Influence of Ag Addition on Thermal Stability and Thermophysical Properties of Ti-Zr-Ni Quasicrystals
Abstract
1. Introduction
2. Materials and Methods
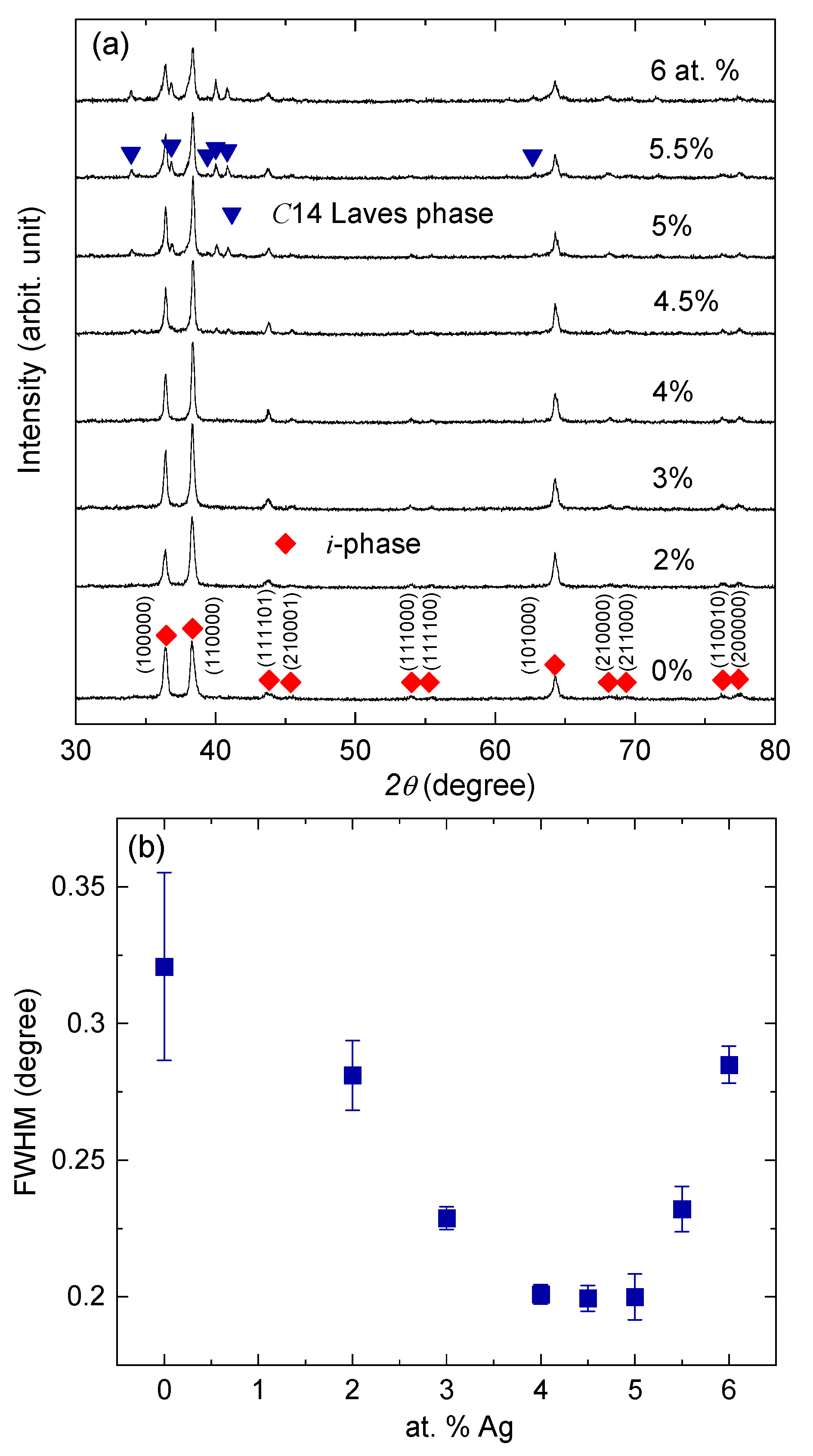
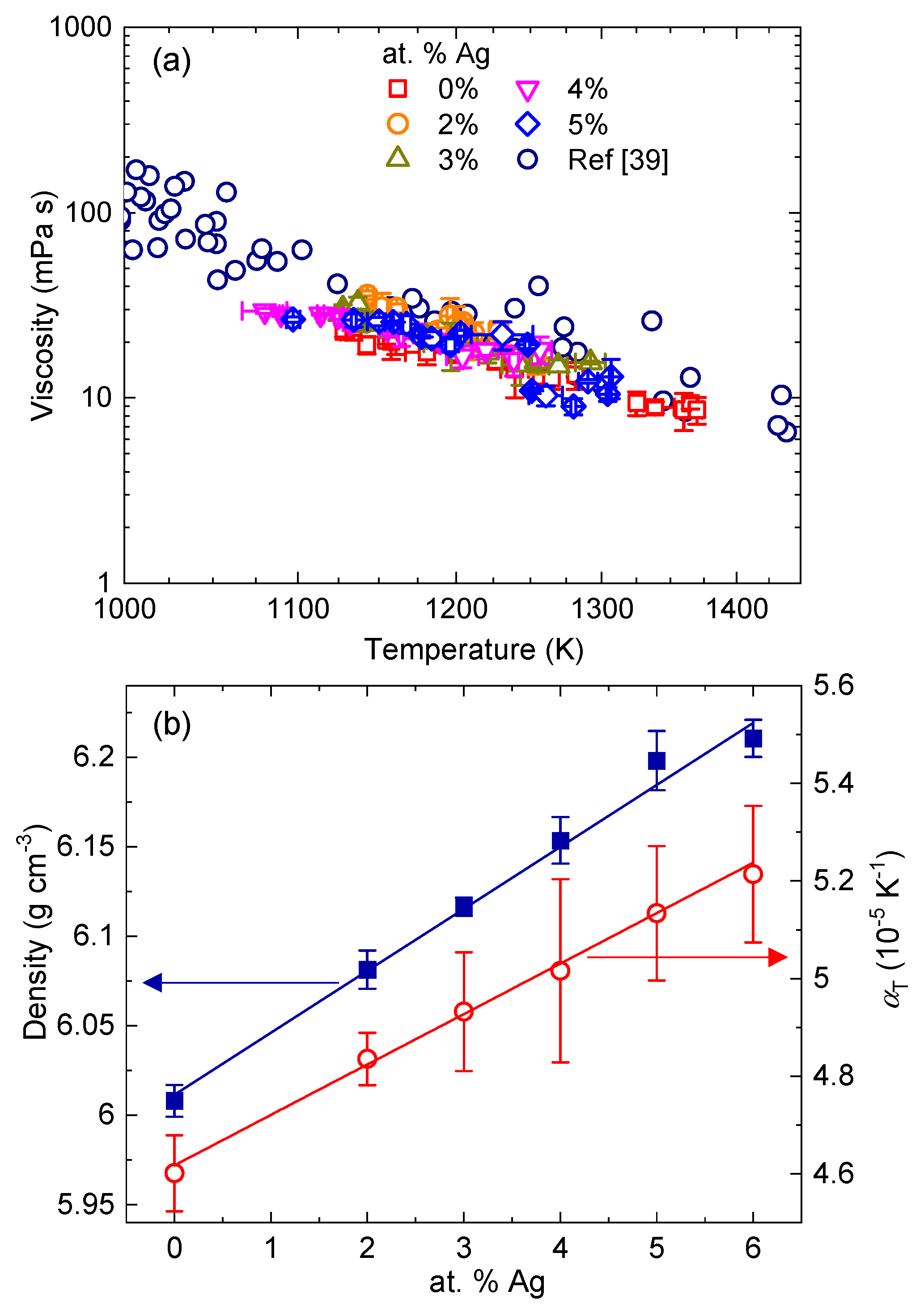
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.H. Roles of minor additions in formation and properties of bulk metallic glasses. Prog. Mater. Sci. 2007, 52, 540–596. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. Role of minor alloying additions in formation of bulk metallic glasses: A review. J. Mater. Sci. 2004, 39, 3965–3974. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wei, S.H.; Guo, G.O.; Wang, J.G.; Yang, L. Structural mechanism of the enhanced glass-forming ability in multicomponent alloys with positive heat of mixing. Sci. Rep. 2016, 6, 38098. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, H.; Liu, X.; Dong, Y.; Yu, C.; Lu, Z. Effects of Al addition on atomic structure of Cu-Zr metallic glass. J. Appl. Phys. 2018, 123, 055101. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, M.; Lee, K.-R.; Lee, J.-C. How can a minor element added to a binary amorphous alloy simultaneously improve the plasticity and glass-forming ability? Acta Mater. 2013, 61, 6597–6608. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.T.; Yang, Y.; Liu, J.B.; Dong, Y.D.; Lu, J. The atomic-scale mechanism for the enhanced glass-forming-ability of a Cu-Zr based bulk metallic glass with minor element additions. Sci. Rep. 2014, 4, 4648. [Google Scholar] [CrossRef]
- Lee, G.W.; Cho, Y.C.; Lee, B.; Kelton, K.F. Interfacial free energy and medium range order: Proof of an inverse of Frank’s hypothesis. Phys. Rev. B 2017, 95, 054202. [Google Scholar] [CrossRef]
