials in Clean Energy Hydrogen Systems; Baranovski, B., Zaginaichenko, S., Schur, D., Skorokhod, V., Veziroglu, A., Eds.; Springer: New York, NY, USA, 2008; pp. 733–741. [Google Scholar]
- Hakobyan, H.; Aleksanyan, A.; Dolukhanyan, S.; Mnatsakanyan, N.; Shekhtman, V. Solid State Phenomena; Bobet, J.L., Ed.; Trans Tech Publications: Stäfa, Switzerland, 2011; p. 354. [Google Scholar]
- Aleksanyan, A.G.; Dolukhanyan, S.K.; Shekhtman, V.S.; Khasanov, S.S.; Ter-Galstyan, O.P.; Martirosyan, M.V. Formation of alloys in the TieNb system by hydride cycle method and synthesis of their hydrides in self-propagating high-temperature synthesis. Int. J. Hydrog. Energy 2012, 37, 14234–14239. [Google Scholar] [CrossRef]
- Wallgram, W.; Schmolzer, T.; Cha, L.; Das, G.; Guther, V.; Clemens, H. Technology and mechanical properties of advanced γ-TiAl based alloys. Int. J. Mater. Res. 2009, 100, 1021–1030. [Google Scholar] [CrossRef]
- Klein, T.; Rashkova, B.; Holec, D.; Clemens, H.; Mayer, S. Advancement of Compositional and Microstructural Design of Intermetallic γ-TiAl Based Alloys Determined by Atom Probe Tomography. Acta Mater. 2016, 110, 236–245. [Google Scholar] [CrossRef]
- Hadi, M.; Shafyei, A.; Meratian, M. A comparative study of microstructure and high temperature mechanical properties of a β-stabilized TiAl alloy modified by lanthanum and erbium. Mater. Sci. Eng. 2015, 624, 1–8. [Google Scholar] [CrossRef]
- Bulanova, M.; Fartushna, I.; Meleshevich, K.; Samelyuk, A. 16. M. Bulanova, I. Fartushna, K. and A. Meleshevich, Samelyuk, Isothermal section at 850°C of the Ti–Dy–Al system in the Ti–TiAl–DyAl2–Dyregion. J. Alloy. Compd. 2014, 598, 61–67. [Google Scholar] [CrossRef]
- Hadi, M.; Shafyei, A.; Meratian, M.; Bayat, O.; Ebrahimzadeh, I. Oxidation Properties of a Beta-Stabilized TiAl Alloy. Oxid. Met. 2018, 90, 421–434. [Google Scholar] [CrossRef]
- Liu, Z.Z.G.; Chai, L.H.; Chen, Y.Y.; Kong, F.T.; Davies, H.A.; Figueroa, I.A. Microstructure evolution in rapidly solidified Y added TiAl ribbons. Intermetallics 2011, 19, 160–164. [Google Scholar] [CrossRef]
- Wang, X.; Luo, R.; Liu, F.; Zhu, F.; Song, S.; Chen, B.; Zhang, X.; Zhang, J.; Chen, M. Characterization of Gd-rich precipitates in a fully lamellar TiAl alloy. Scr. Mater. 2017, 137, 50–54. [Google Scholar] [CrossRef]
- Li, W.; Xia, K. Kinetics of the α grain growth in a binary Ti–44Al alloy and a ternary Ti–44Al-0.15Gd alloy. Mater. Sci. Eng. A 2002, 329–331, 430–434. [Google Scholar] [CrossRef]
- Xia, K.; Wu, X.; Song, D. Effects of Gd addition, lamellar spacing and loading direction on creep behavior of a fully lamellar Ti–44Al–1Mn–2.5Nb alloy. Acta Mater. 2004, 52, 841–849. [Google Scholar] [CrossRef]
- Li, W.; Inkson, B.; Horita, Z.; Xia, K. Microstructure observations in rare earth element Gd-modified Ti-44 at%Al. Intermetallics 2000, 8, 519–523. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Clemens, H.; Lindemann, J.; Mayer, A.S. Hot-working behavior of an advanced intermetallic multi-phase γ-TiAl based alloy. Mater. Sci. Eng. A 2014, 77, 297–310. [Google Scholar] [CrossRef]
- Belgibaeva, A.A.; Erkasov, R.S.; Kurzina, I.A.; Karakchieva, N.I.; Sachkov, V.I.; Abzaev, Y.A. Obtainment of high-strength alloys of the Ti-Al system using “hydride technology”. In Proceedings of the Conference New Materials and Technologies, Barnaul, Russia, 2018; pp. 62–66. [Google Scholar]
- Kosova, N.; Sachkov, V.; Kurzina, I.; Pichugina, A.; Vladimirov, A.; Kazantseva, L.; Sachkova, A. The preparation of the Ti-Al alloys based on intermetallic phases. IOP Conf. Ser. Mater. Sci. Eng. 2016, 112, 012039. [Google Scholar] [CrossRef]
- Abzaev, Y.A.; Syzrantsev, V.V.; Bardakhanov, S.P. Simulation of the Structural State of Amorphous Phases in Nanoscale SiO2 Synthesized via Different Methods. Phys. Solid State 2017, 59, 1874–1878. [Google Scholar] [CrossRef]
- Pecharsky, V.; Zavalij, P. Fundamentals of Powder Diffraction and Structural Characterization of Materials, 2nd ed.; Springer: New York, NY, USA, 2005; p. 713. [Google Scholar]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Young, R.A. The Ritveld Method; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Abdel-hamid, A.A. Influence of Ta, Zr, V and Mo on the growth-morphology of Ti-aluminide crystals. Z. Fur Met. 1991, 82, 383–386. [Google Scholar]
- Crystallography Open Database. Available online: http://www.crystallography.net/cod/search.html (accessed on 13 March 2019).
