Corrosion Resistance and Ion Release of Dental Prosthesis of CoCr Obtained by CAD-CAM Milling, Casting and Laser Sintering
Abstract
1. Introduction
2. Materials and Methods
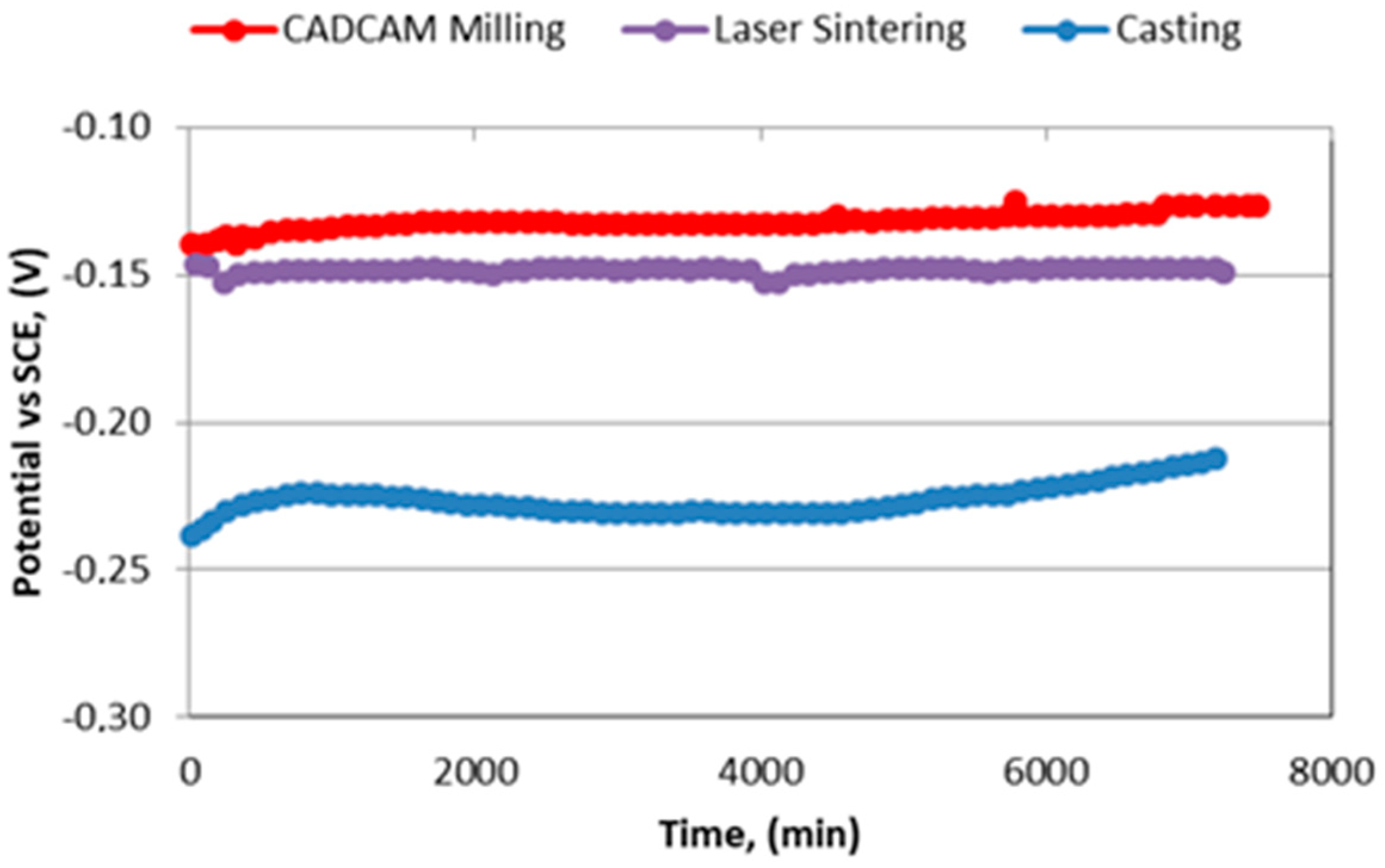
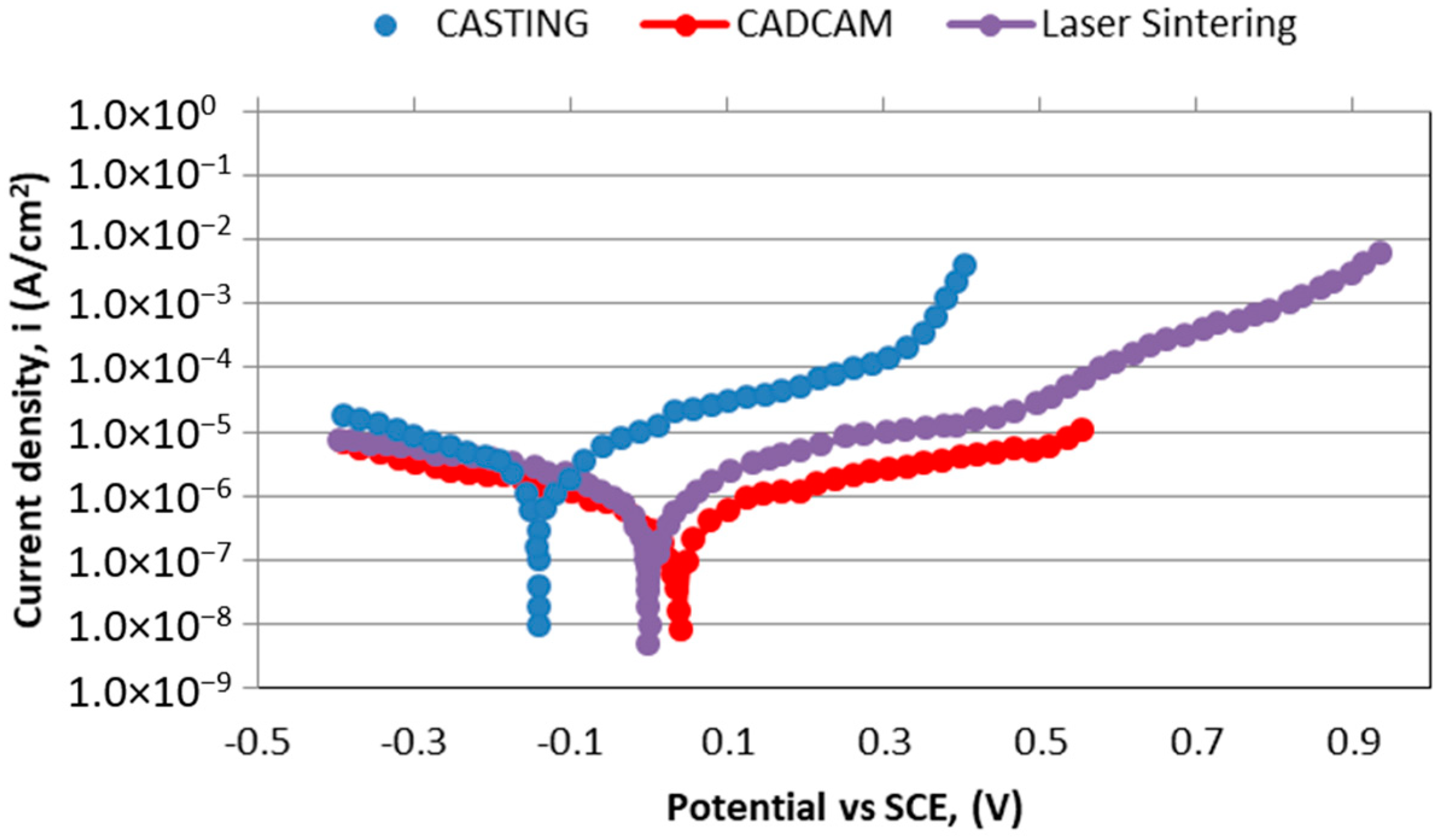