- Chathoth, S.M.; Damaschke, B.; Embs, J.P.; Samwer, K. Giant changes in atomic dynamics on microalloying metallic melt. Appl. Phys. Lett. 2009, 95, 191907. [Google Scholar] [CrossRef]
- Chen, C.J.; Podlesnyak, A.; Mamontov, E.; Wang, W.H.; Chathoth, S.M. Microscopic insight into the origin of enhanced glass-forming ability of metallic melts on micro-alloying. Appl. Phys. Lett. 2015, 107, 131901. [Google Scholar] [CrossRef]
- Saksl, K.; Franz, H.; Jóvári, P.; Klementiev, K.; Welter, E.; Ehnes, A.; Saida, J.; Inoue, A.; Jiang, J.Z. Evidence of icosahedral short-range order in Zr70Cu30 and Zr70Cu29Pd1 and metallic glasses. Appl. Phys. Lett. 2003, 83, 3924–3926. [Google Scholar] [CrossRef]
- Davis, J.P.; Majzoub, E.H.; Simmons, J.M.; Kelton, K.F. Ternary phase diagram studies in Ti-Zr-Ni alloys. Mater. Sci. Eng. 2000, 294, 104–107. [Google Scholar] [CrossRef]
- Hua, N.; Chen, W. Enhancement of glass-forming ability and mechanical property of Zr-based Zr-Al-Ni bulk metallic glasses with addition of Pd. J. Alloy Compd. 2017, 693, 816–824. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, W.; Xie, G.; Inoue, A. Relation between glass and quasicrystal formation in the Zr–Nb–Cu–Ni–Al alloys upon solidification. Appl. Phys. Lett. 2010, 97, 031919. [Google Scholar] [CrossRef]
- Xu, D.; Duan, G.; Johnson, W.L. Unusual Glass-Forming Ability of Bulk Amorphous Alloys Based on Ordinary Metal Copper. Phys. Rev. Lett. 2004, 92, 245504. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.B.; Wang, X.D.; Xu, F.; Ding, S.Q.; Cao, Q.P.; Hono, K.; Jiang, J.Z. 73 mm-diameter bulk metallic glass rod by copper mould casting. Appl. Phys. Lett. 2011, 99, 051910. [Google Scholar] [CrossRef]
- Wang, D.; Tan, H.; Li, Y. Multiple maxima of GFA in three adjacent eutectics in Zr–Cu–Al alloy system—A metallographic way to pinpoint the best glass forming alloys. Acta Mater. 2005, 53, 2969–2979. [Google Scholar] [CrossRef]