- Braun, J.; Ellner, M.; Predel, B.; Splat, Z. Splat-cooling investigations in the binary-system Ti-Al. Z. Fuer Met. 1994, 82, 355–362. [Google Scholar]
- Xie, Y.Q.; Peng, H.J.; Liu, X.B.; Peng, K. Atomic states, potential energies, volumes, stability and brittleness of ordered FCC Ti3Al-type alloys. Phys. B Condens. Matter 2005, 366, 1–17. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hu, Q.K.; Yang, W.P.; Li, X.S. Influence of metal element doping on the mechanical properties of TiAl alloy. Acta Phys. Sin. 2016, 65, 176101. [Google Scholar] [CrossRef]
- Lyakhov, A.O.; Oganov, A.R.; Stokes, H.T.; Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 2013, 184, 1172–1182. [Google Scholar] [CrossRef]
- Abzaev, Y.A.; Starostenkov, M.D.; Klopotov, A.I. First-principles calculations of the concentration dependence of elastic modules in monocrystals Ni3(Ge1-x,Alx). Fundam. Probl. Mod. Mater. Sci. 2014, 11, 56. [Google Scholar]
- Segall, M.D.; Pickard, C.J.; Shah, R.; Payne, M.C. Population analysis in plane wave electronic structure calculations. Mol. Phys. 2010, 89, 571–577. [Google Scholar] [CrossRef]
- Segall, M.D.; Shah, R.; Pickard, C.J.; Payne, M.C. Population analysis of plane-wave electronic structure calculations of bulk materials". Phys. Rev. B 1996, 54, 16317–16320. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W.; Lyakhov, A.O.; Zhu, Q.; Qian, G.R.; Stokes, H.T.; Bushlanov, P.Z.; Allahyari, S. Lepeshkin. Universal Structure Predictor: Evolutionary Xtallography. Manual. 9.7. Appendices.P.108. Electronic access. Available online: http://han.ess.sunysb.edu/ (accessed on 13 March 2019).