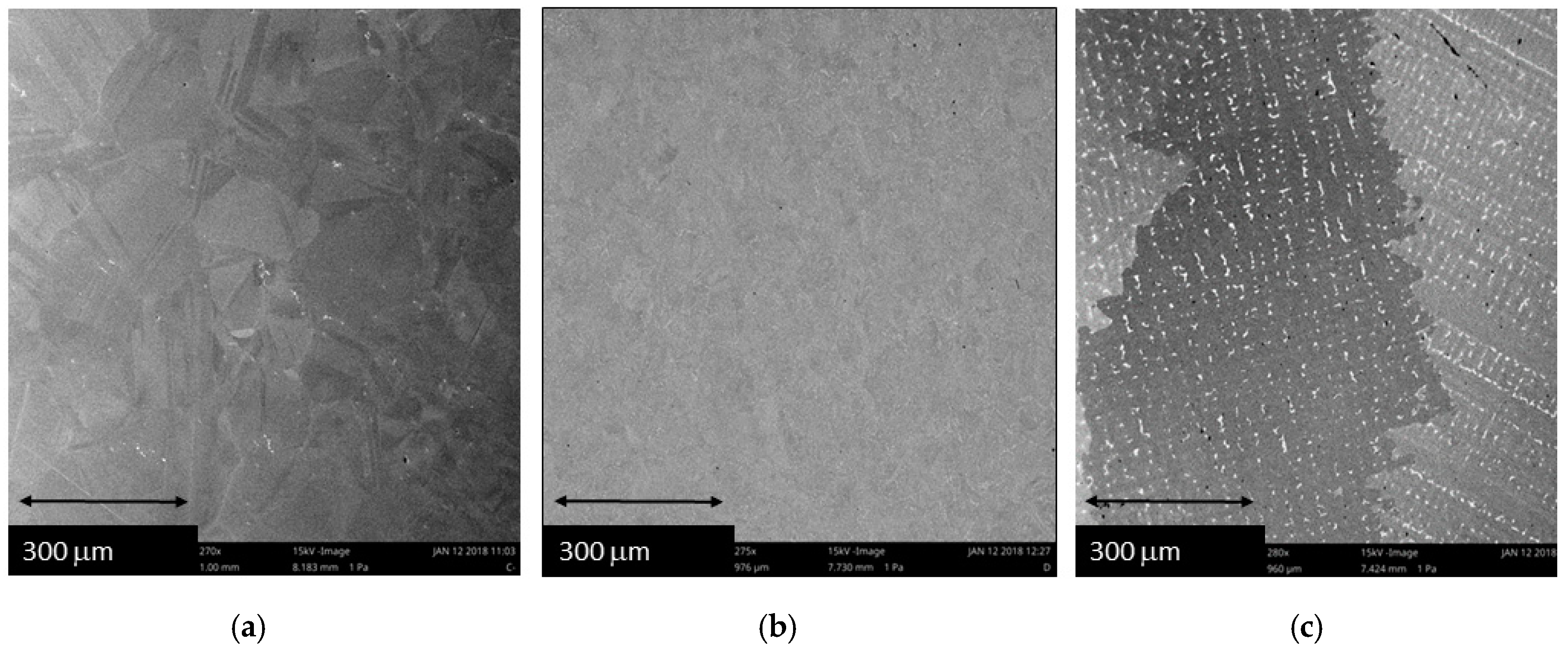
3. Results and Discussion
- Free corrosion potential (EOCP), potential of an electrode measured with respect to a reference electrode or another electrode when no current flows to or from the material.
