Hybrid Structures Made of Polyurethane/Graphene Nanocomposite Foams Embedded within Aluminum Open-Cell Foam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
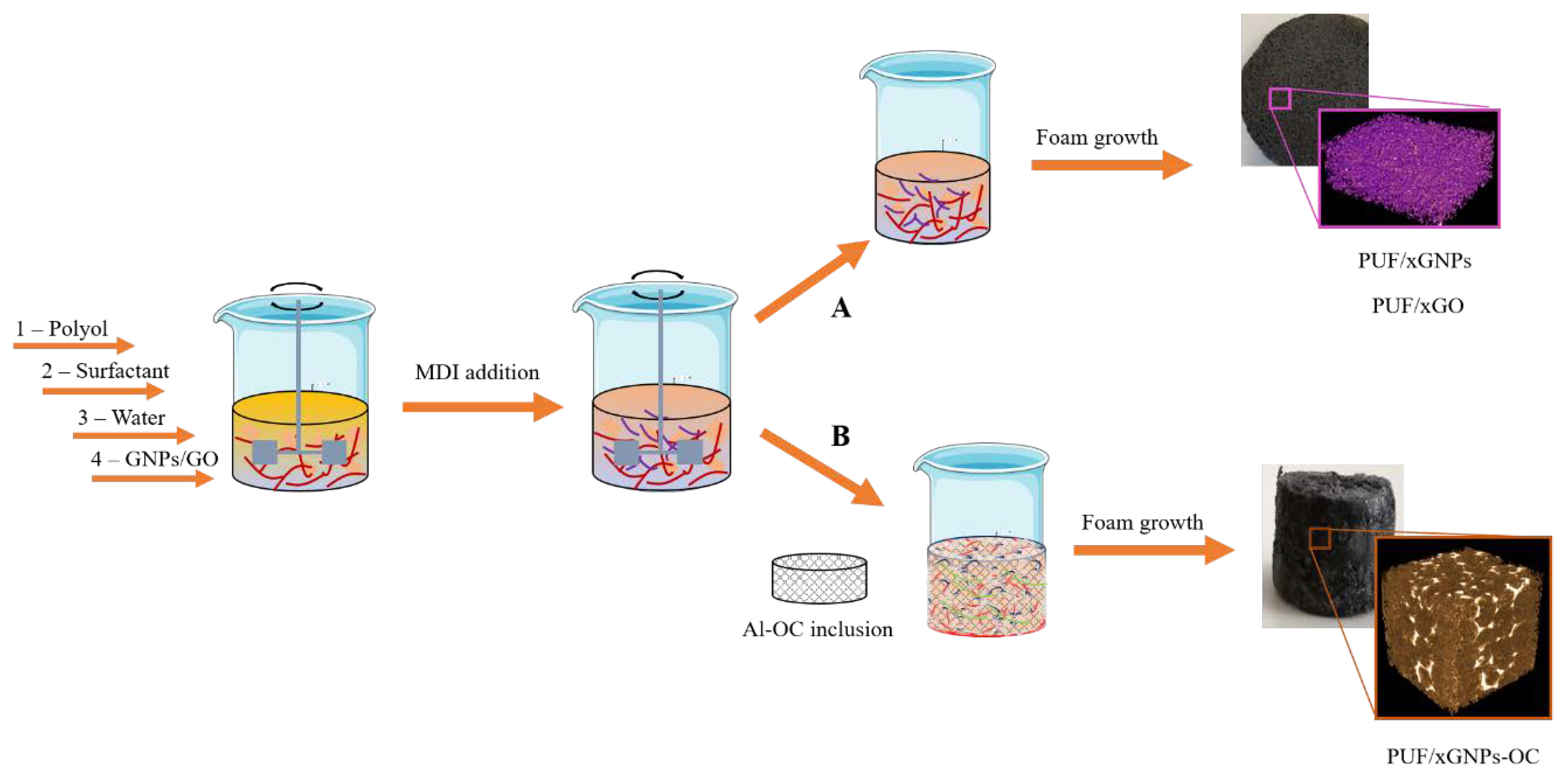
2.2. Sample Preparation
2.3. Sample Characterization
3. Results
3.1. PUFs Nanocomposites
3.1.1. Thermal Stability
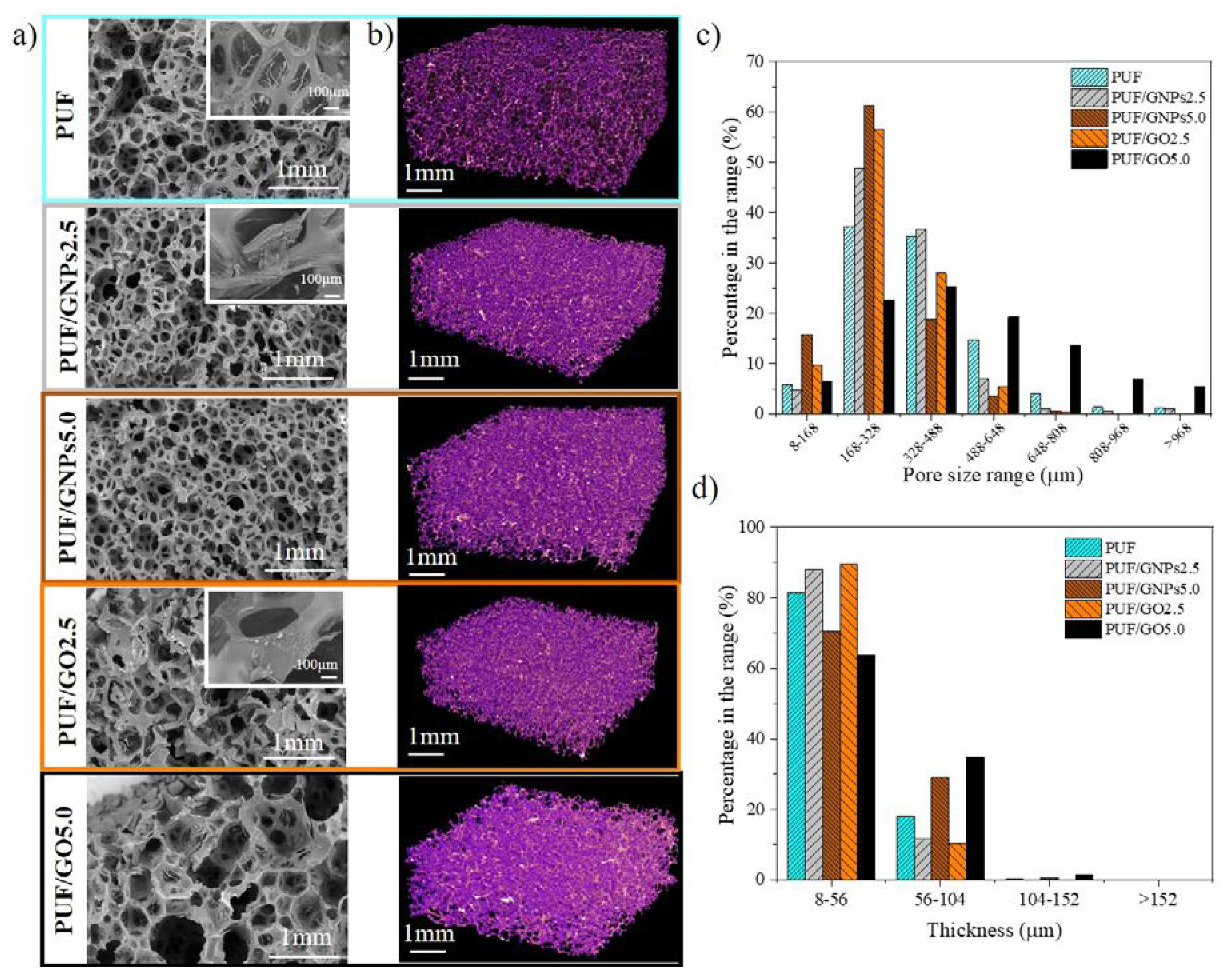
3.1.2. Morphology
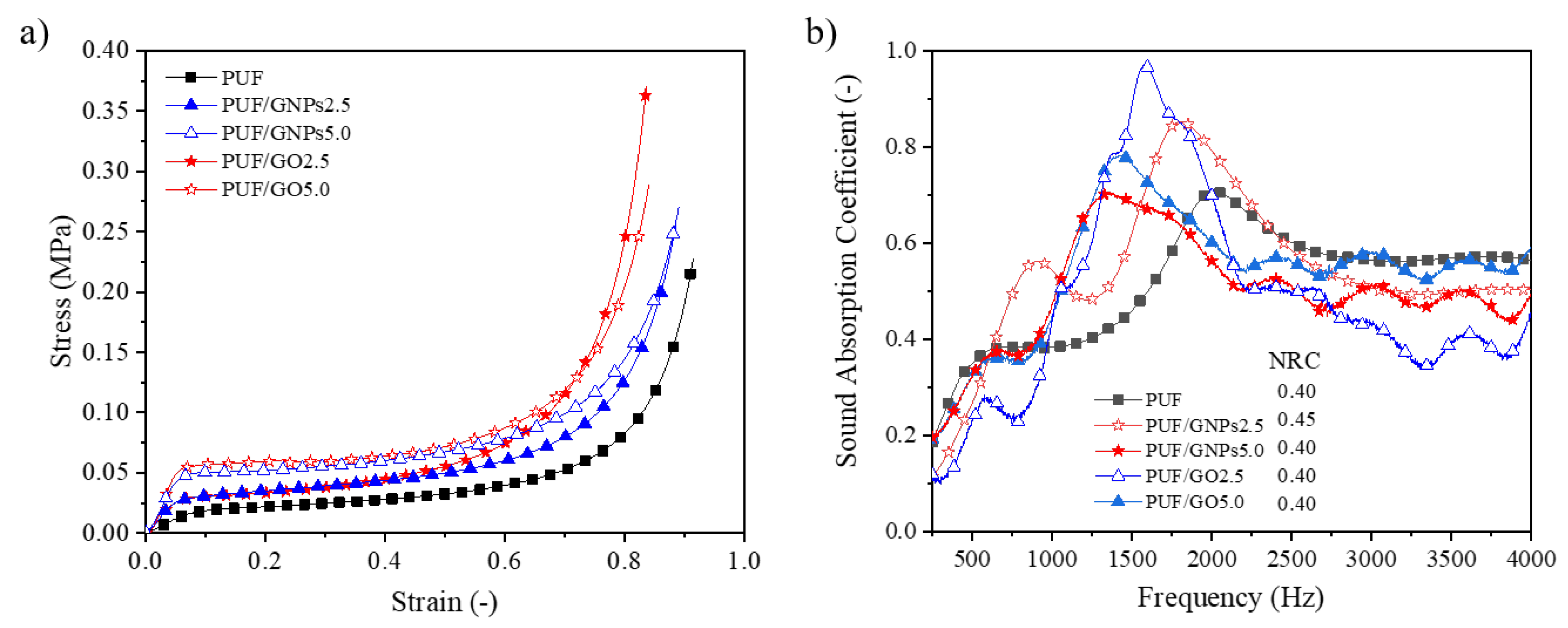
3.1.3. Mechanical Properties
3.1.4. Sound Absorption
3.1.5. Thermal Conductivity
3.2. Hybrid Structures
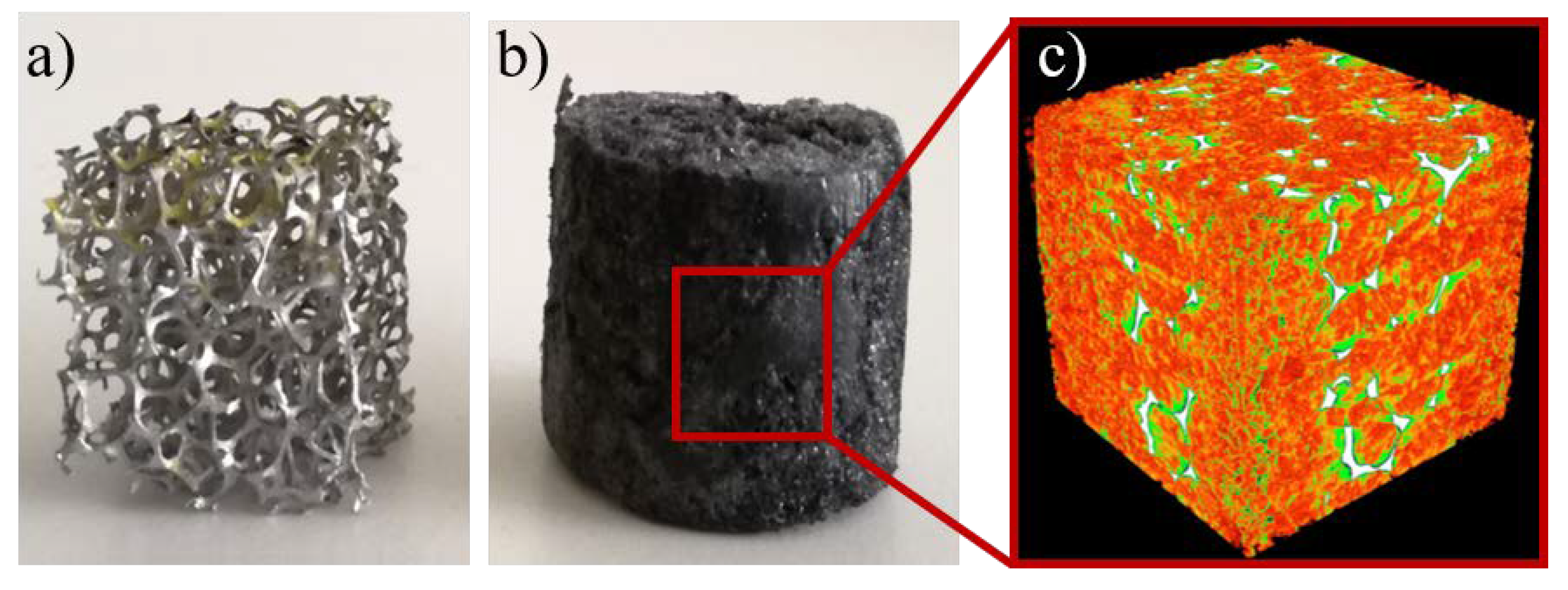
3.2.1. Structure
3.2.2. Mechanical Properties
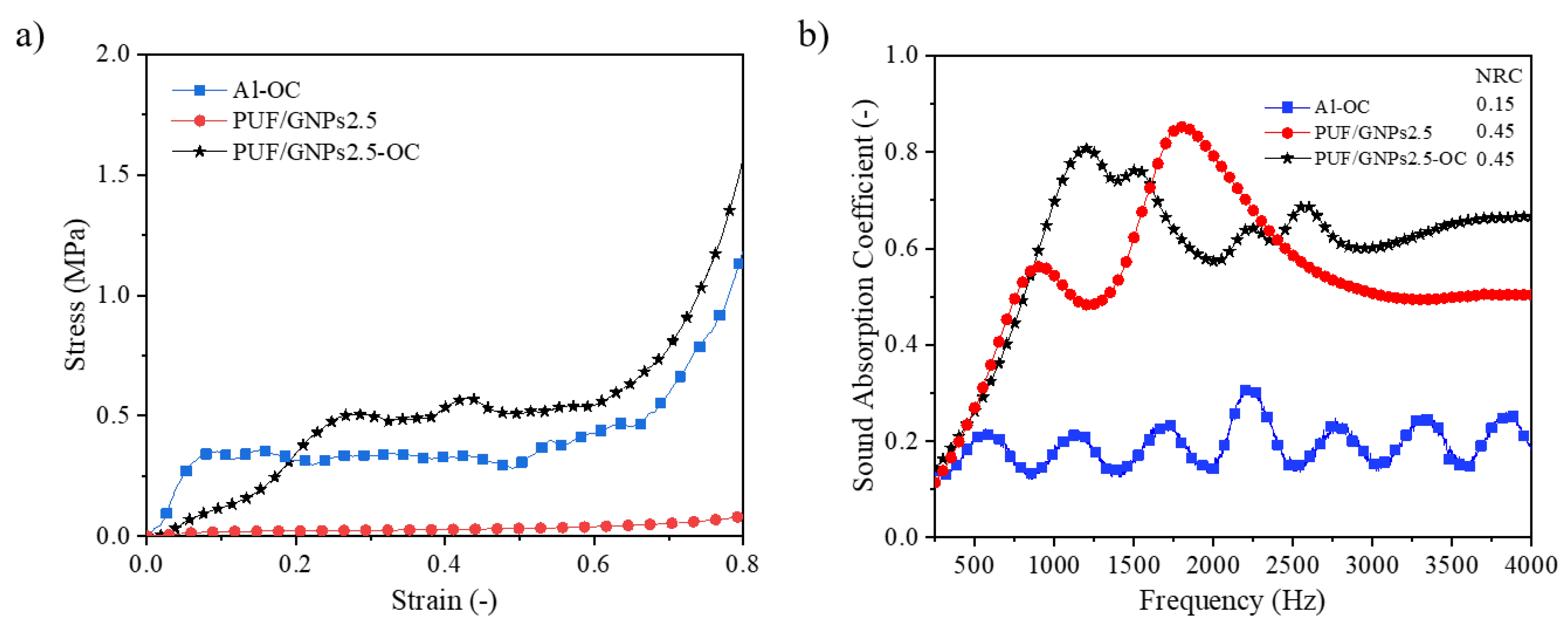
3.2.3. Sound Absorption
3.2.4. Thermal Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, C.Y.; Lu, W.; Tian, Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs). Sol. Energy 2010, 84, 1402–1412. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Wan, L.; Xie, Z.; Easton, C.D.; Bourgeois, L.; Wang, Z.; Bao, Q.; Zhu, Y.; Tao, S.; et al. Construction of porous N-doped graphene layer for efficient oxygen reduction reaction. Chem. Eng. Sci. 2019, 194, 36–44. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xu, H.; Dufresne, A.; Lin, N. High-adsorption, self-extinguishing, thermal, and acoustic-resistance aerogels based on organic and inorganic waste valorization from cellulose nanocrystals and red mud. ACS Sustain. Chem. Eng. 2018, 6, 7168–7180. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Z.; Yang, X.; Tu, J.; Wu, R.; Wang, W. Green synthesis of amphipathic graphene aerogel constructed by using the framework of polymer-surfactant complex for water remediation. Appl. Surf. Sci. 2018, 444, 399–406. [Google Scholar] [CrossRef]
- Sing, K.S. Characterization of porous solids: An introductory survey. Stud. Surf. Sci. Catal. 1991, 62, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Xu, F.; Li, S.; Ma, P.; Zhang, X.; Liu, Q.; Fu, R.; Wu, D. Porous Polymers as Multifunctional Material Platforms toward Task-Specific Applications. Adv. Mater. 2018, 31, 1802922. [Google Scholar] [CrossRef]