- Sereda, B.; Zherebtsov, A.; Belokon’, Y. The Modeling and Processes Research of Titan Aluminides Structurization Received by SHS Technology; TMS: Seattle, WA, USA, 2010; pp. 99–108. [Google Scholar]
- Sereda, B.; Kruglyak, I.; Zherebtsov, A.; Belokon’, Y. The Processes Research of Structurization of Titan Aluminides Received by SHS; Materials Science & Technology: Pittsburg, CA, USA, 2009; pp. 2069–2073. [Google Scholar]
- Kurzina, I.A.; Kozlov, E.V.; Sharkeev, Y.P.; Ryabchikov, A.I.; Stepanov, I.B.; Bozhko, I.A.; Kalashnikov, M.P.; Sivin, D.O.; Fortuna, S.V. Influence of ion implantation on nanoscale intermetallic-phase formation in Ti–Al, Ni–Al and Ni–Ti systems. Surf. Coat. Technol. 2007, 201, 8463. [Google Scholar] [CrossRef]
- Imayev, V.M.; Imayev, R.M.; Oleneva, T.I. Current status of γ-TiAl intermetallic alloys investigations and prospects for the technology developments. Lett. Mater. 2011, 1, 25. [Google Scholar] [CrossRef]
- Cordell, T. Titanium Aluminide Intermetallics. Advanced Materials and Processes Technology. AMPTIAC Newsl. 2000, 4, 9. [Google Scholar]
- Raghavan, V. Al-Ti-Y (Aluminum-Titanium-Yttrium). Phase Equilibria Diffus. 2005, 26, 191. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, W.; Yuan, S.; Yan, J. The 500 °C Isothermal Section of the Al-Dy-Ti Ternary System. Alloy. Compd. 2002, 336, 218–222. [Google Scholar] [CrossRef]