- Corrosion Potential (Ecorr), potential calculated at the intersection where the total oxidation rate is equal to the total reduction rate.
- Corrosion current density (iCORR), current divided by the surface of the electrode. It is the size of the anodic component of the current which flows at the corrosion potential Ecorr. Since by definition the resulting current is equal to zero at that potential, the cathodic component is of equal size, but of opposite sign.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Branemark, P.; Adell, R.; Albrektsson, T. Osseointegrated titanium implants in the rehabilitation of the edentulous patient. Adv. Biomater. 1982, 4, 133–141. [Google Scholar]
- Ericsson, I.; Lekholm, U.; Branemark, P.; Lindhe, J.; Glantz, P.O.; Nyman, S. A clinical evaluation of fixed bridge restorations supported by the combination of teeth and osseointegrated titanium implants. J. Clin. Periodontol. 1986, 13, 307–312. [Google Scholar] [CrossRef]
- Reclaru, L.; Meyer, J.M. Study of corrosion between a titanium implant and dental alloys. J. Dent. 1994, 22, 159–168. [Google Scholar] [CrossRef]
- Geis-Gerstorfer, J. In vitro corrosion measurements of dental alloys. J. Dent. 1994, 22, 247–251. [Google Scholar] [CrossRef]
- Denizoğlu, S.; YeşLilDuymuş, Z.; Akyalçin, S. Evaluation of Ion Release from Two Base-Metal Alloys at Various pH Levels. J. Int. Med. Res. 2004, 1. [Google Scholar] [CrossRef]
- Benatti, O.F.M.; Miranda, W.G.; Muench, A. In vitro and in vivo corrosion evaluation of nickel-chromium and copper-aluminum-based alloys. J. Prosthet. Dent. 2000, 84, 360–363. [Google Scholar] [CrossRef]
- Wataha, J.C.; Lockwood, P.E.; Khajotia, S.S. Effect of pH on element release from dental casting alloys. J. Prosthet. Dent. 1998, 80, 691–698. [Google Scholar] [CrossRef]
- Gil, F.J.; Rodríguez, D.; Planell, J.A.; Cortada, M.; Giner, L.; Costa, S. Galvanic corrosion behaviour of Titanium implants coupled to dental alloys. J. Mat. Sci. Mat. Med. 2000, 11, 287–293. [Google Scholar]