- Ferreira, A.D.B.; Nóvoa, P.R.; Marques, A.T. Multifunctional material systems: A state-of-the-art review. Compos. Struct. 2016, 151, 3–35. [Google Scholar] [CrossRef]
- Duarte, I.; Ferreira, J.M.F. Composite and Nanocomposite Metal Foams. Materials 2016, 9, 79. [Google Scholar] [CrossRef]
- Duarte, I.; Vesenjak, M.; Krstulović-Opara, L.; Ren, Z. Crush performance of multifunctional hybrid foams based on an aluminium alloy open-cell foam skeleton. Polym. Test. 2018, 67, 246–256. [Google Scholar] [CrossRef]
- Pinto, S.C.; Marques, P.A.; Vesenjak, M.; Vicente, R.; Godinho, L.; Krstulović-Opara, L.; Duarte, I. Characterization and physical properties of aluminium foam-polydimethylsiloxane nanocomposite hybrid structures. Compos. Struct. 2019, 230, 111521. [Google Scholar] [CrossRef]
- Pinto, S.; Marques, P.A.; Vesenjak, M.; Vicente, R.; Godinho, L.; Krstulović-Opara, L.; Duarte, I. Mechanical, thermal, and acoustic properties of aluminum foams impregnated with epoxy/graphene oxide nanocomposites. Metals 2019, 9, 1214. [Google Scholar] [CrossRef]
- Li, A.; Li, A.; He, S.; Xuan, P. Cyclic compression behavior and energy dissipation of aluminum foam–polyurethane interpenetrating phase composites. Compos. Part A Appl. Sci. Manuf. 2015, 78, 35–41. [Google Scholar] [CrossRef]
- Reinfried, M.; Stephani, G.; Luthardt, F.; Adler, J.; John, M.; Krombholz, A. Hybrid foams—A new approach for multifunctional applications. Adv. Eng. Mater. 2011, 13, 1031–1036. [Google Scholar] [CrossRef]
- Pinto, S.C.; Silva, N.H.; Pinto, R.J.; Freire, C.S.; Duarte, I.; Vicente, R.; Vesenjak, M.; Marques, P.A. Multifunctional hybrid structures made of open-cell aluminum foam impregnated with cellulose/graphene nanocomposites. Carbohydr. Polym. 2020, 238, 116197. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane composite foams in high-performance applications: A review. Polym. Technol. Eng. 2017, 57, 346–369. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Carvalho, A.; Ferreira, A.; Barros-Timmons, A. Sound absorption properties of polyurethane foams derived from crude glycerol and liquefied coffee grounds polyol. Polym. Test. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Zheng, X. Research on highly flame-retardant rigid PU foams by combination of nanostructured additives and phosphorus flame retardants. Polym. Degrad. Stab. 2015, 111, 142–150. [Google Scholar] [CrossRef]
- Piszczyk, Ł.; Danowska, M.; Mietlarek-Kropidłowska, A.; Szyszka, M.; Strankowski, M. Synthesis and thermal studies of flexible polyurethane nanocomposite foams obtained using nanoclay modified with flame retardant compound. J. Therm. Anal. Calorim. 2014, 118, 901–909. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, K.; Jin, X.; Sun, S.; Tian, Y.; Wang, F.; Liu, P.; Yang, M. Investigation of flame retardant flexible polyurethane foams containing DOPO immobilized titanium dioxide nanoparticles. Polymers 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Nikje, M.M.A.; Moghaddam, S.T.; Noruzian, M. Preparation of novel magnetic polyurethane foam nanocomposites by using core-shell nanoparticles. Polímeros 2016, 26, 297–303. [Google Scholar] [CrossRef]
- Huang, J.; Tang, Q.; Liao, W.; Wang, G.; Wei, W.; Li, C. Green preparation of expandable graphite and its application in flame-resistance polymer elastomer. Ind. Eng. Chem. Res. 2017, 56, 5253–5261. [Google Scholar] [CrossRef]
- Pandey, G.; Thostenson, E.T. Carbon nanotube-based multifunctional polymer nanocomposites. Polym. Rev. 2012, 52, 355–416. [Google Scholar] [CrossRef]
- Caglayan, C.; Gurkan, I.; Gungor, S.; Cebeci, H. The effect of CNT-reinforced polyurethane foam cores to flexural properties of sandwich composites. Compos. Part A Appl. Sci. Manuf. 2018, 115, 187–195. [Google Scholar] [CrossRef]