- Das, K.; Das, S. A Review of the Ti-Al-Ta (Titanium-Aluminum-Tantalum) System. Phase Equilibria Diffus. 2005, 26, 322–329. [Google Scholar]
- Sridharan, S.; Nowotny, H. Studies in the Ternary System Ti-Ta-Al and in the Quaternary System Ti-Ta-Al-C. Z. Fuer Met. 1983, 74, 468–472. [Google Scholar]
- McCullough, C.; Valencia, J.J.; Levi, C.G.; Mehrabian, R.; Maloney, M.; Hecht, R. Solidification Paths of Ti-Ta-Al Alloys. Acta Metall. Mater. 1991, 39, 2745–2758. [Google Scholar] [CrossRef]
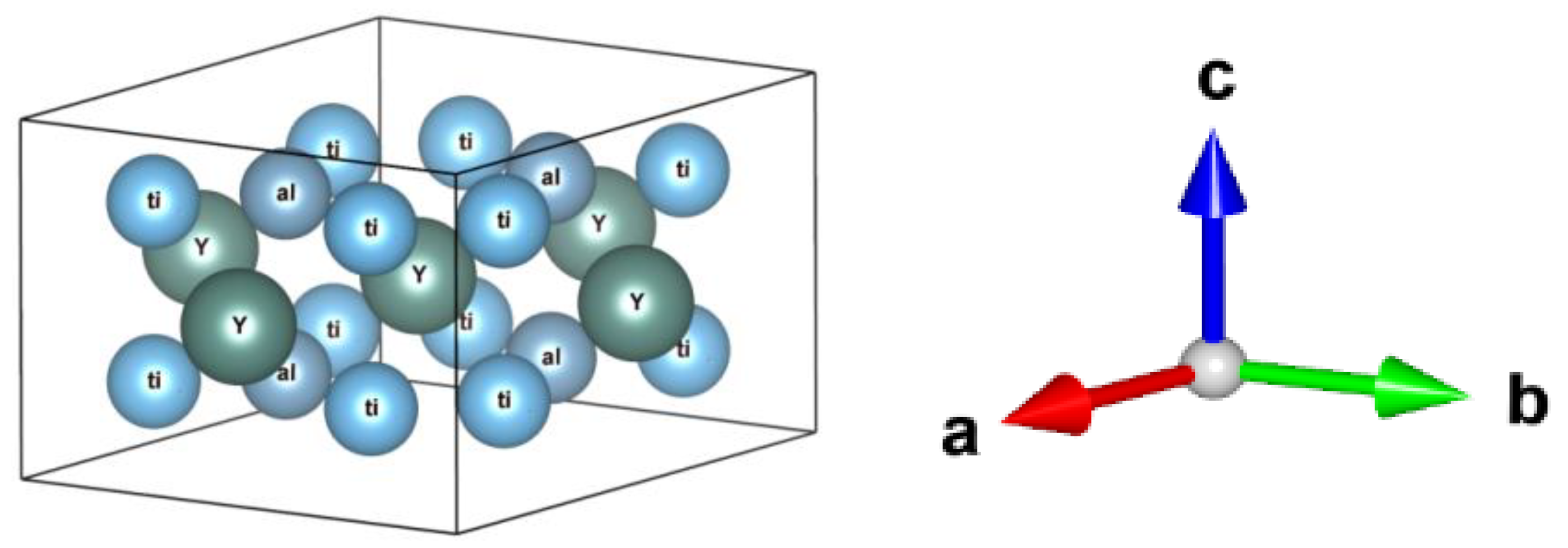
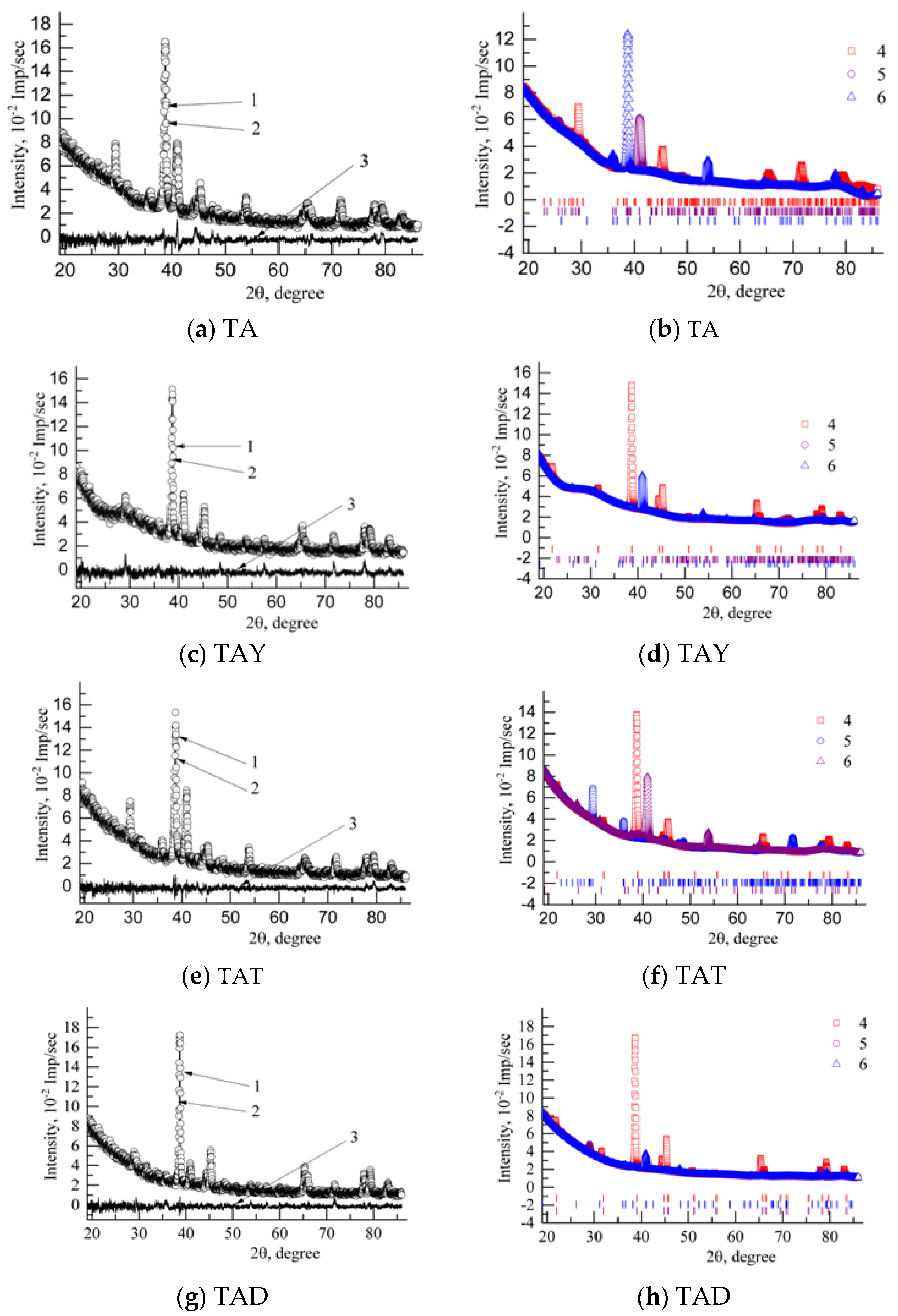
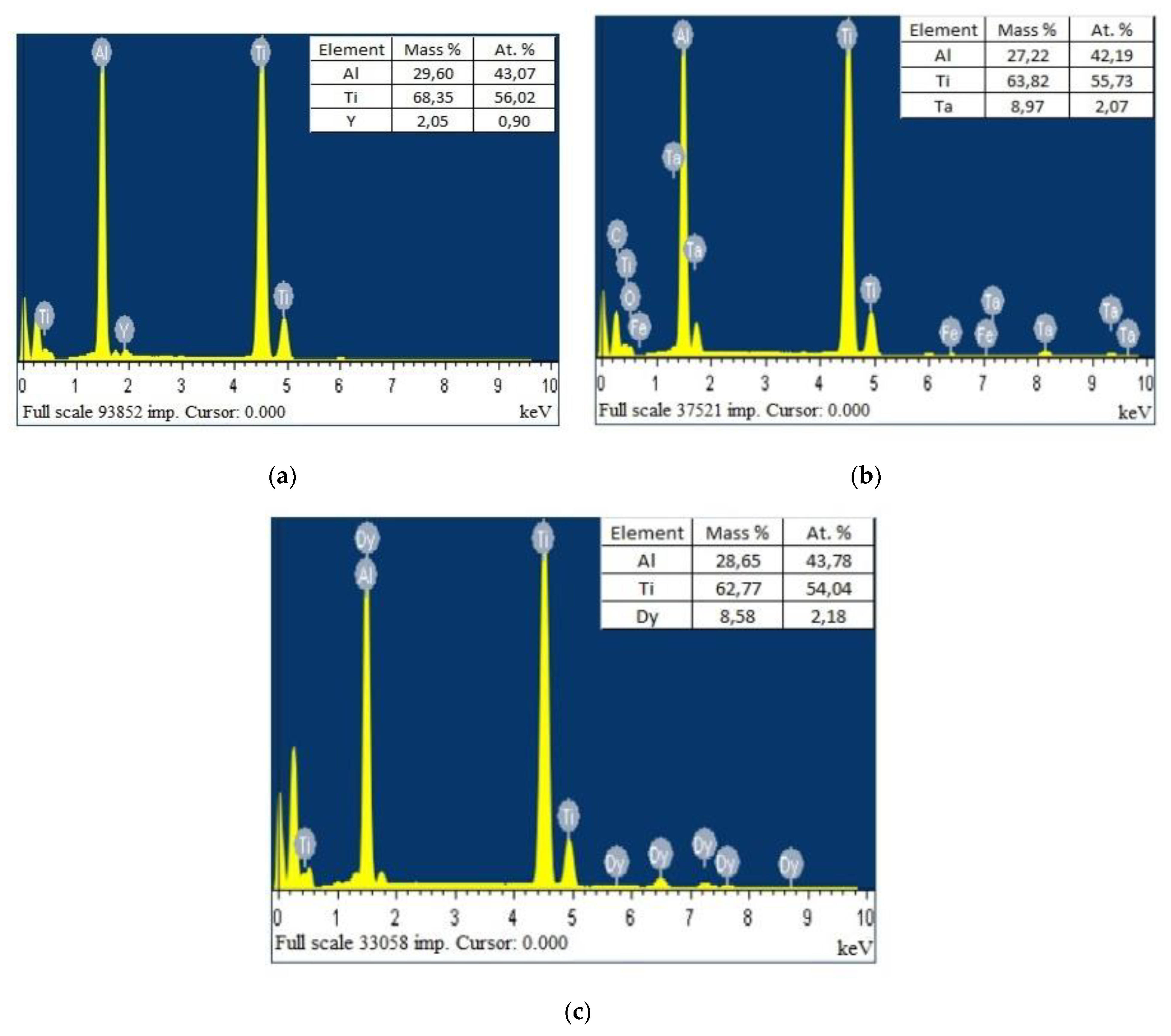
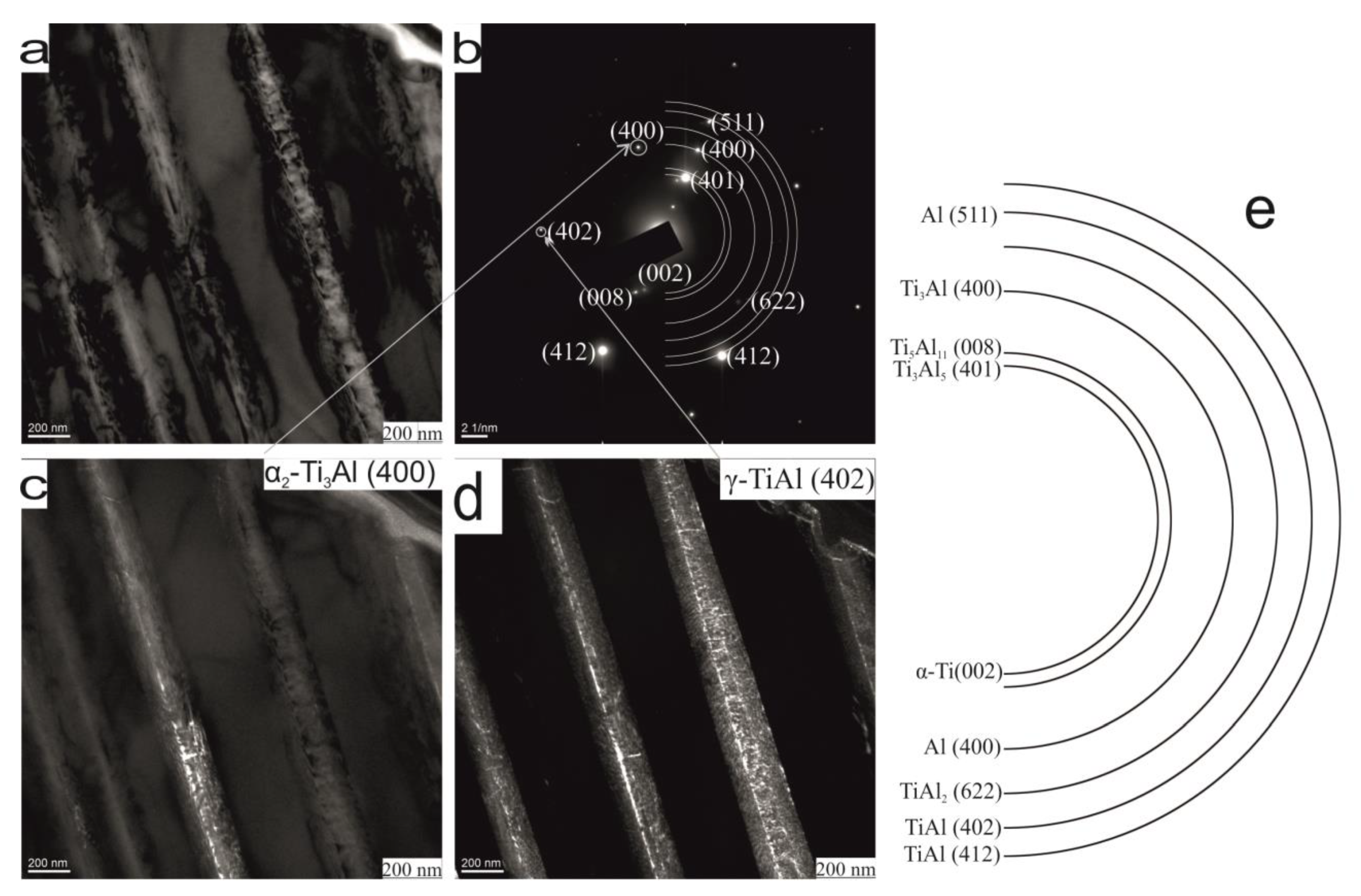
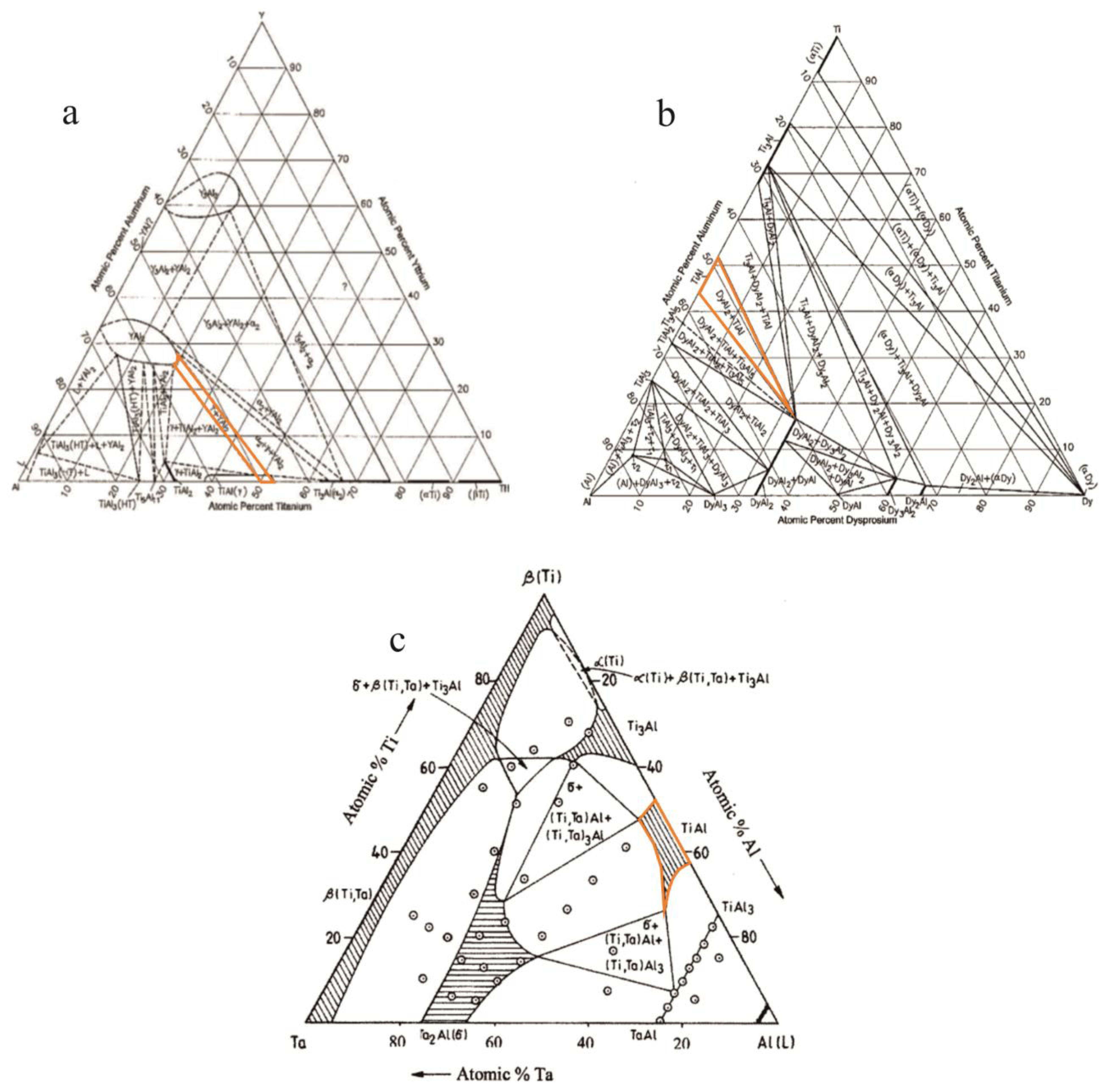

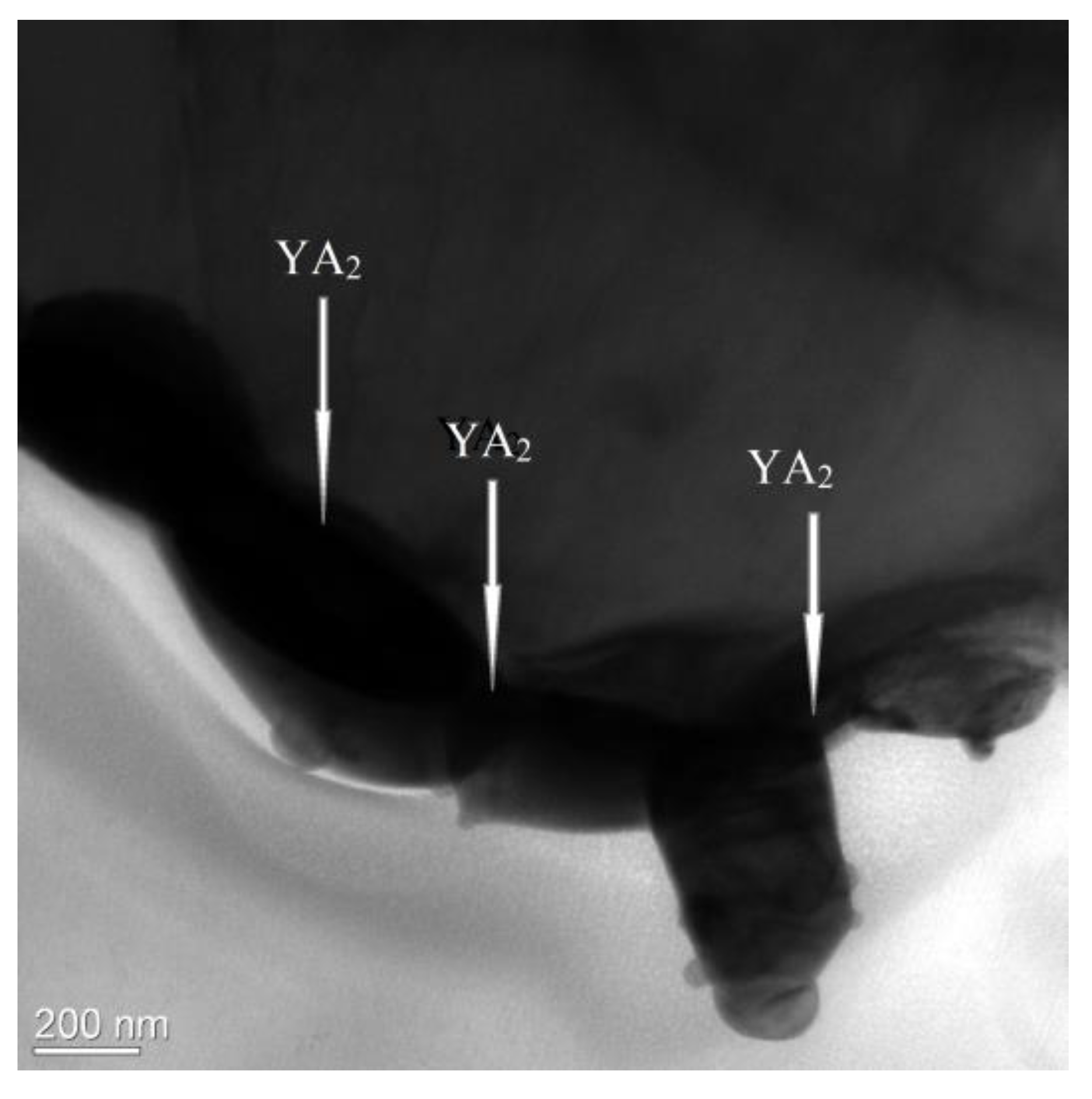
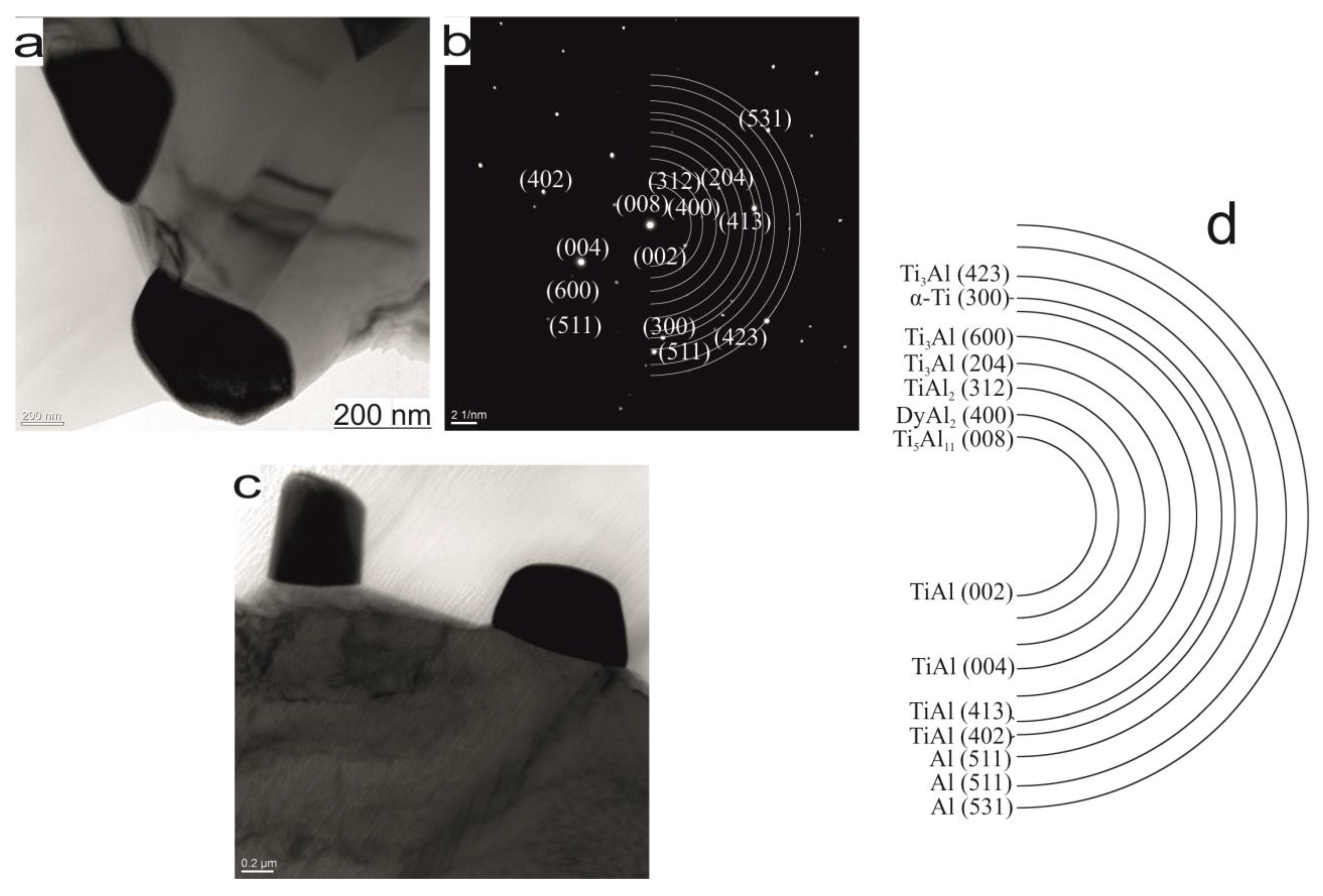
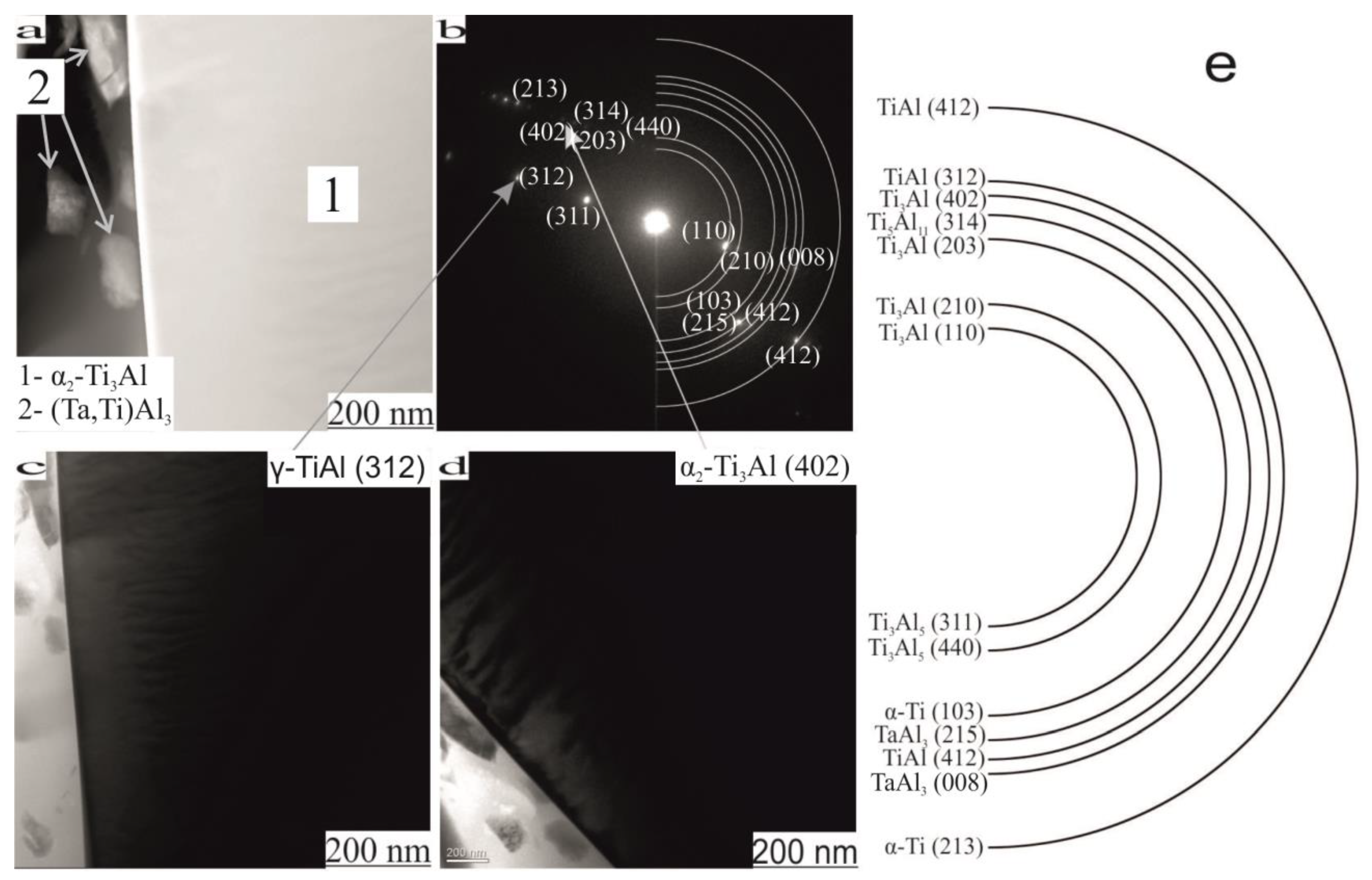
| Phase | State | a, Å | b, Å | c, Å | Alpha | Beta | Gamma | V, A^3 | Space Group | Share, % | E, eV | Rwp, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AlTi3-2768 | Init. | 5.764 | 5.764 | 4.664 | 90.00 | 90.00 | 120.00 | 132.56 | P6/mmm, Hexagonal | 22.33 | −19,317.484 | 7.193 |
| Spec. | 5.763 | 5.763 | 4.645 | 90.00 | 90.00 | 120.00 | 131.996 | |||||
| TiAl-Struct2-GeomOpt | Init. | 6.339 | 4.150 | 4.234 | 113.36 | 93.36 | 92.52 | 132.56 | P1, Triclinic | 49.80 | −4810.6263 | |
| Spec. | 6.129 | 4.237 | 4.017 | 113.62 | 88.24 | 92.42 | 133.711 | |||||
| TiAl-Struct2 | Init. | 6.339 | 4.150 | 4.234 | 113.36 | 93.36 | 92.52 | 101.791 | P1, Triclinic | 24.08 | −4975.776 | |
| Spec. | 6.194 | 4.119 | 4.215 | 113.07 | 92.88 | 91.97 | 98.64 |