- Branemark, P.; Hansson, I.; Adell, R.; Lindstrom, U.; Hallen, J.; Ohman, O. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Adell, R.; Lekholm, U.; Rocker, U.; Branemark, P. A 15-year study of osseointegrated implants in the treatment of the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 6, 387–416. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Fuys, R.A.; Stanford, J.W. Applications of electrochemical techniques to characterize the corrosion of dental alloys. In Corrosion and Degradation of Implant Materials; Syrett, B.C., Acharya, A., Eds.; ASTM: Philadelphia, PA, USA, 1979; pp. 277–294. [Google Scholar]
- Tuna, S.H.; Pekmez, N.O.; Kürkçüoğlu, I. Corrosion resistance assessment of Co-Cr alloy frameworks fabricated by CAD/CAM milling, laser sintering, and casting methods. J. Prosthet. Dent. 2015, 114, 725–734. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hotta, Y.; Kunii, J.; Kuriyama, S.; Tamaki, Y. A review of dental CAD/CAM: Current status and future perspectives from 20 years of experience. Dent. Mater. J. 2009, 28, 44–56. [Google Scholar] [CrossRef]
- Lin, W.S.; Harris, B.T.; Zandinejad, A.; Morton, D. Use of digital data acquisition and CAD/CAM technology for the fabrication of a fixed complete dental prosthesis on dental implants. J. Prosthet. Dent. 2014, 111, 1–5. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, W.C.; Kim, H.Y.; Kim, J.H. An evaluation of marginal fit of three-unit fixed dental prostheses fabricated by direct metal laser sintering system. Dent. Mater. 2013, 29, 91–96. [Google Scholar] [CrossRef]
- Akova, T.; Ucar, Y.; Tukay, A.; Balkaya, M.C.; Brantley, W.A. Comparison of the bond strength of laser-sintered and cast base metal dental alloys to porcelain. Dent. Mater. 2008, 24, 1400–1404. [Google Scholar] [CrossRef]
- Ucar, Y.; Akova, T.; Akyil, M.S.; Brantley, W.A. Internal fit evaluation of crowns prepared using a new dental crown fabrication technique: Laser-sintered Co-Cr crowns. J. Prosthet. Dent. 2009, 102, 253–259. [Google Scholar] [CrossRef]
- Atzeni, E.; Salmi, A. Evaluation of additive manufacturing (AM) techniques for the production of metal–ceramic dental restorations. J. Manuf. Process. 2015, 20, 40–45. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Chen, X.; Zhang, C.; Zhang, G.; Xu, Z. Design and manufacture of customized dental implants by using reverse engineering and selective laser melting technology. J. Prosthet. Dent. 2014, 112, 1088–1095. [Google Scholar] [CrossRef]
