Mechanism of Nickel, Magnesium, and Iron Recovery from Olivine Bearing Ore during Leaching with Hydrochloric Acid Including a Carbonation Pre-Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Analysis Methods
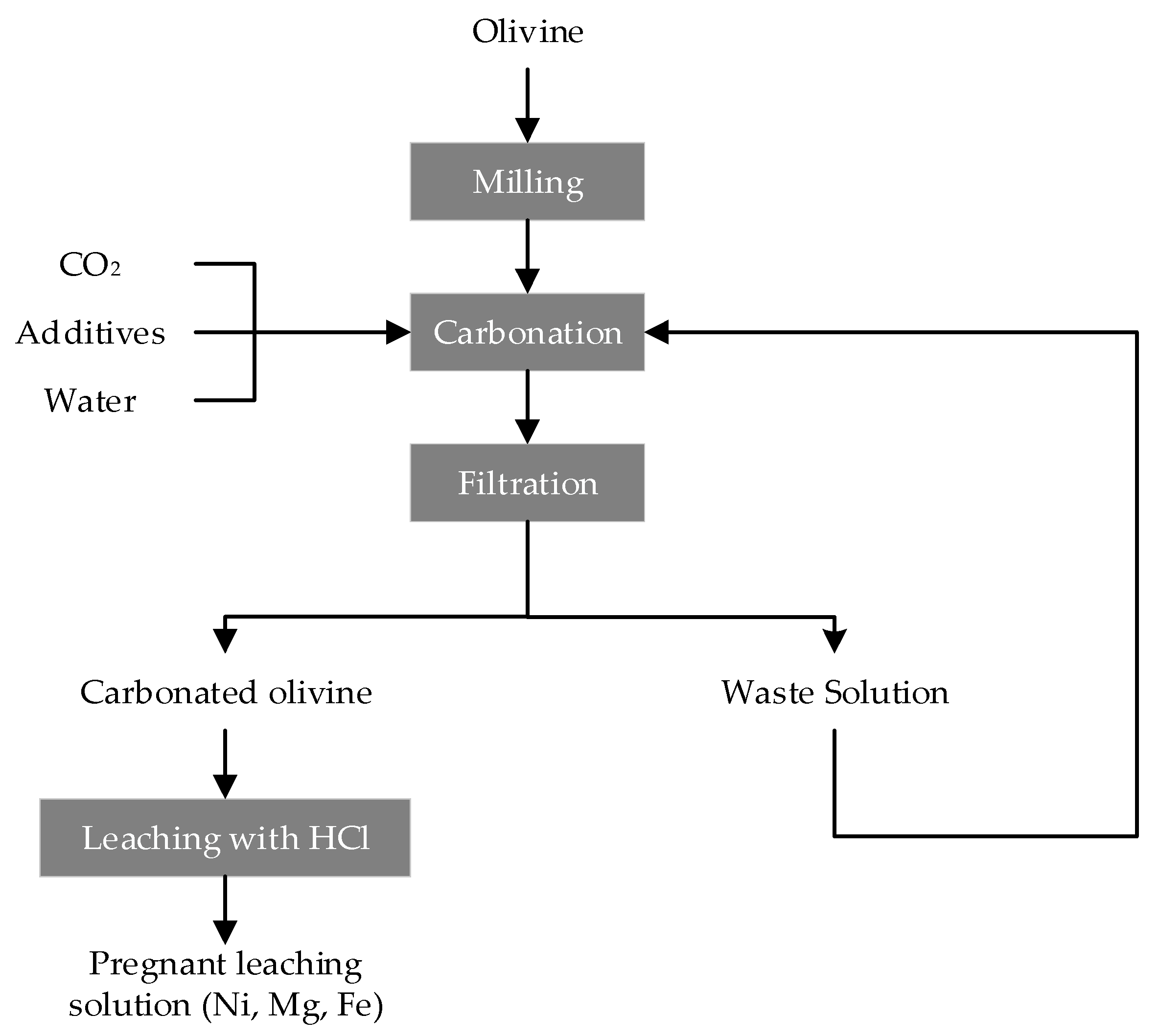
2.2. Leaching of Olivine Bearing Ore without Carbonation Pre-Treatment
2.3. Leaching of the Carbonated Olivine
3. Results
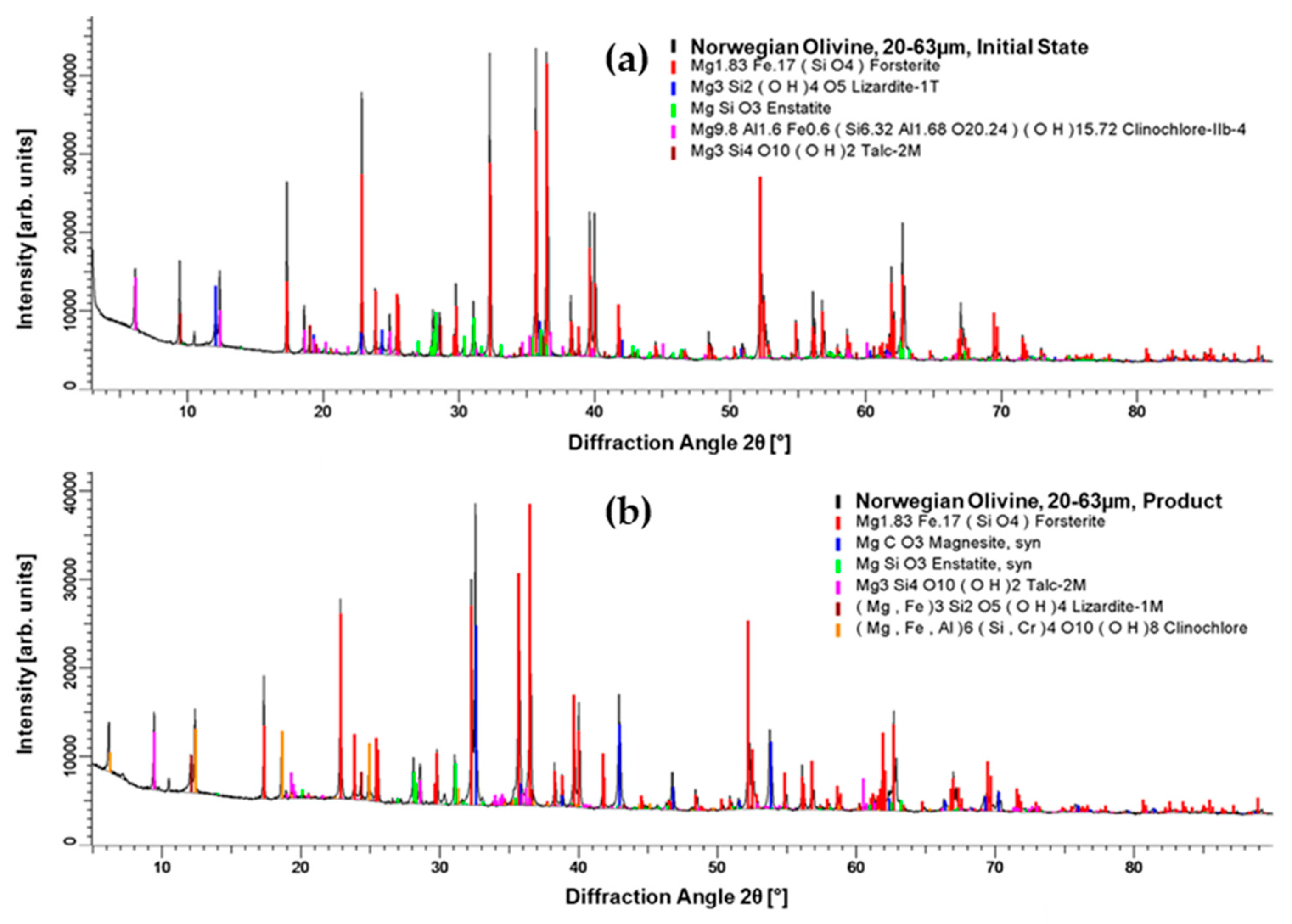
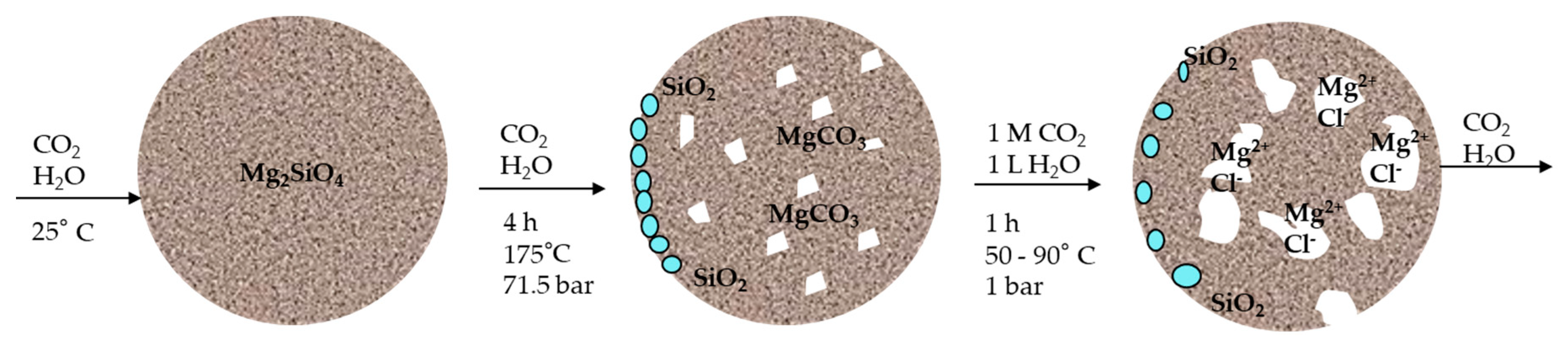
3.1. Material Analysis after Carbonation as a Pre-Treatment Step
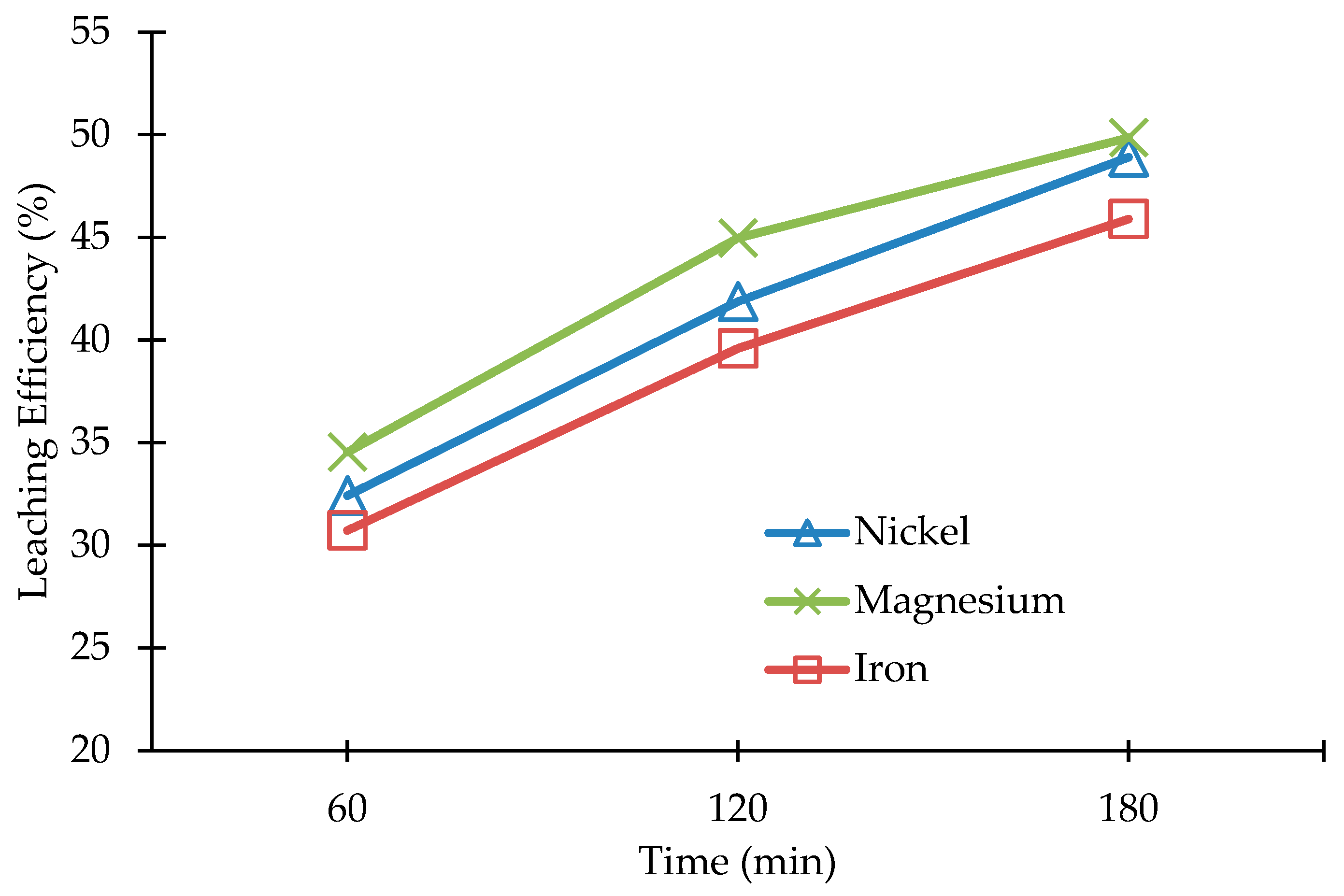
3.2. Leaching of Olivine Bearing Ore without Carbonation Pre-Treatment
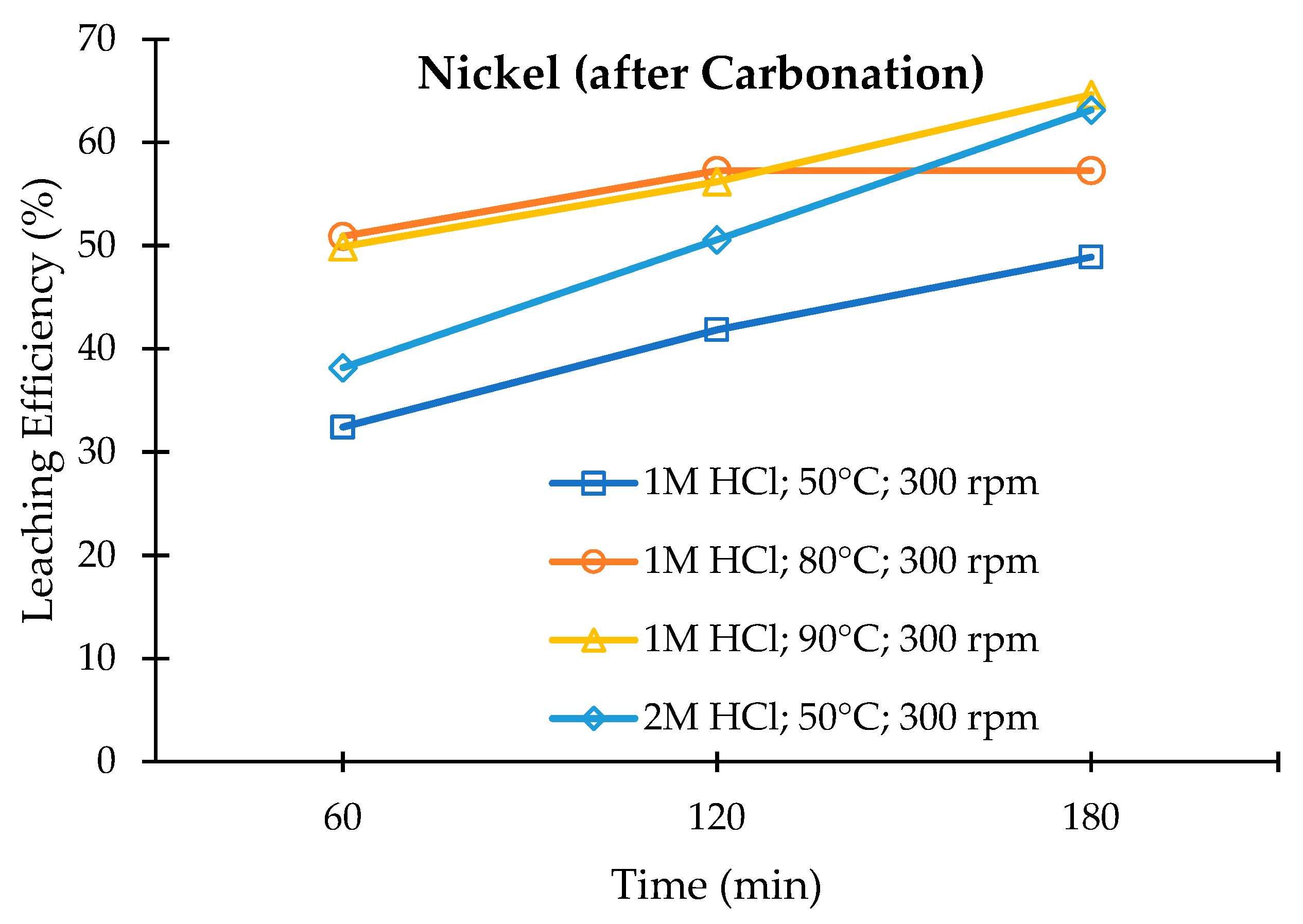
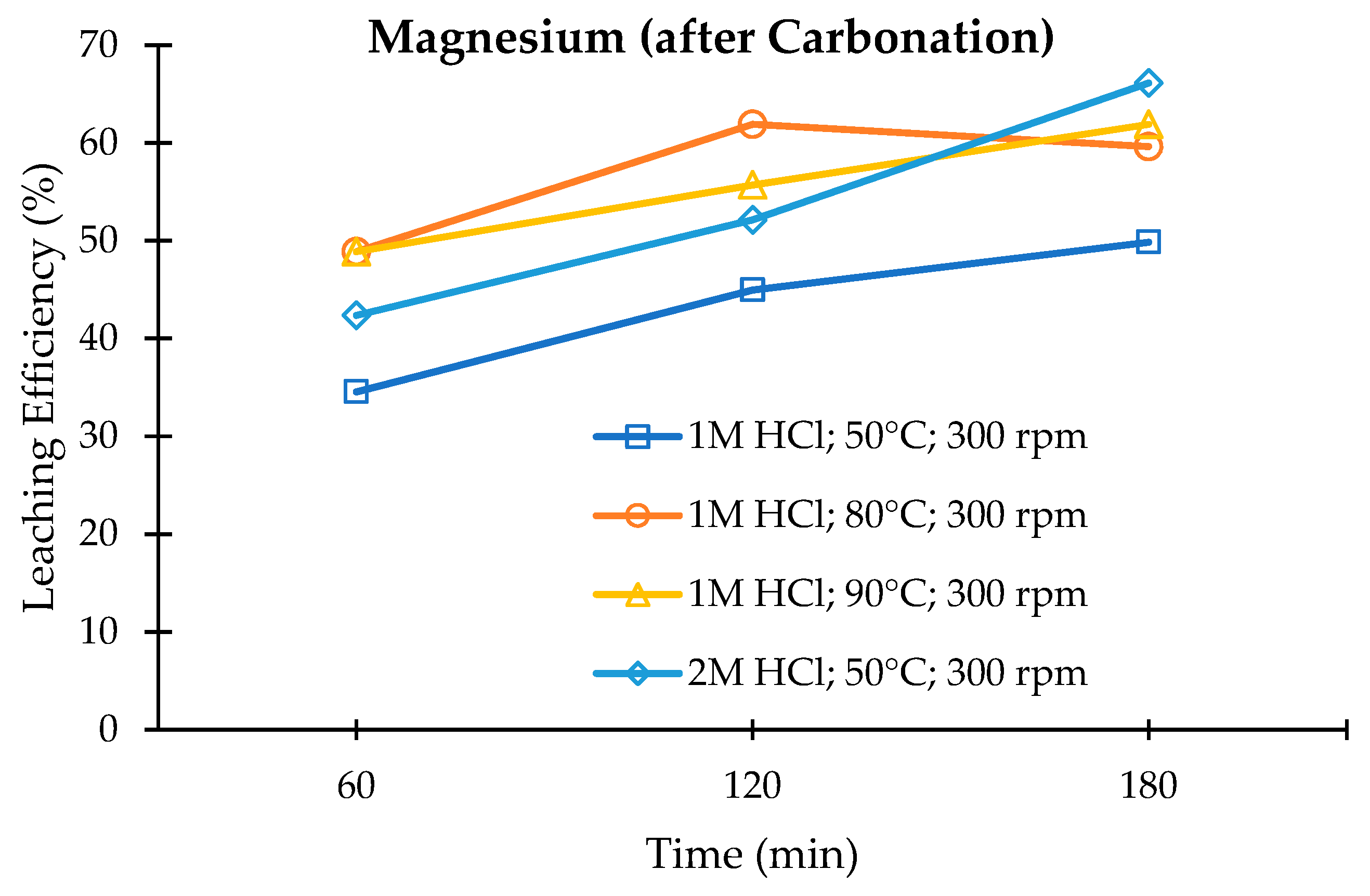
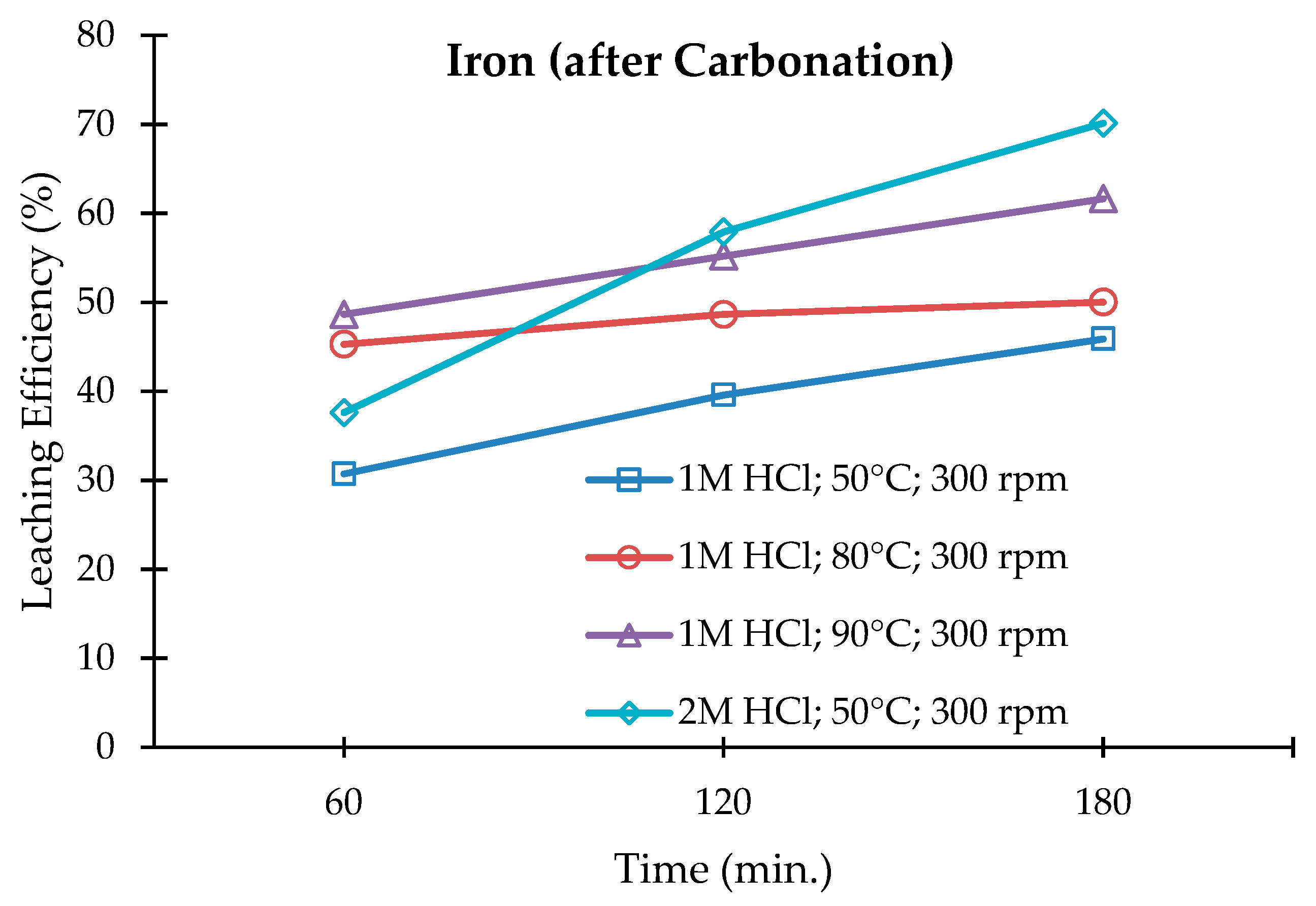
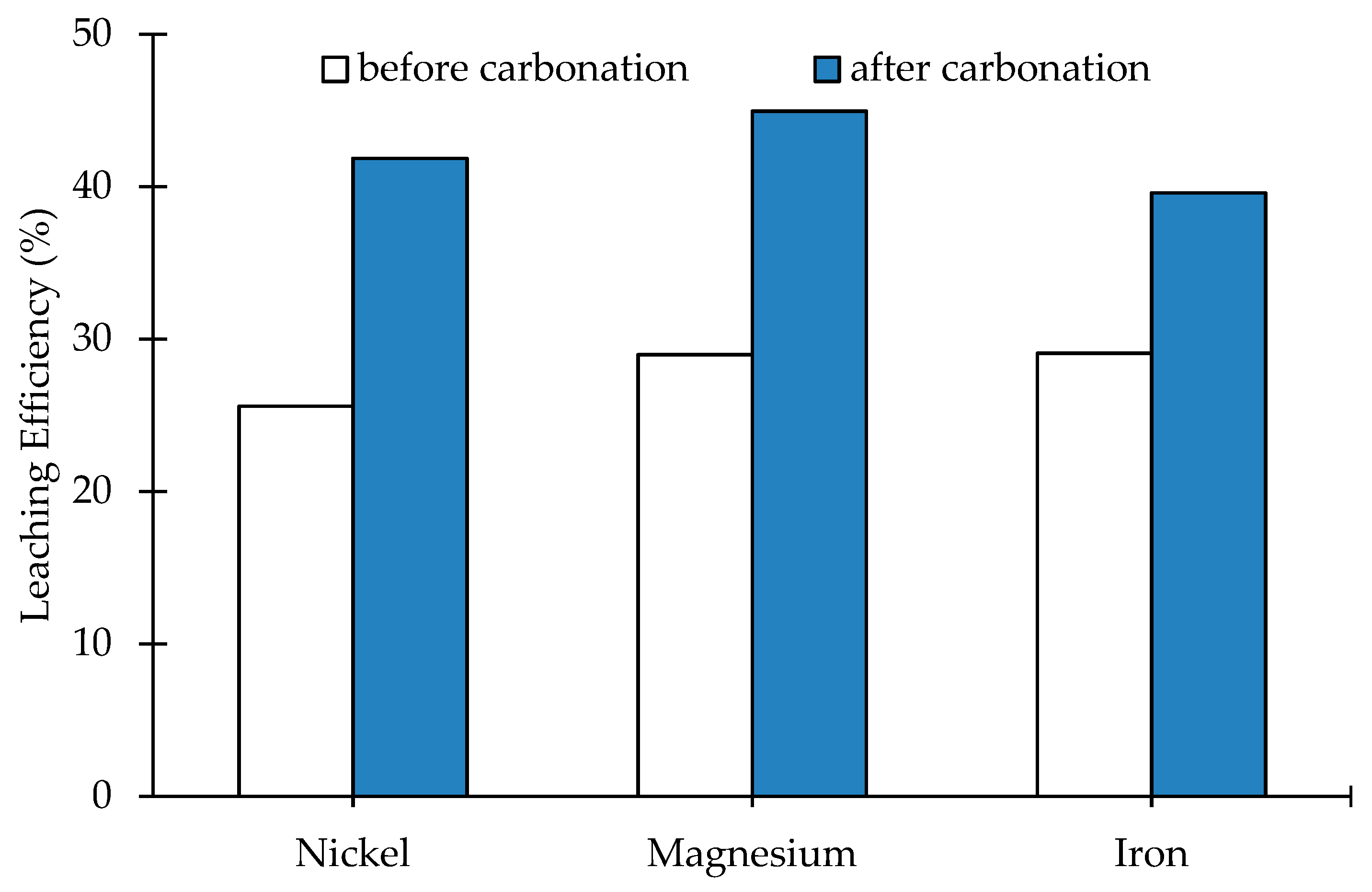
3.3. Leaching of the Carbonated Olivine
4. Discussion