| Phase | State | a, Å | b, Å | c, Å | Alpha | Beta | Gamma | V, A^3 | Space Group | Share % | E, eV | Rwp, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AlTi3-2768 | Init. | 5.764 | 5.764 | 4.664 | 90.00 | 90.00 | 120.00 | 132.56 | P6/mmm, Hexagonal | 26.99 | −19,603.151 | 6.317 |
| Spec. | 5.736 | 5.736 | 4.626 | 90.00 | 90.00 | 120.00 | 131.828 | |||||
| AlTi-2770 | Init. | 2.837 | 2.837 | 4.059 | 90.00 | 90.00 | 90.00 | 32.677 | P4/mmm, Tetragonal | 41.04 | −1660.340 | |
| Spec. | 2.824 | 2.824 | 4.070 | 90.00 | 90.00 | 90.00 | 32.466 | |||||
| TiAl-Struct2 | Init. | 6.339 | 4.150 | 4.234 | 113.36 | 93.36 | 92.52 | 101.791 | P1, Triclinic | 27.63 | −4954.073 | |
| Spec. | 6.245 | 4.128 | 4.319 | 114.67 | 91.27 | 93.84 | 100.802 |
| Phase | State | a, Å | b, Å | c, Å | Alpha | Beta | Gamma | V, A^3 | Space Group | Share, % | E, eV | Rwp, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AlTi3-2768 | Init. | 5.764 | 5.764 | 4.664 | 90.00 | 90.00 | 120.00 | 132.56 | P6/mmm, Hexagonal | 16.66 | −19,705.872 | 6.701 |
| Spec. | 5.791 | 5.791 | 4.736 | 90.00 | 90.00 | 120.00 | 137.565 | |||||
| AlTi-2770 | Init. | 2.837 | 2.837 | 4.059 | 90.00 | 90.00 | 90.00 | 32.677 | P4/mmm, Tetragonal | 50.61 | −1660.341 | |
| Spec. | 2.831 | 2.831 | 4.067 | 90.00 | 90.00 | 90.00 | 32.609 | |||||
| TiAl-Struct2 | Init. | 6.339 | 4.150 | 4.234 | 113.36 | 93.36 | 92.52 | 101.791 | P1, Triclinic | 25.79 | −4978.726 | |
| Spec. | 6.568 | 4.133 | 4.093 | 160.27 | 95.85 | 92.64 | 33.821 |