- Hongfu, Z.; Qin, F. 3D reconstruction and SLM survey for dental implants. J. Mech. Med. Biol. 2017, 17, 1750084. [Google Scholar]
- Beuer, F.; Aggstaller, H.; Ritcher, J.; Edelhoff, D.; Gernet, W. Influence of preparation angle on marginal and internal fit of CAD/CAM fabricated zirconia crowns copings. Quintessence Int. 2009, 40, 419–424. [Google Scholar]
- Borba, M.; Cesar, P.F.; Griggs, J.A.; Della Bona, A. Adaptation of all-ceramic fixed partial dentures. Dent. Mater. 2011, 27, 1119–1126. [Google Scholar] [CrossRef]
- Coli, P.; Karlsson, S. Fit of a new pressure sintered zirconium dioxide coping. Int. J. Prosthod. 2004, 17, 59–64. [Google Scholar]
- Colpani, J.T.; Borba, M.; Della Bona, A. Evaluation of marginal and internal fit of ceramic crown copings. Dent. Mater. 2013, 29, 174–180. [Google Scholar] [CrossRef]
- Naert, I.; van Der Donck, A.; Beckers, L. Precision of fit and clinical evaluation of all-ceramic full restorations followed between 0.5 and 5 years. J. Oral Rehabil. 2005, 32, 51–57. [Google Scholar] [CrossRef]
- Rödiger, M.; Schneider, L.; Rinke, S. Influence of Material Selection on the Marginal Accuracy of CAD/CAM-Fabricated Metal- and All-Ceramic Single Crown Copings. Biomed. Res. Int. 2018, 2018, 2143906. [Google Scholar] [CrossRef]
- Sannino, G.; Gloria, F.; Schiavetti, R.; Ottria, L.; Barlattani, A. Dental wings cad/cam system precision: An internal and marginal fit sperimental analisys. Oral Implantol. 2009, 2, 11–20. [Google Scholar]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef]
- Nedelcu, R.; Olsson, P.; Nyström, I.; Thor, A. Finish line distinctness and accuracy in 7 intraoral scanners versus conventional impression: An In Vitro descriptive comparison. BMC Oral Health 2018, 18, 27. [Google Scholar] [CrossRef]
- Aldegheishem, A.; Ioannidis, G.; Att, W.; Petridis, H. Success and survival of various types of all-ceramic single crowns: A critical review and analysis of studies with a mean follow-up of 5 years or longer. Int. J. Prosthodont. 2017, 30, 168–181. [Google Scholar] [CrossRef]
- Rinke, S.; Fornefett, D.; Gersdor, N.; Lange, K.; Roediger, M. Multifactorial analysis of the impact of different manufacturing processes on the marginal t of zirconia copings. Dent. Mater. 2012, 31, 601–609. [Google Scholar] [CrossRef]