- Colloca, M.; Gupta, N.; Porfiri, M. Tensile properties of carbon nanofiber reinforced multiscale syntactic foams. Compos. Part. B Eng. 2013, 44, 584–591. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Desai, S. Nanofiller-reinforced polymer nanocomposites. Polym. Adv. Technol. 2008, 19, 947–959. [Google Scholar] [CrossRef]
- Pinto, D.; Bernardo, L.; Amaro, A.; Lopes, S. Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement—A review. Constr. Build. Mater. 2015, 95, 506–524. [Google Scholar] [CrossRef]
- Cao, Z.-J.; Liao, W.; Wang, S.-X.; Zhao, H.-B.; Wang, Y.-Z. Polyurethane foams with functionalized graphene towards high fire-resistance, low smoke release, superior thermal insulation. Chem. Eng. J. 2019, 361, 1245–1254. [Google Scholar] [CrossRef]
- Gharehbaghi, A.; Bashirzadeh, R.; Ahmadi, Z. Polyurethane flexible foam fire resisting by melamine and expandable graphite: Industrial approach. J. Cell. Plast. 2011, 47, 549–565. [Google Scholar] [CrossRef]
- Yan, D.-X.; Xu, L.; Chen, C.; Tang, J.; Ji, X.; Li, Z. Enhanced mechanical and thermal properties of rigid polyurethane foam composites containing graphene nanosheets and carbon nanotubes. Polym. Int. 2012, 61, 1107–1114. [Google Scholar] [CrossRef]
- Piszczyk, Ł.; Kosmela, P.; Strankowski, M. Elastic polyurethane foams containing graphene nanoplatelets. Adv. Polym. Technol. 2017, 37, 1625–1634. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, H.; Kim, J.; Lee, J.W.; Kim, W.N. Effect of graphene on the sound damping properties of flexible polyurethane foams. Macromol. Res. 2017, 25, 190–196. [Google Scholar] [CrossRef]
- Lee, J.; Jung, I. Tuning sound absorbing properties of open cell polyurethane foam by impregnating graphene oxide. Appl. Acoust. 2019, 151, 10–21. [Google Scholar] [CrossRef]
- Silva, J. da Metodologias Experimentais Para a Determinação do Coeficiente de Absorção de Sonora em Materiais de Construção. Master’s Thesis, University of Coimbra, Coimbra, Portugal, July 2008. [Google Scholar]