| Phase | State | a, Å | b, Å | c, Å | Alpha | Beta | GAMMA | V, A^3 | Space Group | Share, % | E, eV | Rwp, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AlTi3-2768 | Init. | 5.764 | 5.764 | 4.664 | 90.00 | 90.00 | 120.00 | 132.56 | P6/mmm, Hexagonal | 11.20 | −31,228.526 | 6.504 |
| Spec. | 5.771 | 5.771 | 4.657 | 90.00 | 90.00 | 120.00 | 134.34 | |||||
| AlTi-2770 | Init. | 2.837 | 2.837 | 4.059 | 90.00 | 90.00 | 90.00 | 32.677 | P4/mmm, Tetragonal | 65.04 | −1660.341 | |
| Spec. | 2.826 | 2.826 | 4.074 | 90.00 | 90.00 | 90.00 | 32.537 | |||||
| TiAl-Struct2-GeomOpt | Init. | 6.339 | 4.145 | 4.234 | 113.36 | 93.36 | 92.52 | 101.79 | P1, Triclinic | 16.88 | −4978.606 | |
| Spec. | 6.294 | 4.139 | 4.260 | 115.23 | 92.72 | 91.94 | 100.09 |
| Symbol of the Atom | x | y | z | Displacement Parameters, (U_iso) | Occupancy |
|---|---|---|---|---|---|
| Ti1 | 0.310 | −0.359 | −0.678 | 0.0127 | 1.0 |
| Ti2 | −0.500 | 0.334 | 0.667 | 0.0127 | 1.0 |
| Ti3 | −0.311 | 0.027 | 0.012 | 0.0127 | 1.0 |
| Ti4 | 0.227 | −0.017 | −0.036 | 0.0127 | 1.0 |
| Al5 | −0.000 | 0.334 | −0.333 | 0.0127 | 1.0 |
| Al6 | −0.227 | −0.316 | 0.370 | 0.0127 | 1.0 |
| Element | x | y | z | U_iso | Occupancy |
|---|---|---|---|---|---|
| Ti | 0.833 | 0.167 | 0.25 | 0.0127 | 1 |
| Al | 0.333 | 0.667 | 0.25 | 0.0127 | 1 |
| Dy; Ta; Y | 0.5 | 0.5 | 0.5 | 0.0127 | 1 |
| Composition | Space Group | Syngony | CSR Volume, Å^3 | Weight Fraction, % | Lattice Parameters, Å | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| TiAl | P4/mmm | tetragonal | 33 ± 5 | 38.4 | 2.8234 | 2.8234 | 4.0768 |