- Brugirard, J. Etude du Comportament Électrochimique des Métaux et Alliages Dentaires; J Prelat: Paris, France, 1970; pp. 62–68. [Google Scholar]
- Correa, C.B.; Pires, J.R.; Fernandes-Filho, R.B.; Sartori, R.; Vaz, L.G. Fatigue and fluoride corrosion on Streptococcus mutans adherence to titanium-based implant/component surfaces. J. Prosthodont. 2009, 18, 382–387. [Google Scholar] [CrossRef]
- ASTM-E3-11. Standard Guide for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Baboian, R. Electrochemical techniques for predicting galvanic corrosion. In Galvanic and Pitting Corrosion-Field and Laboratory Studies; Baboian, R., France, W., Rowel, L., Ryniewicz, J., Eds.; ASTM: Philadelphia, PA, USA, 1976; pp. 5–19. [Google Scholar]
- Mansfeld, F.; Kenkel, J.V. Laboratory studies of galvanic corrosion of aluminium alloys. In Galvanic and Pitting Corrosion-Field and Laboratory Studies; Baboian, R., France, W., Roew, L., Rynewicz, J., Eds.; ASTM: Philadelphia, PA, USA, 1976; pp. 20–47. [Google Scholar]
- Mansfeld, F. The Polarization Resistance Technique for Measuring Corrosion Currents. In Advances in Corrosion Science and Technology; Fontana, M.G., Staehle, R.W., Eds.; Springer: Boston, MA, USA, 1976; pp. 89–92. [Google Scholar]
- Canay, S.; Öktemer, M. In Vitro corrosion behaviour of 13 prosthodontic alloys. Dent. Res. 1992, 23, 279–287. [Google Scholar]
- American Society for Testing and Materials. Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements; Technical Report No. ASTM G5-14e1; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- IOS. ISO 10993-5:2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneve, Switzerland, 2009. [Google Scholar]
- Al Jabbari, Y.S. Physico-mechanical properties and prosthodontic applications of Co-Cr dental alloys: A review of the literature. J. Adv. Prosthodont. 2014, 6, 138–145. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S.; Barmpagadaki, X.; Psarris, I.; Zinelis, S. Microstructural, mechanical, ionic realese and tarnish resistance characterization of porcelain fused to metal Co-Cr alloys manufactured via casting and three different CAD/CAM techniques. J. Prosthod. Res. 2019, 63, 150–156. [Google Scholar] [CrossRef]