- Colomer, J.-F.; Marega, R.; Traboulsi, H.; Meneghetti, M.; Van Tendeloo, G.; Bonifazi, D. Microwave-assisted bromination of double-walled carbon nanotubes. Chem. Mater. 2009, 21, 4747–4749. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, S.; Hu, P.; Zhao, G.; Li, Y.; Zhang, X.-H.; Han, W. Enhanced mechanical, thermal, and electric properties of graphene aerogels via supercritical ethanol drying and high-temperature thermal reduction. Sci. Rep. 2017, 7, 1439. [Google Scholar] [CrossRef]
- Malucelli, G. The role of graphene in flame retardancy of polymeric materials: Recent advances. Curr. Graphene Sci. 2018, 2, 27–34. [Google Scholar] [CrossRef][Green Version]
- Idumah, C.I.; Hassan, A.; Affam, A.C. A review of recent developments in flammability of polymer nanocomposites. Rev. Chem. Eng. 2015, 31, 149–177. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.; Li, X.-H.; Yu, L.-G.; Zhang, Z.-J. Graphene-based flame retardants: A review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Qu, H.; Zhang, X.; Zhu, J.; Zhao, G.; Lopera, H.C.; Yu, J.; Sun, L.; Bhana, S.; et al. Magnetic graphene oxide nanocomposites: Nanoparticles growth mechanism and property analysis. J. Mater. Chem. C 2014, 2, 9478–9488. [Google Scholar] [CrossRef]
- Liu, H.; Dong, M.; Huang, W.; Gao, J.; Liu, Y.; Zheng, G.; Liu, C.; Shen, C.; Guo, Z. Lightweight conductive graphene/thermoplastic polyurethane foams with ultrahigh compressibility for piezoresistive sensing. J. Mater. Chem. C 2017, 5, 73–83. [Google Scholar] [CrossRef]
- Gama, N.V.; Amaral, C.; Silva, T.; Vicente, R.; Coutinho, J.A.P.; Barros-Timmons, A.; Ferreira, A. Thermal energy storage and mechanical performance of crude glycerol polyurethane composite foams containing phase change materials and expandable graphite. Materials 2018, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.; Bouhfid, R.; Qaiss, A.K. Functionalized graphene nanocomposites and their derivatives: Synthesis, processing and applications. In Micro and Nano Technologies; Elsevier Science: Amsterdam, The Nertherlands, 2018; ISBN 9780128145531. [Google Scholar]