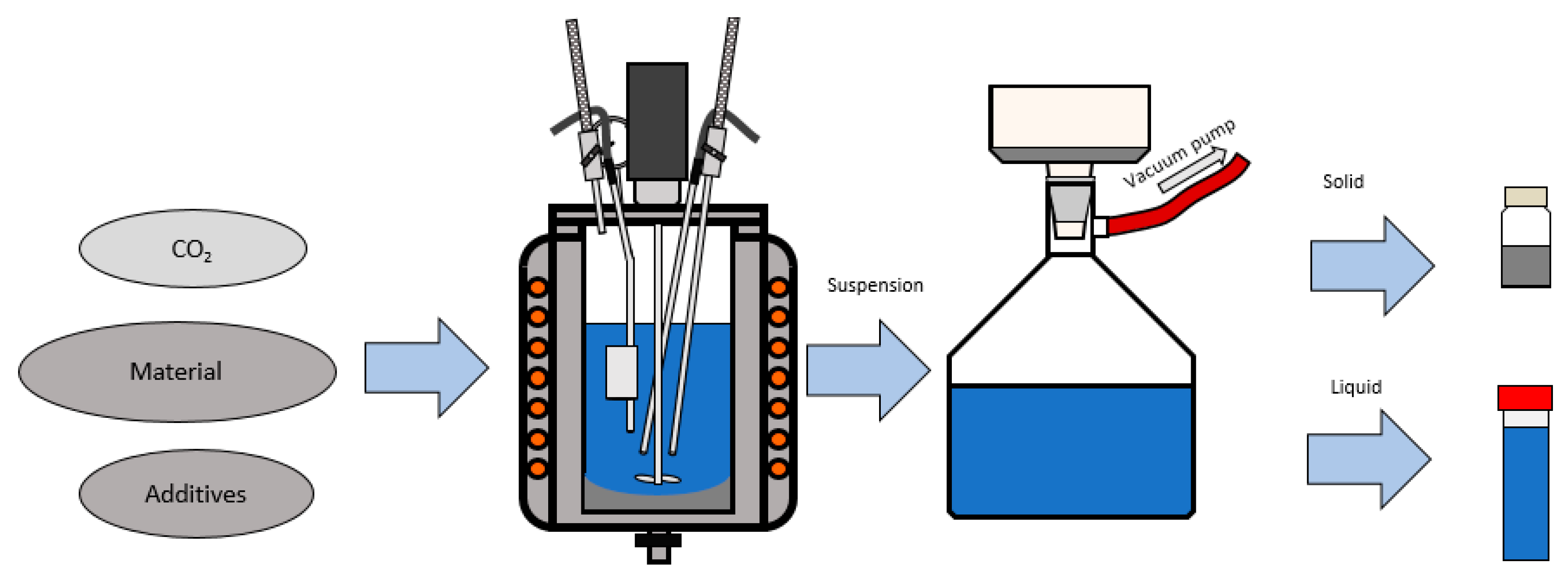
- Injection of carbon dioxide and water in an autoclave with olivine and additives and a heating of the suspension to 175 °C in order to reach 71.5 bar.
- Carbonation process at previously mentioned conditions in duration of 4 h.
- Cooling of a water solution to an atmospheric pressure and temperature of 80 °C.
- Addition of hydrochloric acid and leaching of the carbonated product in duration of 1 h in the same way as for the untreated olivine.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harben, P.; Smith, C., Jr. Industrial Minerals & Rocks: Commodities, Markets, and Uses; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; SME: Dearborn, MI, USA, 2006. [Google Scholar]
- Smyth, J.; Frost, D.; Nestola, F.; Holl, C.; Bromiley, G. Olivine hydration in the deep upper mantle: Effects of temperature and silica activity. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Kremer, D.; Etzold, S.; Boldt, J.; Blaum, P.; Hahn, K.; Wotruba, H.; Telle, R. Geological Mapping and Characterization of Possible Primary Input Materials for the Mineral Sequestration of Carbon Dioxide in Europe. Minerals 2019, 9, 485. [Google Scholar] [CrossRef]
- Turri, L.; Muhr, H.; Rech, C.; Lapicque, F. Flotation of Chromite as Pre-Treatment of Olivine Before Carbonation for CO2 Sequestration. Open Chem. Eng. J. 2017, 11, 1–16. [Google Scholar] [CrossRef][Green Version]
- Olerud, S. Method for Manufacturing Spherical Silica from Olivine. U.S. Patent No. 5,780,005, 14 July 1998. [Google Scholar]
- Hansen, T.; Zander, N. Extraction of Silica and Magnesium Compounds from Olivine. WO Patent WO/2002/048,036, 20 June 2002. [Google Scholar]
- Crundwell, F. The mechanism of dissolution of forsterite, olivine and minerals of the orthosilicate group. Hydrometallurgy 2014, 150, 68–82. [Google Scholar] [CrossRef]
- Oelkers, E.; Declercq, J.; Saldi, G.; Gislason, S.; Schott, J. Olivine dissolution rates. A critical review. Chem. Geol. 2018, 500, 1–19. [Google Scholar] [CrossRef]
- Stopic, S.; Dertmann, C.; Modolo, G.; Kegler, P.; Neumeier, S.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Rosani, D.; et al. Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave. Metals 2018, 8, 993. [Google Scholar] [CrossRef]
- Stopic, S.; Dertmann, C.; Koiwa, I.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Knops, P.; Friedrich, B. Synthesis of Nanosilica via Olivine Mineral Carbonation under High Pressure in an Autoclave. Metals 2019, 9, 708. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Turri, L.; Muhr, H.; Rijnsburger, K.; Knops, P.; Lapicque, F. CO2 sequestration by high pressure reaction with olivine in a rocking batch autoclave. Chem. Eng. Sci. 2017, 171, 27–31. [Google Scholar] [CrossRef]