- Gil, F.J.; Sánchez, L.A.; Espias, A.; Planell, J.A. In vitro corrosion behaviour and metallic ion release of different prosthodontic alloys. Int. Dent. J. 1999, 49, 347–351. [Google Scholar] [CrossRef]
- Aparicio, C.; Rodriguez, R.; Manero, J.M.; Andrés, A.; Arandés, J.; Planell, J.A. Aleaciones Ligeras; Gil, F.J., Ed.; Ediciones UPC: Barcelona, Spain, 2001; pp. 480–487. [Google Scholar]
- Porter, D.A.; Easterling, K.E.; Sheriff, M. Phase Transformation in Metals and Alloys, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Velasco, E.; Monsalve-Guil, L.; Jimenez, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pegueroles, M.; Perez, R.; Gil, F.J. Importance of the Roughness and Residual Stresses of Dental Implants on Fatigue and Osseointegration Behavior. In Vivo Study in Rabbits. J. Oral Inv. 2016, 42, 469–476. [Google Scholar] [CrossRef]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent Mater. 2007, 23, 679–687. [Google Scholar] [CrossRef]
- Galo, R.; Rocha, L.A.; Faria, A.C.; Silveira, R.R.; Ribeiro, R.F.; de Mattos, M.D.G. Influence of the casting processing route on the corrosion behavior of dental alloys. Mater. Sci. Eng. 2014, 45, 519–523. [Google Scholar] [CrossRef]
- Molina, C.; Nogués, L.l.; Martinez-Gomis, J.; Peraire, M.; Salsench, J.; Sevilla, P.; Gil, F.J. Dental casting alloys behaviour during power toothbrushing with toothpastes with various abrasivities. Part II: Corrosion and ion release. J. Mater. Sci. Mater. Med. 2008, 19, 3015–3019. [Google Scholar] [CrossRef][Green Version]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Gil, F.J. Localized corrosion of 316L stainless steel with SiO2-CaO films obtained by means of sol-gel treatment. J. Biomed. Mater. Res. 2003, 67, 674–678. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, S.H.; Kim, Y.K.; Son, J.S.; Min, B.K. Microstructures and mechanical properties of CoCr dental alloys fabricated by three CAD/CAM based processing techniques. Materials 2016, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar]
- Subbu, R.; Sagoo, R.; Goswami, V.; Goswami, K.; Langman, G.; Bhatt, K.; Roy-Choudhury, S. Metallosis in metal-on-metal hip resurfacing: An unusual presentation. Eur. J. Radiol. Extra 2011, 78, 101–104. [Google Scholar] [CrossRef]