- Li, Y.; Zou, J.; Zhou, S.; Chen, Y.; Zou, H.; Liang, M.; Luo, W. Effect of expandable graphite particle size on the flame retardant, mechanical, and thermal properties of water-blown semi-rigid polyurethane foam. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Wang, G.; Fu, Y.; Guo, A.; Mei, T.; Wang, J.; Li, J.; Wang, X. Reduced graphene oxide—Polyurethane nanocomposite foam as a reusable photoreceiver for efficient solar steam generation. Chem. Mater. 2017, 29, 5629–5635. [Google Scholar] [CrossRef]
- Najib, N.; Ariff, Z.M.; Bakar, A.; Sipaut, C. Correlation between the acoustic and dynamic mechanical properties of natural rubber foam: Effect of foaming temperature. Mater. Des. 2011, 32, 505–511. [Google Scholar] [CrossRef]
- Arjunan, A.; Baroutaji, A.; Praveen, A.S.; Olabi, A.G.; Wang, C.J. Acoustic performance of metallic foams. In Reference Module in Materials Science and Materials Engineering; Elsevier BV: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gwon, J.G.; Kim, S.K.; Kim, J.H. Sound absorption behavior of flexible polyurethane foams with distinct cellular structures. Mater. Des. 2016, 89, 448–454. [Google Scholar] [CrossRef]
- Sung, G.; Kim, J.W.; Kim, J.H. Fabrication of polyurethane composite foams with magnesium hydroxide filler for improved sound absorption. J. Ind. Eng. Chem. 2016, 44, 99–104. [Google Scholar] [CrossRef]
- Al-Ajlan, S.A. Measurements of thermal properties of insulation materials by using transient plane source technique. Appl. Therm. Eng. 2006, 26, 2184–2191. [Google Scholar] [CrossRef]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions—Properties, requirements and possibilities. Energy Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- Vesenjak, M.; Krstulović-Opara, L.; Ren, Z. Characterization of irregular open-cell cellular structure with silicone pore filler. Polym. Test. 2013, 32, 1538–1544. [Google Scholar] [CrossRef]
- Lu, T.J.; Hess, A.; Ashby, M.F. Sound absorption in metallic foams. J. Appl. Phys. 1999, 85, 7528–7539. [Google Scholar] [CrossRef]