- Santos, R.; van Audenaerde, A.; Chiang, Y.; Iacobescu, R.; Knops, P.; van Gerven, T. Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment. Metals 2015, 5, 1620–1644. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S. Synthesis and characterization of silica nanoparticles from clay. J. Asian Ceram. Soc. 2018, 4, 91–96. [Google Scholar] [CrossRef]

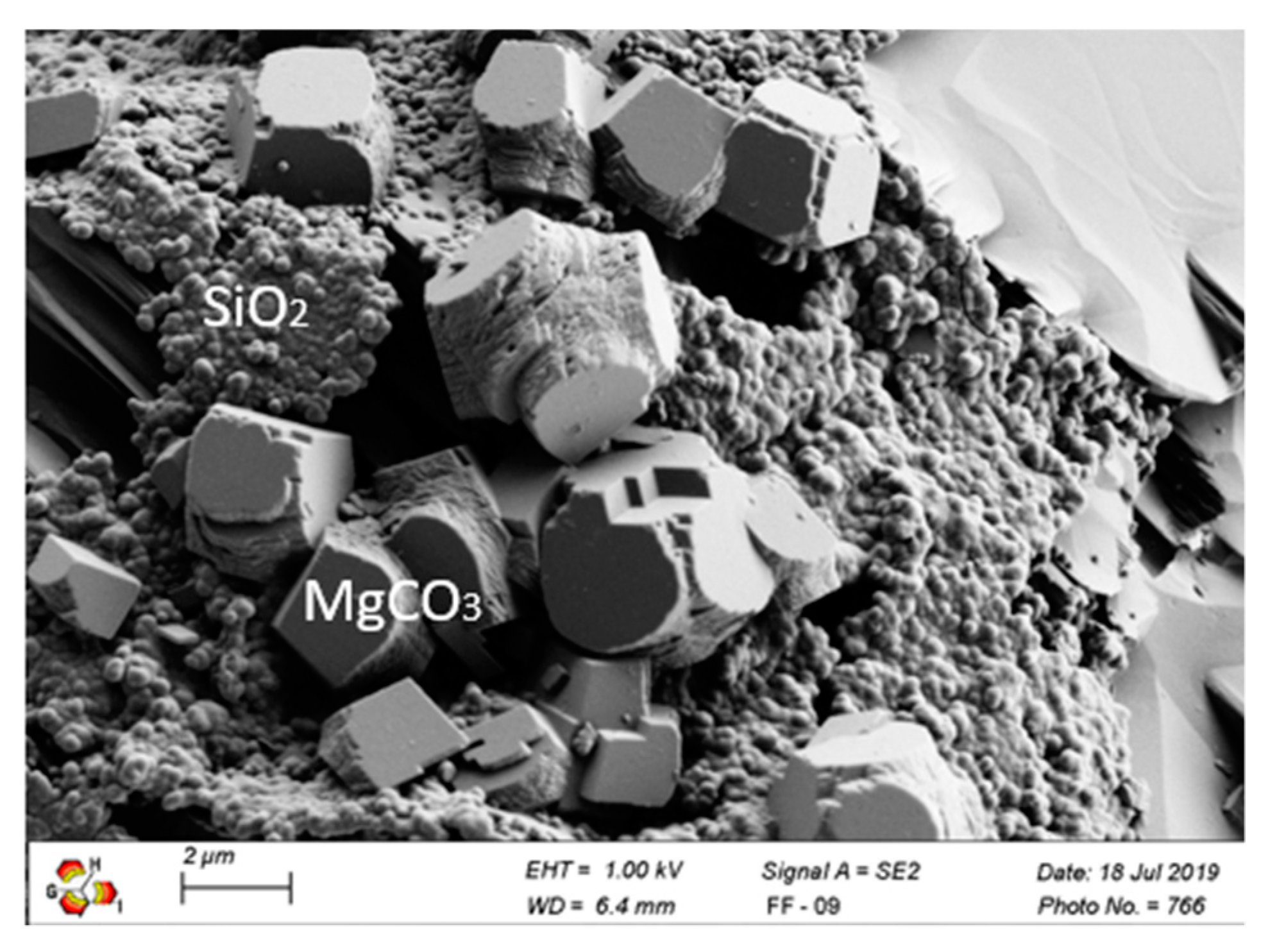
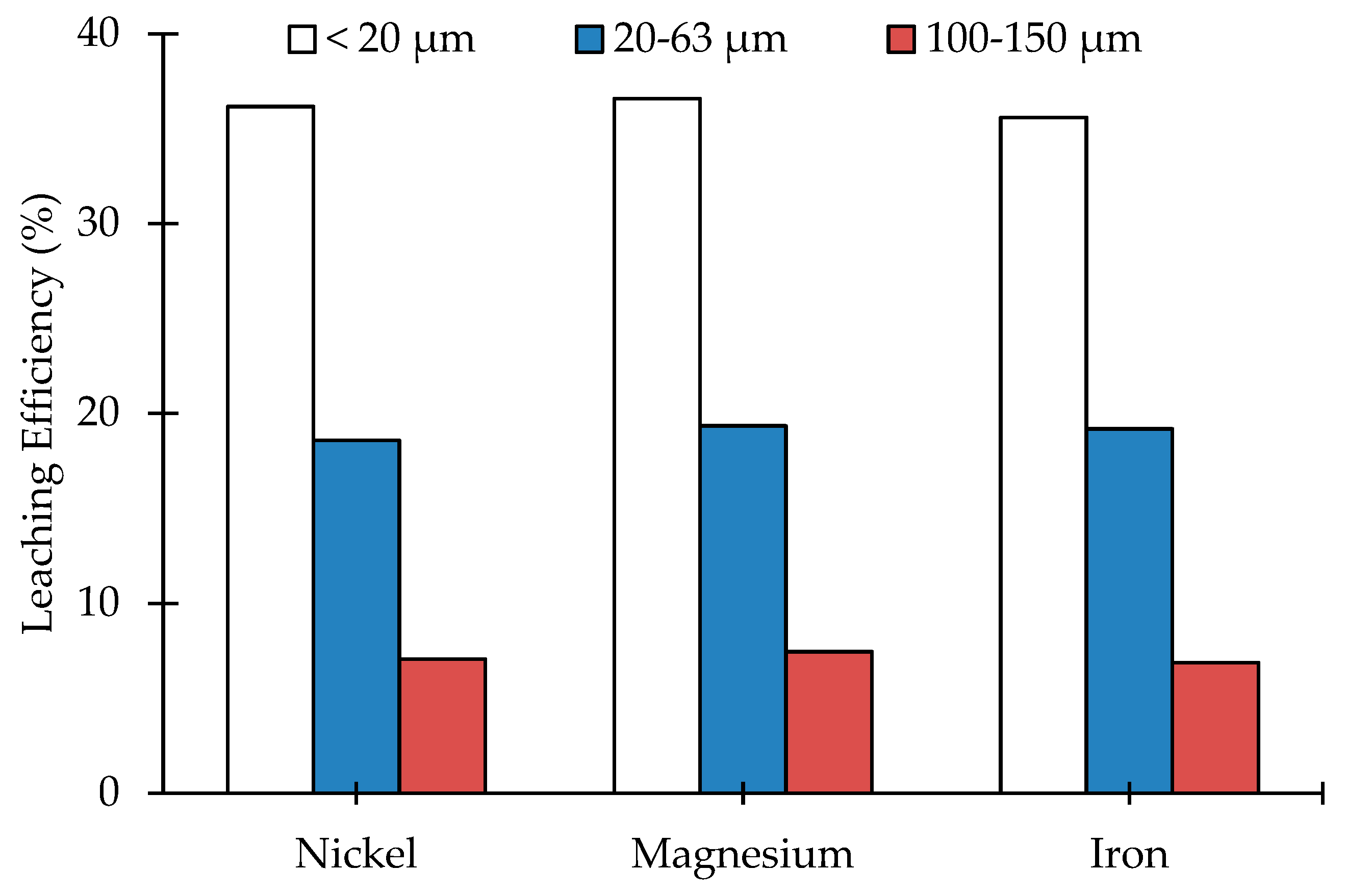
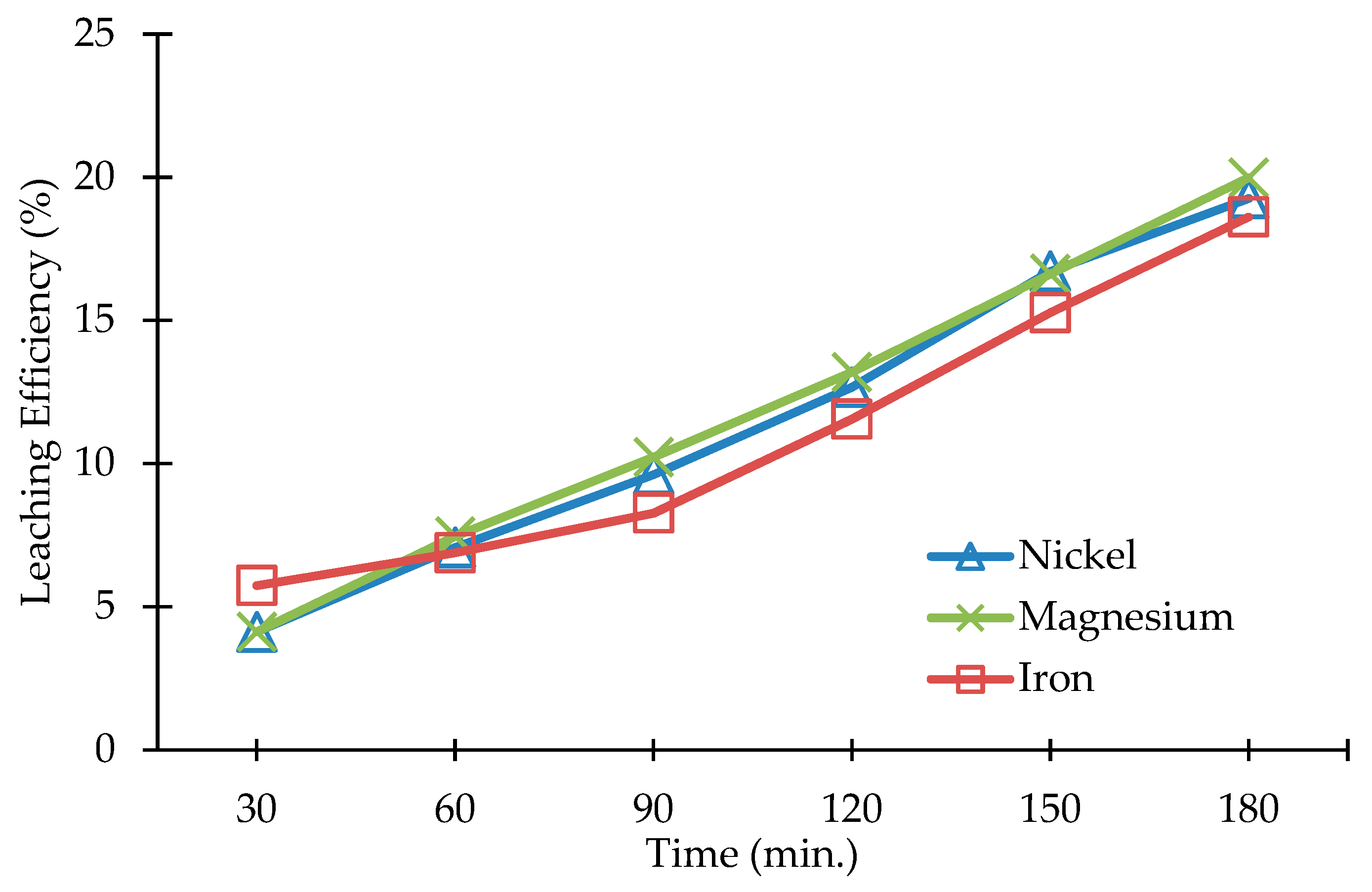
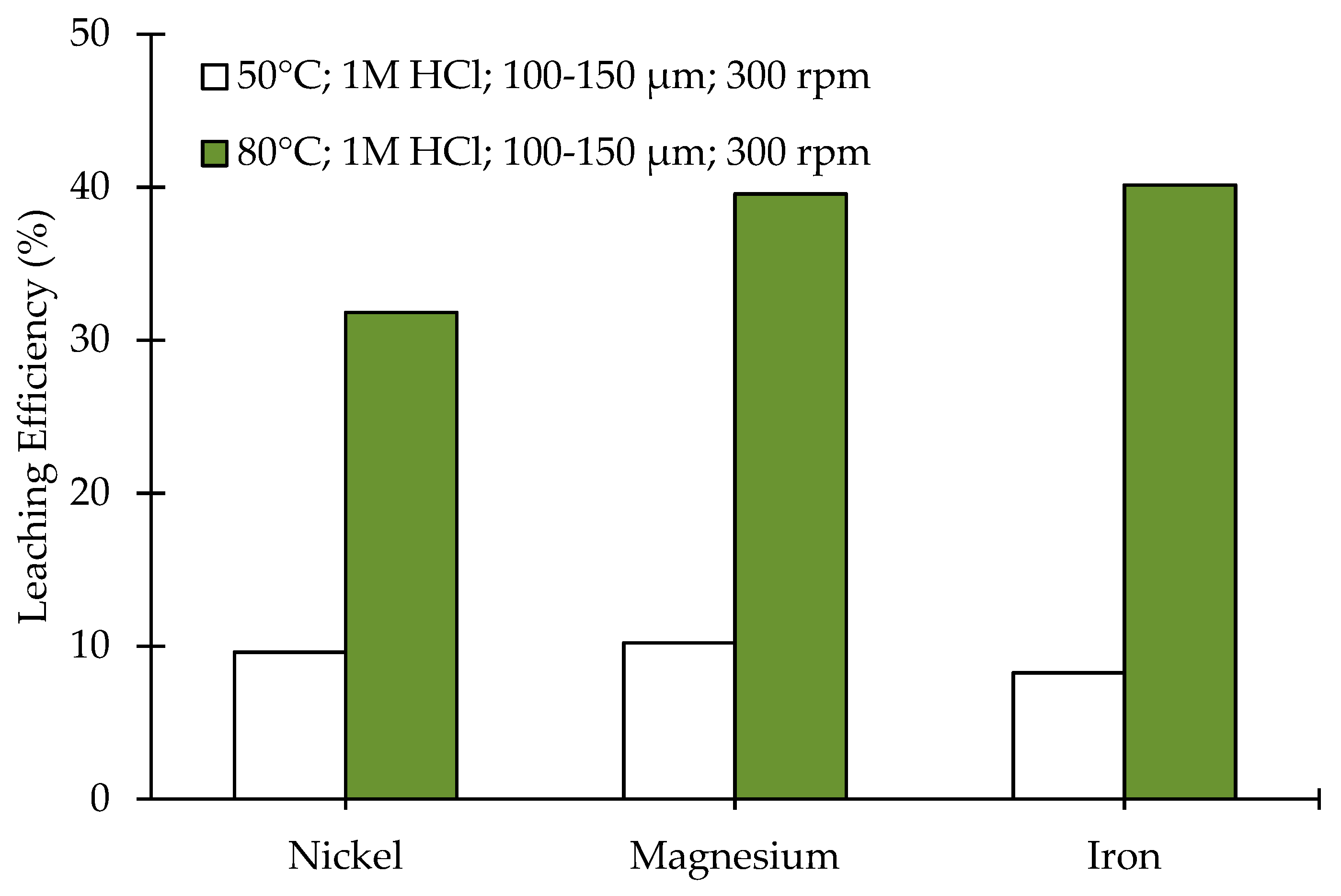
| Compound (wt.%) | <20 µm | 20–63 µm | 100–150 µm |
|---|---|---|---|
| SiO2 | 47.91 | 47.31 | 47.72 |
| Al2O3 | 0.58 | 0.47 | 0.42 |
| Fe2O3 | 7.02 | 7.41 | 7.38 |
| CaO | 0.72 | 0.17 | 0.11 |
| MgO | 42.49 | 43.58 | 43.42 |