| Ti3Al | P63/mmc | tetragonal | 134 ± 5 | 25.2 | 5.7671 | 5.7671 | 4.64646 |
| α-Ti | C6/mmc | hexagonal | 31 ± 5 | 18.0 | 2.9253 | 2.9253 | 4.6184 |
| TiAl2 | C/mmm | rhombic | 195 ± 5 | 11.8 | 12.0187 | 4.0232 | 4.0253 |
| Ti5Al11 | P/mmm | rhombic | 261 ± 5 | 8.3 | 3.9522 | 3.9522 | 17.3119 |
| Ti3Al5 | P/mmm | rhombic | 61 ± 5 | 2.9 | 3.8675 | 3.8212 | 4.1445 |
| Ti2Al5 | P4/mmm | tetragonal | 440 ± 5 | 2.5 | 3.8164 | 3.8164 | 30.1785 |
| Composition | Space Group | Syngony | CSR Volume, Å^3 | Weight Fraction, % | Lattice Parameters, Å | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Ti3Al5 | P/mmm | rhombic | 65 ± 5 | 70.3 | 4.0040 | 4.0049 | 4.0710 |
| Ti3Al | P63/mmc | tetragonal | 134 ± 5 | 18.3 | 5.7661 | 5.7661 | 4.6371 |
| Al | Fm-3m | cubic | 66±5 | 8.6 | 4.0311 | 4.0311 | 4.0311 |
| α-Ti | P63/mmc | hexagonal | 31 ± 5 | 1.4 | 2.9186 | 2.9186 | 4.6006 |
| TiAl | P4/mmm | tetragonal | 40 ± 5 | 1.2 | 2.7453 | 2.7453 | 5.3402 |
| Y | P63/mmc | hexagonal | 67± 5 | 0.3 | 3.6689 | 3.6689 | 5.7302 |
| Alloy | Composition | Space Group | Syngony | Weight Fraction, % | Alloy | Composition | Space Group | Syngony | Weight Fraction, % |
|---|---|---|---|---|---|---|---|---|---|
| TAD | TiAl | P4/mmm | tetragonal | 74.3 | TAT | TiAl | P4/mmm | tetragonal | 30.1 |
| Ti3Al5 | P/mmm | rhombic | 10.9 | Ti3Al | P63/mmc | tetragonal | 22.9 | ||
| TiAl2 | C/mmm | rhombic | 5.0 | Ti3Al5 | P/mmm | rhombic | 26.4 | ||
| Al | Fm-3m | cubic | 3.9 | Ti5Al11 | P/mmm | rhombic | 3.9 | ||