- Kang, D.-H.; Zhang, H.; Yoo, H.; Lee, H.H.; Lee, S.; Lee, G.W.; Lou, H.; Wang, X.; Cao, Q.; Zhang, D.; et al. Interfacial free energy controlling glass-forming ability of Cu-Zr alloys. Sci. Rep. 2014, 4, 5167. [Google Scholar] [CrossRef]
- Yang, L.; Guo, G.Q.; Chen, L.Y.; Huang, C.L.; Ge, T.; Chen, D.; Liaw, P.K.; Saksl, K.; Ren, Y.; Zeng, Q.S.; et al. Atomic-scale mechanisms of the glass-forming ability in metallic glasses. Phys. Rev. Lett. 2012, 109, 105502. [Google Scholar] [CrossRef]
- Bendert, J.C.; Gangopadhyay, A.K.; Mauro, N.A.; Kelton, K.F. Volume expansion measurements in metallic liquids and their relation to fragility: An energy landscape interpretation. Phys. Rev. Lett. 2012, 109, 185901. [Google Scholar] [CrossRef]
- Ganorkar, S.; Lee, S.; Lee, Y.-H.; Ishikawa, T.; Lee, G.W. Origin of glass forming ability of Cu-Zr alloys: A link between compositional variation and stability of liquid and glass. Phys. Rev. Mater. 2018, 2, 115606. [Google Scholar] [CrossRef]
- Park, E.S.; Kang, H.G.; Kim, W.T.; Kim, D.H. The effect of Ag addition on the glass-forming ability of Mg–Cu–Y metallic glass alloys. J. Non-Cryst. Solids 2011, 279, 154–160. [Google Scholar] [CrossRef]
- Fujita, T.; Konno, K.; Zhang, W.; Kumar, V.; Matsuura, M.; Inoue, A.; Sakurai, T.; Chen, M.W. Atomic-scale heterogeneity of a multicomponent bulk metallic. Phys. Rev. Lett. 2009, 103, 075502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jia, F.; Zhang, Q.; Inoue, A. Effects of additional Ag on the thermal stability and glass-forming ability of Cu–Zr binary glassy alloys. Mater. Sci. Eng. 2007, 459, 330–336. [Google Scholar] [CrossRef]