| MnO | 0.11 | 0.12 | 0.11 |
| Cr2O3 | 0.41 | 0.38 | 0.38 |
| NiO | 0.41 | 0.39 | 0.41 |
| Others | 0.35 | 0.17 | 0.05 |
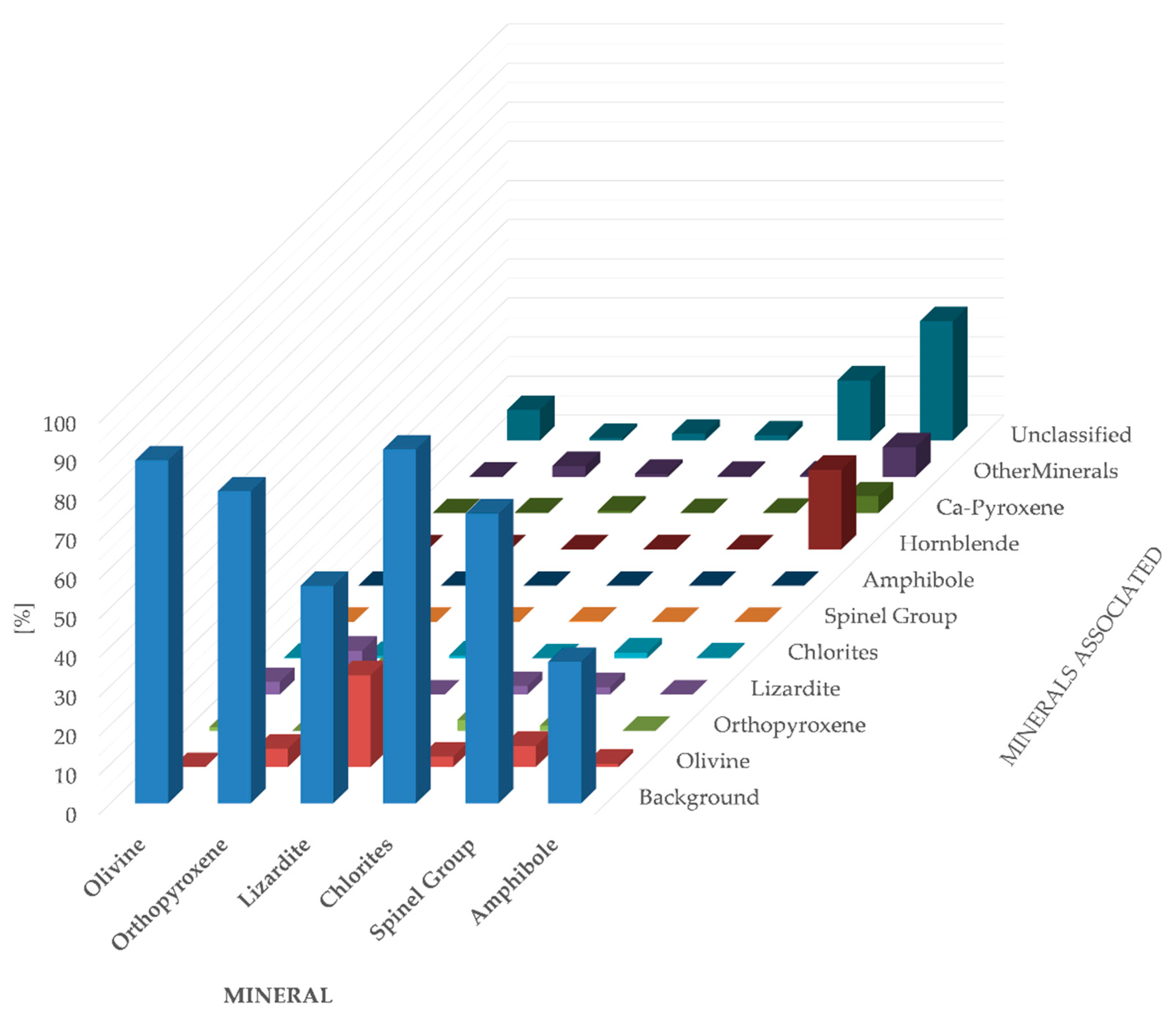
| Mineral | Content (vol.%) |
|---|---|
| Lizardite | 6.24 |
| Biotite | 0.06 |
| Chlorites | 3.40 |
| Spinel Group | 0.33 |
| Amphibole | 0.04 |
| Orthopyroxene | 13.55 |
| Hornblende | 0.07 |
| Olivine | 71.52 |
| Ca-Pyroxene | 0.96 |
| Other Minerals | 1.14 |
| Unclassified | 2.69 |
| Experiment | T (°C) | t (min) | Particle Size (µm) | Concentration (mol/L) | Leaching Agent |
|---|---|---|---|---|---|
| Leaching (without carbonation pre-treatment) | |||||
| 1 | 50 | 30, 60 | <20 | 1 | HCl |
| 2 | 50 | 30, 60, 90 | 20–63 | 1 | HCl |
| 3 | 50 | 30, 60, 90, 120, 150, 180 | 100–150 | 1 | HCl |
| 4 | 80 | 30, 60, 90 | 100–150 | 1 | HCl |
| Leaching (after carbonation pre-treatment) | |||||