| Ti5Al11 | I4/mmm | rhombic | 1.4 | α-Ti | P63/mmc | hexagonal | 3.9 | ||
| α-Ti | P63/mmc | hexagonal | 1.4 | TaAl3 | F-43m | cubic | 2.8 | ||
| Dy | P63/mmc | hexagonal | 1,6 | Ti2Al5 | P4/mmm | tetragonal | 1.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belgibayeva, A.; Abzaev, Y.; Karakchieva, N.; Erkasov, R.; Sachkov, V.; Kurzina, I. The Structural and Phase State of the TiAl System Alloyed with Rare-Earth Metals of the Controlled Composition Synthesized by the “Hydride Technology”. Metals 2020, 10, 859. https://doi.org/10.3390/met10070859
Belgibayeva A, Abzaev Y, Karakchieva N, Erkasov R, Sachkov V, Kurzina I. The Structural and Phase State of the TiAl System Alloyed with Rare-Earth Metals of the Controlled Composition Synthesized by the “Hydride Technology”. Metals. 2020; 10(7):859. https://doi.org/10.3390/met10070859
Chicago/Turabian StyleBelgibayeva, Akbayan, Yuri Abzaev, Natalia Karakchieva, Rakhmetulla Erkasov, Victor Sachkov, and Irina Kurzina. 2020. "The Structural and Phase State of the TiAl System Alloyed with Rare-Earth Metals of the Controlled Composition Synthesized by the “Hydride Technology”" Metals 10, no. 7: 859. https://doi.org/10.3390/met10070859
APA StyleBelgibayeva, A., Abzaev, Y., Karakchieva, N., Erkasov, R., Sachkov, V., & Kurzina, I. (2020). The Structural and Phase State of the TiAl System Alloyed with Rare-Earth Metals of the Controlled Composition Synthesized by the “Hydride Technology”. Metals, 10(7), 859. https://doi.org/10.3390/met10070859