- Handbook of Cellular Metals; Degischer, H., Kriszt, B., Eds.; Wiley: Weinheim, Germany, 2002; ISBN 9783527303397. [Google Scholar]
- Pedroso, M.; De Brito, J.; Silvestre, J. Characterization of eco-efficient acoustic insulation materials (traditional and innovative). Constr. Build. Mater. 2017, 140, 221–228. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Effective thermal conductivity of open-cell metal foams impregnated with pure paraffin for latent heat storage. Int. J. Therm. Sci. 2014, 81, 94–105. [Google Scholar] [CrossRef]
- Li, W.; Qu, Z.; He, Y.; Tao, W. Experimental and numerical studies on melting phase change heat transfer in open-cell metallic foams filled with paraffin. Appl. Therm. Eng. 2012, 37. [Google Scholar] [CrossRef]
- Fiedler, T.; Sulong, M.A.; Vesenjak, M.; Higa, Y.; Belova, I.V.; Öchsner, A.; Murch, G.E. Determination of the thermal conductivity of periodic APM foam models. Int. J. Heat Mass Transf. 2014, 73, 826–833. [Google Scholar] [CrossRef]

| Samples | T5% (°C) | T50% (°C) | Tmax (°C) | Residue 750 °C (%) |
|---|---|---|---|---|
| PUF | 173.6 | 349.8 | 312.3 | 5.68 |
| PUF/GNPs2.5 | 197.7 | 404.6 | 314.7 | 5.18 |
| PUF/GNPs5.0 | 188.5 | 392.9 | 316.5 | 5.12 |
| PUF/GO2.5 | 165.4 | 360.7 | 311.4 | 2.42 |
| PUF/GO5.0 | 149.2 | 355.6 | 312.9 | 1.88 |
| Samples | Apparent Density (kg/m3) | Compressive Modulus (kPa) | Compressive Strength (kPa) (Strain: 0.1) | |||
|---|---|---|---|---|---|---|
| Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | |
| PUF | 44 | 1.1 | 247 | 3.6 | 21.7 | 0.7 |
| PUF/GNPs2.5 | 47 | 0.8 | 570 | 12.8 | 28.3 | 5.8 |
| PUF/GNPs5.0 | 56 | 2.0 | 966 | 81.9 | 48.3 | 5.4 |
| PUF/GO2.5 | 50 | 1.2 | 598 | 46.4 | 35.3 | 0.3 |
| PUF/GO5.0 | 60 | 3.6 | 1510 | 165.4 | 56.3 | 5.0 |
| Samples | Thermal Conductivity (W∙m−1∙K−1) | Thermal Diffusivity (mm2∙s−1) | Specific Heat (MJ∙m−3∙K−1) | |||
|---|---|---|---|---|---|---|
| Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | |
| PUF | 0.0352 | 0.0004 | 0.8577 | 0.0065 | 0.0410 | 0.0003 |
| PUF/GNPs2.5 | 0.0361 | 0.0001 | 0.8037 | 0.0004 | 0.0448 | 0.0001 |
| PUF/GNPs5.0 | 0.0393 | 0.0002 | 0.7672 | 0.0119 | 0.0486 | 0.0010 |
| PUF/GO2.5 | 0.0355 | 0.0001 | 0.8276 | 0.0060 | 0.0429 | 0.0003 |
| PUF/GO5.0 | 0.0366 | 0.0001 | 1.001 | 0.0060 | 0.0360 | 0.0002 |
| Samples | Apparent Density (kg∙m−3) | Stress Peak (MPa) | EAD (MJ∙m−3) Strain: 0.8 | SEA (MJ∙m−3∙kg−1) Strain: 0.8 | Thermal Conductivity (W∙m−1∙K−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Value | Std. Dev. | Mean Value | Std. Dev. | Mean Value | Std. Dev. | Mean Value | Std. Dev. | Mean Value | Std. Dev. | |
| Al-OC | 110 | 1.6 | 0.312 | 0.015 | 0.317 | 0.004 | 249 | 1.5 | 1.1781 | 0.0002 |
| PUF/GNPs2.5 | 50 | 1.2 | 0.028 | 0.004 | 0.028 | 0.004 | 445 | 31 | 0.0355 | 0.0001 |
| PUF/GNPs2.5-OC | 116 | 6.4 | 0.396 | 0.005 | 0.401 | 0.002 | 127 | 8.8 | 0.0377 | 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, S.C.; Marques, P.A.A.P.; Vicente, R.; Godinho, L.; Duarte, I. Hybrid Structures Made of Polyurethane/Graphene Nanocomposite Foams Embedded within Aluminum Open-Cell Foam. Metals 2020, 10, 768. https://doi.org/10.3390/met10060768
Pinto SC, Marques PAAP, Vicente R, Godinho L, Duarte I. Hybrid Structures Made of Polyurethane/Graphene Nanocomposite Foams Embedded within Aluminum Open-Cell Foam. Metals. 2020; 10(6):768. https://doi.org/10.3390/met10060768
Chicago/Turabian StylePinto, Susana C., Paula A. A. P. Marques, Romeu Vicente, Luís Godinho, and Isabel Duarte. 2020. "Hybrid Structures Made of Polyurethane/Graphene Nanocomposite Foams Embedded within Aluminum Open-Cell Foam" Metals 10, no. 6: 768. https://doi.org/10.3390/met10060768
APA StylePinto, S. C., Marques, P. A. A. P., Vicente, R., Godinho, L., & Duarte, I. (2020). Hybrid Structures Made of Polyurethane/Graphene Nanocomposite Foams Embedded within Aluminum Open-Cell Foam. Metals, 10(6), 768. https://doi.org/10.3390/met10060768