| 5 | 50 | 60, 120, 180, 240 | 20–63 | 1 | HCl |
| 6 | 80 | 60, 120, 180 | 20–63 | 1 | HCl |
| 7 | 90 | 60, 120, 180, 240 | 20–63 | 1 | HCl |
| 8 | 50 | 60, 120, 180 | 20–63 | 2 | HCl |
| Element | Ni | Mg | Ca | Fe | Al | Si | Mn |
|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 1.27 | 76.2 | <1 | 2.92 | <1 | 74.8 | <1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matus, C.; Stopic, S.; Etzold, S.; Kremer, D.; Wotruba, H.; Dertmann, C.; Telle, R.; Friedrich, B.; Knops, P. Mechanism of Nickel, Magnesium, and Iron Recovery from Olivine Bearing Ore during Leaching with Hydrochloric Acid Including a Carbonation Pre-Treatment. Metals 2020, 10, 811. https://doi.org/10.3390/met10060811
Matus C, Stopic S, Etzold S, Kremer D, Wotruba H, Dertmann C, Telle R, Friedrich B, Knops P. Mechanism of Nickel, Magnesium, and Iron Recovery from Olivine Bearing Ore during Leaching with Hydrochloric Acid Including a Carbonation Pre-Treatment. Metals. 2020; 10(6):811. https://doi.org/10.3390/met10060811
Chicago/Turabian StyleMatus, Carlos, Srecko Stopic, Simon Etzold, Dario Kremer, Hermann Wotruba, Christian Dertmann, Rainer Telle, Bernd Friedrich, and Pol Knops. 2020. "Mechanism of Nickel, Magnesium, and Iron Recovery from Olivine Bearing Ore during Leaching with Hydrochloric Acid Including a Carbonation Pre-Treatment" Metals 10, no. 6: 811. https://doi.org/10.3390/met10060811
APA StyleMatus, C., Stopic, S., Etzold, S., Kremer, D., Wotruba, H., Dertmann, C., Telle, R., Friedrich, B., & Knops, P. (2020). Mechanism of Nickel, Magnesium, and Iron Recovery from Olivine Bearing Ore during Leaching with Hydrochloric Acid Including a Carbonation Pre-Treatment. Metals, 10(6), 811. https://doi.org/10.3390/met10060811