- Hosoki, M.; Bando, E.; Asaoka, K.; Takeuchi, H.; Nishigawa, K. Assessment of allergic hypersensivity to dental materials. Biomed. Mater. Eng. 2009, 19, 53–61. [Google Scholar]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and bioocorrosion on implant survival and complications? A critical review. Clin. Oral Impl. Res. 2018, 29, 37–53. [Google Scholar] [CrossRef]

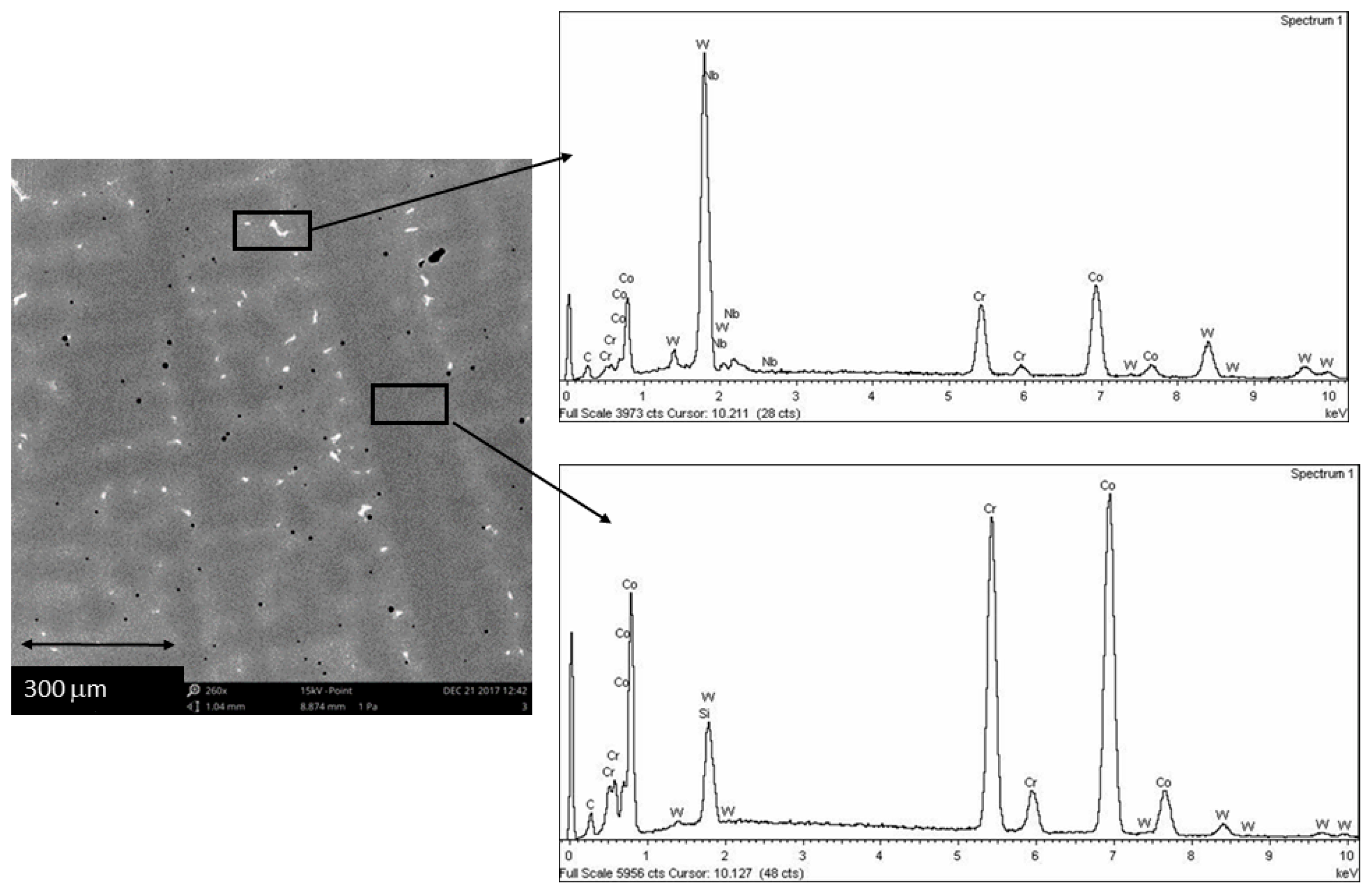
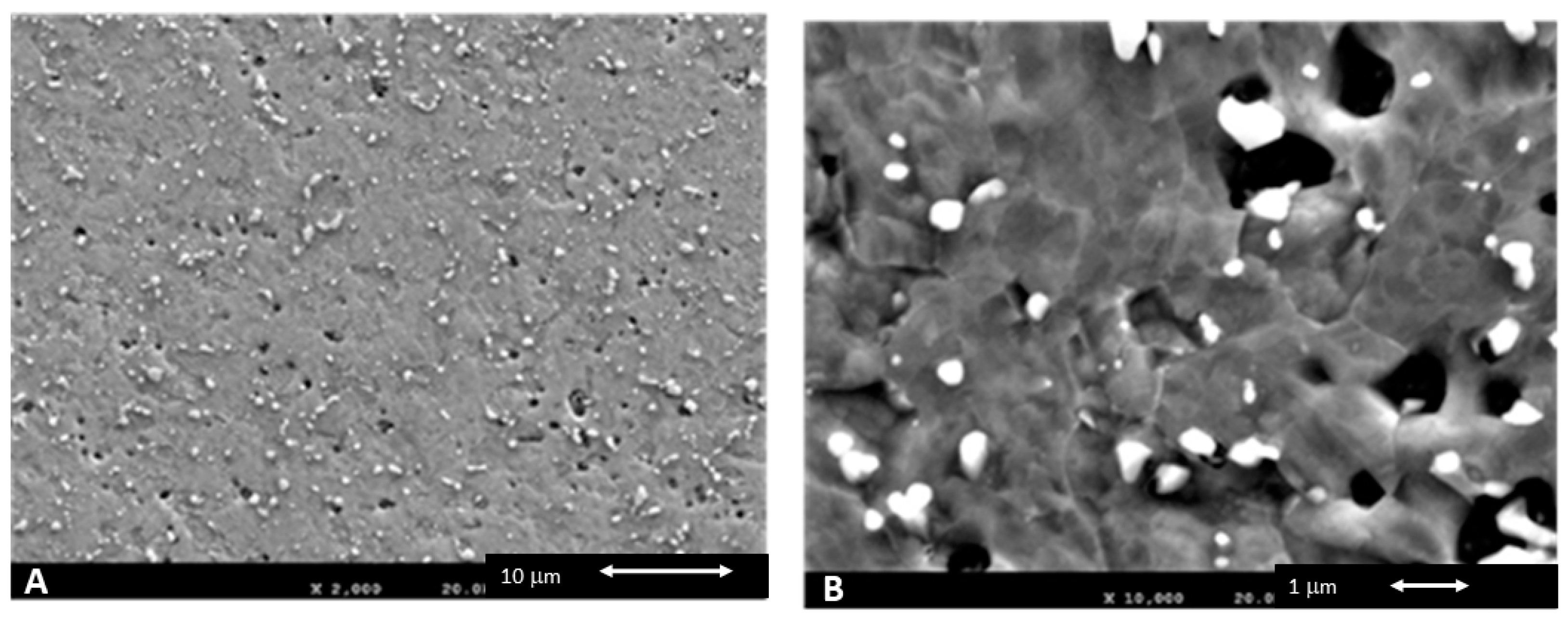
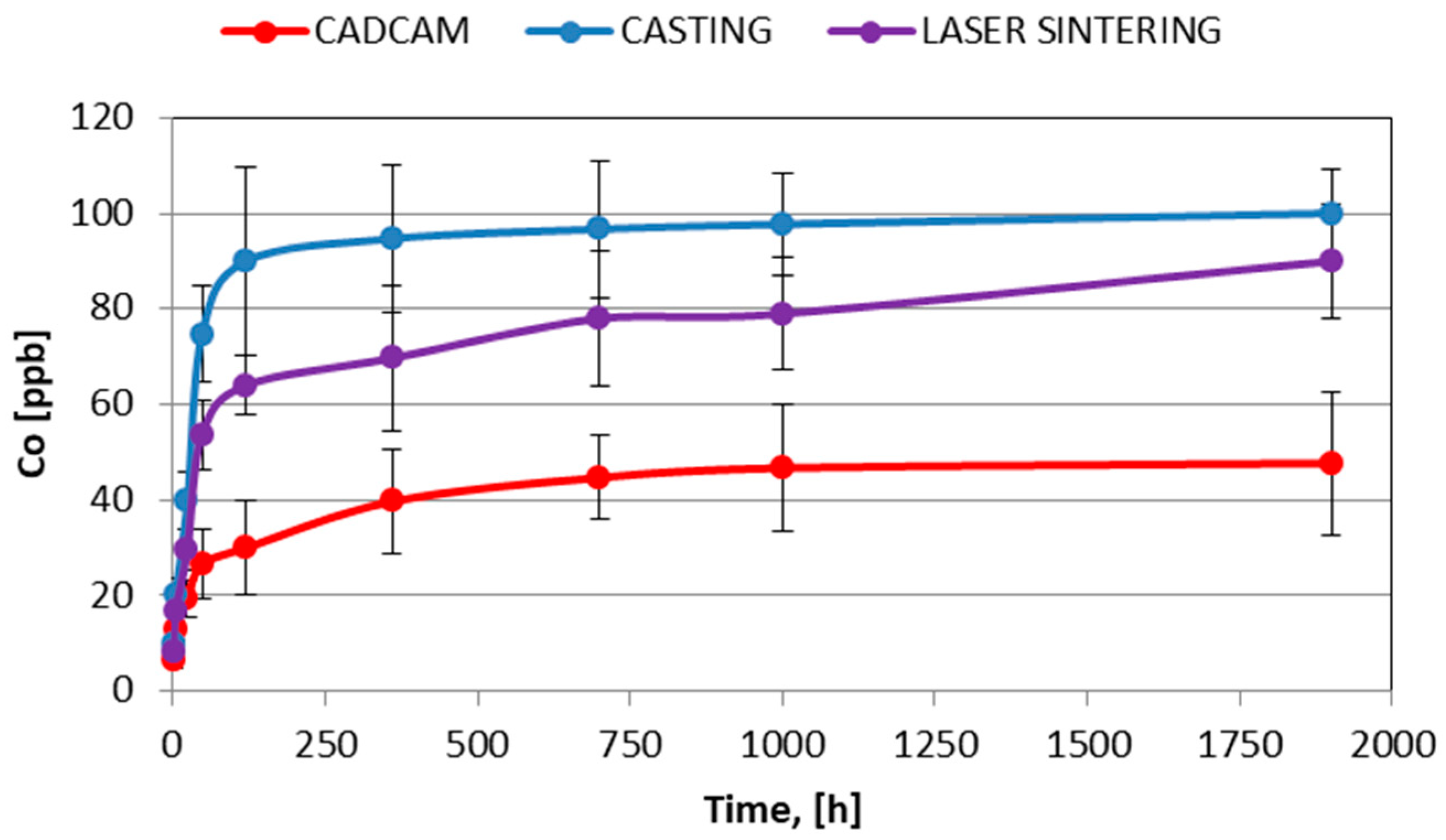
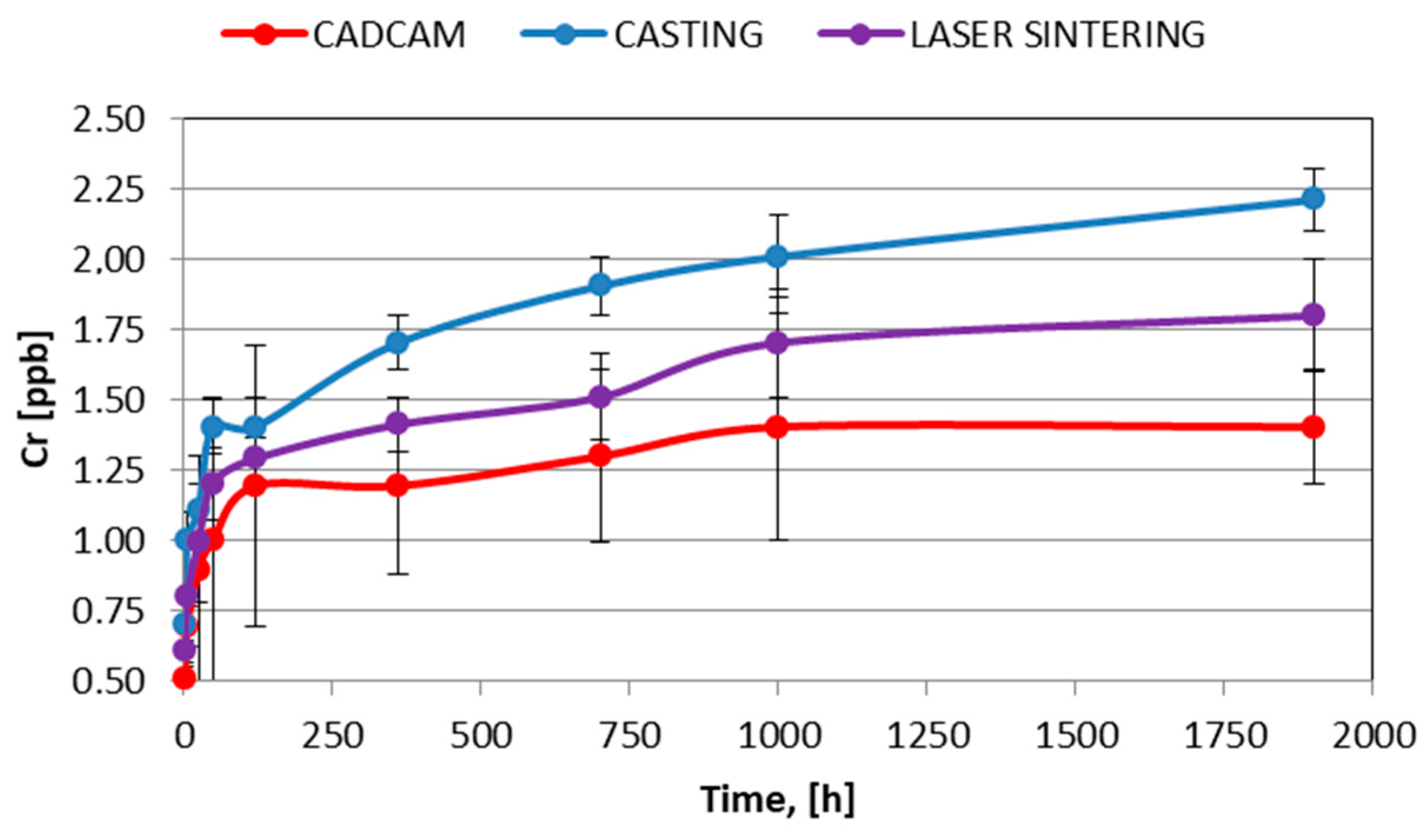
| Co | Cr | W | Si | C | Nb |
|---|---|---|---|---|---|
| 56.53 ± 2.11 | 27.11 ± 1.31 | 9.64 ± 0.79 | 1.27 ± 0.80 | <1% | <1.5% |
| Chemical Product | Composition (g/dm3) |
|---|---|
| K2HPO4 | 0.22 |
| KCI | 1.19 |
| KSCN | 0.29 |
| Na2HPO4 | 0.26 |
| NaCl | 0.69 |
| NaHCO3 | 1.49 |
| Urea | 1.49 |
| Lactic acid | up to pH = 6.8 |
| CoCr alloy | icorr (μA/cm2) | ip (μA/cm2) | Ecorr (V) |
|---|---|---|---|
| Casting | 0.009 | 0.65 | −0.18 |
| Laser sintering | 0.008 | 0.51 | −0.01 |
| CAD-CAM milling | 0.005 | 0.90 | +0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padrós, R.; Giner-Tarrida, L.; Herrero-Climent, M.; Punset, M.; Gil, F.J. Corrosion Resistance and Ion Release of Dental Prosthesis of CoCr Obtained by CAD-CAM Milling, Casting and Laser Sintering. Metals 2020, 10, 827. https://doi.org/10.3390/met10060827
Padrós R, Giner-Tarrida L, Herrero-Climent M, Punset M, Gil FJ. Corrosion Resistance and Ion Release of Dental Prosthesis of CoCr Obtained by CAD-CAM Milling, Casting and Laser Sintering. Metals. 2020; 10(6):827. https://doi.org/10.3390/met10060827
Chicago/Turabian StylePadrós, Roberto, Luís Giner-Tarrida, Mariano Herrero-Climent, Miquel Punset, and Francisco Javier Gil. 2020. "Corrosion Resistance and Ion Release of Dental Prosthesis of CoCr Obtained by CAD-CAM Milling, Casting and Laser Sintering" Metals 10, no. 6: 827. https://doi.org/10.3390/met10060827
APA StylePadrós, R., Giner-Tarrida, L., Herrero-Climent, M., Punset, M., & Gil, F. J. (2020). Corrosion Resistance and Ion Release of Dental Prosthesis of CoCr Obtained by CAD-CAM Milling, Casting and Laser Sintering. Metals, 10(6), 827. https://doi.org/10.3390/met10060827