- Gammer, C.; Escher, B.; Ebner, C.; Minor, A.M.; Karnthaler, H.P.; Eckert, J.; Pauly, S.; Rentenberger, C. Influence of the Ag concentration on the medium-range order in a CuZrAlAg bulk metallic glass. Sci. Rep. 2017, 7, 44903. [Google Scholar] [CrossRef] [PubMed]
- Nicula, R.; Ponkratz, U.; Jianu, A.; Schick, C.; Burkel, E. Icosahedral phase formation in Ag-substituted Ti–Zr–Ni rapidly-quenched alloys. Mater. Sci. Eng. 2000, 294, 90–92. [Google Scholar] [CrossRef]
- Lee, G.W.; Gangopadhyay, A.K.; Kelton, K.F. Effect of microalloying on the formation and stability of the Ti-Zr-Ni icosahedral quasicrystal. J. Alloy Compd. 2012, 537, 171–174. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, X.; Li, J.F. Effects of Ag addition on crystallization, microstructure and mechanical properties of Zr–Cu–Ni–Al–Ag bulk metallic glasses. J. Alloy Compd. 2013, 552, 102–106. [Google Scholar] [CrossRef]
- Lee, G.W.; Jeon, S.; Kang, D.-H. Crystal–liquid interfacial free energy of supercooled liquid Fe using a containerless technique. Cryst. Growth Des. 2013, 13, 1786–1792. [Google Scholar] [CrossRef]
- Ishikawa, T.; Paradis, P.-F.; Yoda, S. New sample levitation initiation and imaging techniques for the processing of refractory metals with an electrostatic levitator furnace. Rev. Sci. Instrum. 2001, 72, 2490–2495. [Google Scholar] [CrossRef]
- Yoo, H.; Park, C.; Jeon, S.; Lee, S.; Lee, G.W. Uncertainty evaluation for density measurements of molten Ni, Zr, Nb and Hf by using a containerless method. Metrologia 2015, 52, 677–684. [Google Scholar] [CrossRef]
- Lee, G.W.; Jeon, S.; Park, C.; Kang, D.-H. Crystal–liquid interfacial free energy and thermophysical properties of pure liquid Ti using electrostatic levitation: Hypercooling limit, specific heat, total hemispherical emissivity, density, and interfacial free energy. J. Chem. Thermodyn. 2013, 63, 1–6. [Google Scholar] [CrossRef]
- Lee, G.W.; Croat, T.K.; Gangopadhyay, A.K.; Kelton, K.F. Icosahedral-phase formation in as-cast Ti-Zr-Ni alloys. Philos. Mag. Lett. 2002, 82, 199–205. [Google Scholar] [CrossRef]
- Lee, G.W.; Gangopadhyay, A.K.; Croat, T.K.; Rathz, T.J.; Hyers, R.W.; Rogers, J.R.; K. F.; Kelton, K.F. Link between liquid structure and the nucleation barrier for icosahedral quasicrystal, polytetrahedral, and simple crystalline phases in Ti-Zr-Ni alloys: Verification of Frank’s hypothesis. Phys. Rev. B 2005, 72, 174107. [Google Scholar] [CrossRef]
- Lee, G.W.; Gangopadhyay, A.K.; Kelton, K.F. Phase diagram studies of Ti-Zr-Ni alloys containing less than 40 at.% Ni and estimated critical cooling rate for icosahedral quasicrystal formation from the liquid. Acta Mater. 2011, 12, 4964–4973. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray diffraction methods. J. Appl. Phys. 1941, 12, 375–383. [Google Scholar] [CrossRef]
- Park, G.H.; Hong, S.H.; Kim, J.T.; Park, H.J.; Seo, Y.; Suh, J.Y.; Hong, S.; Park, J.M.; Kim, K.B. Phase transformation and mechanical properties of as-cast Ti41.5Zr41.5Ni17 quasicrystalline composites. J. Non-Cryst. Solids 2014, 392, 6–10. [Google Scholar] [CrossRef]
- Kelton, K.F.; Lee, G.W.; Gangophdhyay, A.K.; Hyers, R.W.; Rathz, T.J.; Rogers, J.R.; Robinson, M.B.; Robinson, D.S. First X-ray scattering studies on electrostatically levitated metallic liquids: Demonstrated influence of local icosahedral order on the nucleation barrier. Phys. Rev. Lett. 2003, 90, 195504. [Google Scholar] [CrossRef]
- Arroyave, R.; Eagar, T.W.; Kaufman, L. Thermodynamic assessment of the Cu-Ti-Zr system. J. Alloy Compd. 2003, 351, 158–170. [Google Scholar] [CrossRef]
- Hyers, R.W.; Bradshaw, R.C.; Rogers, J.R.; Rathz, T.J.; Lee, G.W.; Gangopadhyay, A.K.; Kelton, K.F. Surface tension and viscosity of quasicrystal-forming Ti–Zr–Ni Alloys. Int. J. Thermophys. 2004, 25, 1155–1162. [Google Scholar] [CrossRef]
- Cho, Y.C.; Kim, B.S.; Yoo, H.; Kim, J.Y.; Lee, S.; Lee, Y.-H.; Lee, G.W.; Jeong, S.Y. Successful melting and density measurements of Cu and Ag single crystals with an electrostatic levitation (ESL) system. CrystEngComm 2014, 16, 7575–7579. [Google Scholar] [CrossRef]
- Jeon, S.; Kang, D.-H.; Lee, Y.H.; Lee, S.; Lee, G.W. Effect of atomic size on undercoolability of binary solid solution alloy liquids with Zr, Ti, and Hf using electrostatic levitation. J. Chem. Phys. 2016, 145, 174504. [Google Scholar] [CrossRef] [PubMed]
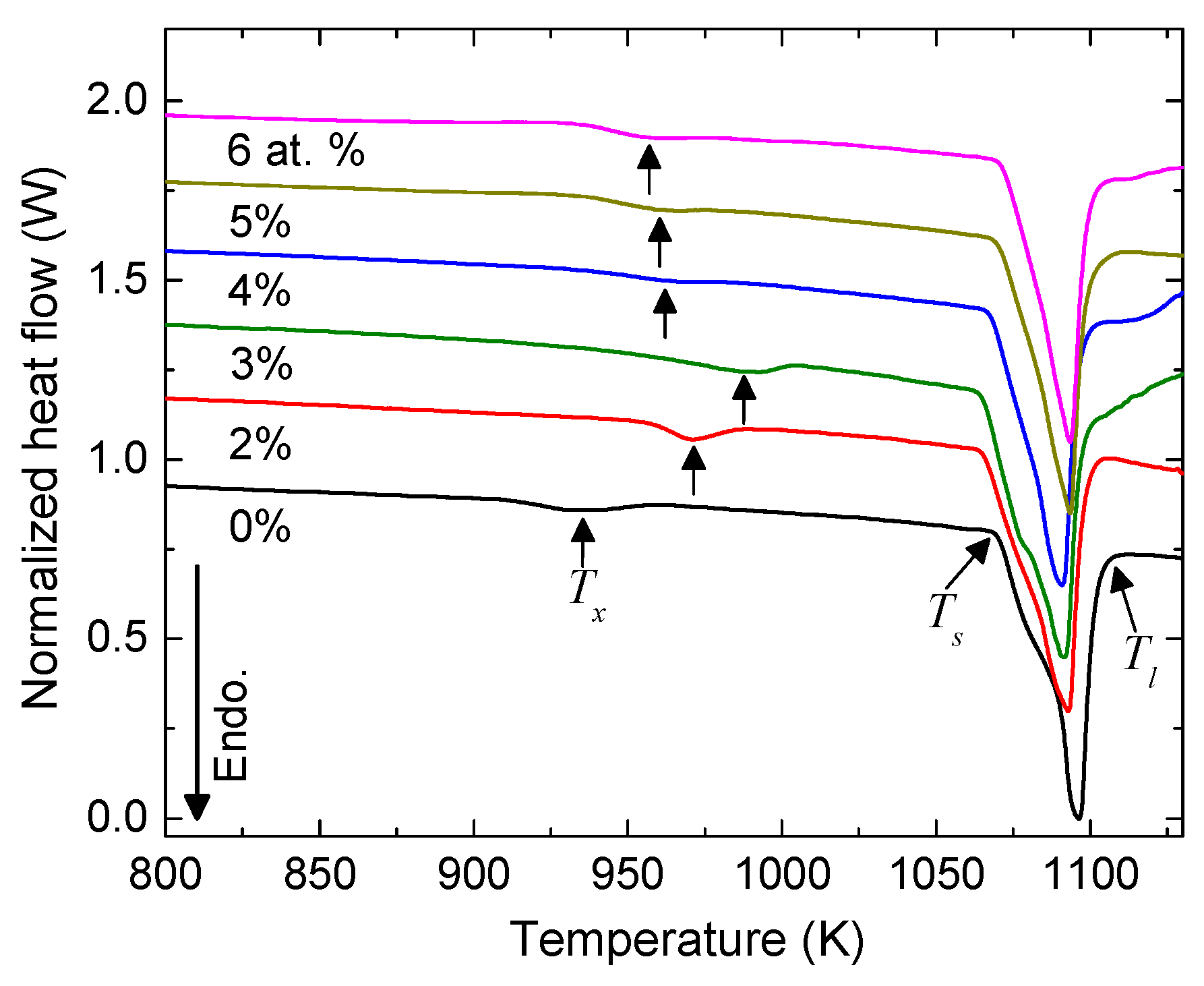


| at.% Ag | 0 | 2 | 3 | 4 | 5 | 6 |
| Tx (K) | 936 | 971 | 992 | 967 | 963 | 960 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Cho, Y.C.; Kim, Y.-I.; Lee, Y.-H.; Lee, S.; Lee, G.W. Influence of Ag Addition on Thermal Stability and Thermophysical Properties of Ti-Zr-Ni Quasicrystals. Metals 2020, 10, 760. https://doi.org/10.3390/met10060760
Jeon S, Cho YC, Kim Y-I, Lee Y-H, Lee S, Lee GW. Influence of Ag Addition on Thermal Stability and Thermophysical Properties of Ti-Zr-Ni Quasicrystals. Metals. 2020; 10(6):760. https://doi.org/10.3390/met10060760
Chicago/Turabian StyleJeon, Sangho, Yong Chan Cho, Yong-Il Kim, Yun-Hee Lee, Sooheyong Lee, and Geun Woo Lee. 2020. "Influence of Ag Addition on Thermal Stability and Thermophysical Properties of Ti-Zr-Ni Quasicrystals" Metals 10, no. 6: 760. https://doi.org/10.3390/met10060760
APA StyleJeon, S., Cho, Y. C., Kim, Y.-I., Lee, Y.-H., Lee, S., & Lee, G. W. (2020). Influence of Ag Addition on Thermal Stability and Thermophysical Properties of Ti-Zr-Ni Quasicrystals. Metals, 10(6), 760. https://doi.org/10.3390/met10060760






