Recovery of Diamond and Cobalt Powder from Polycrystalline Drawing Die Blanks via Ultrasound-Assisted Leaching Process—Part 1: Process Design and Efficiencies
Abstract
1. Introduction
2. Experimental
2.1. Material
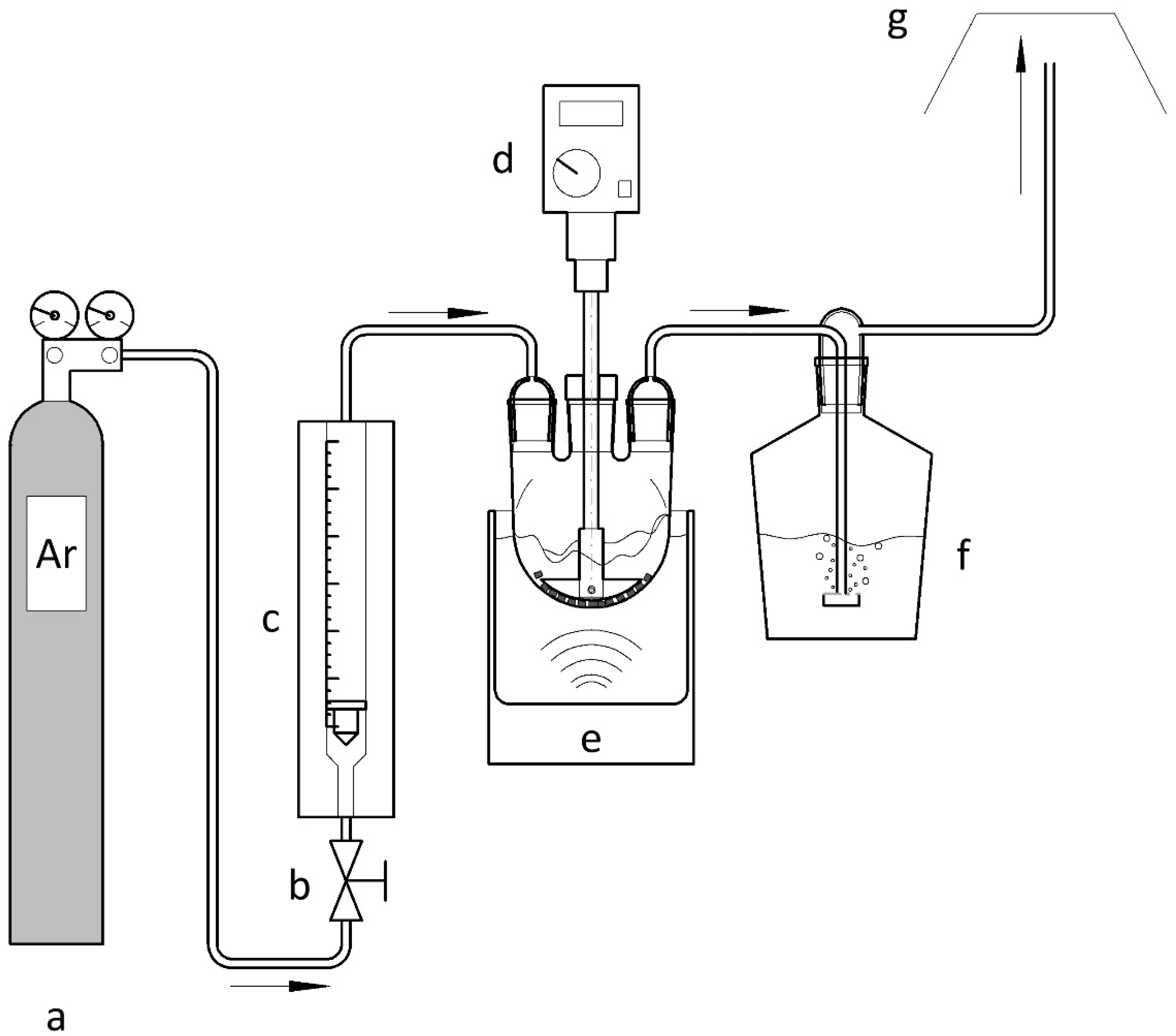
2.2. Procedure
2.2.1. Preparation of Samples
2.2.2. Conduct of Experiments
2.2.3. Sampling
2.2.4. Weighing of Sample
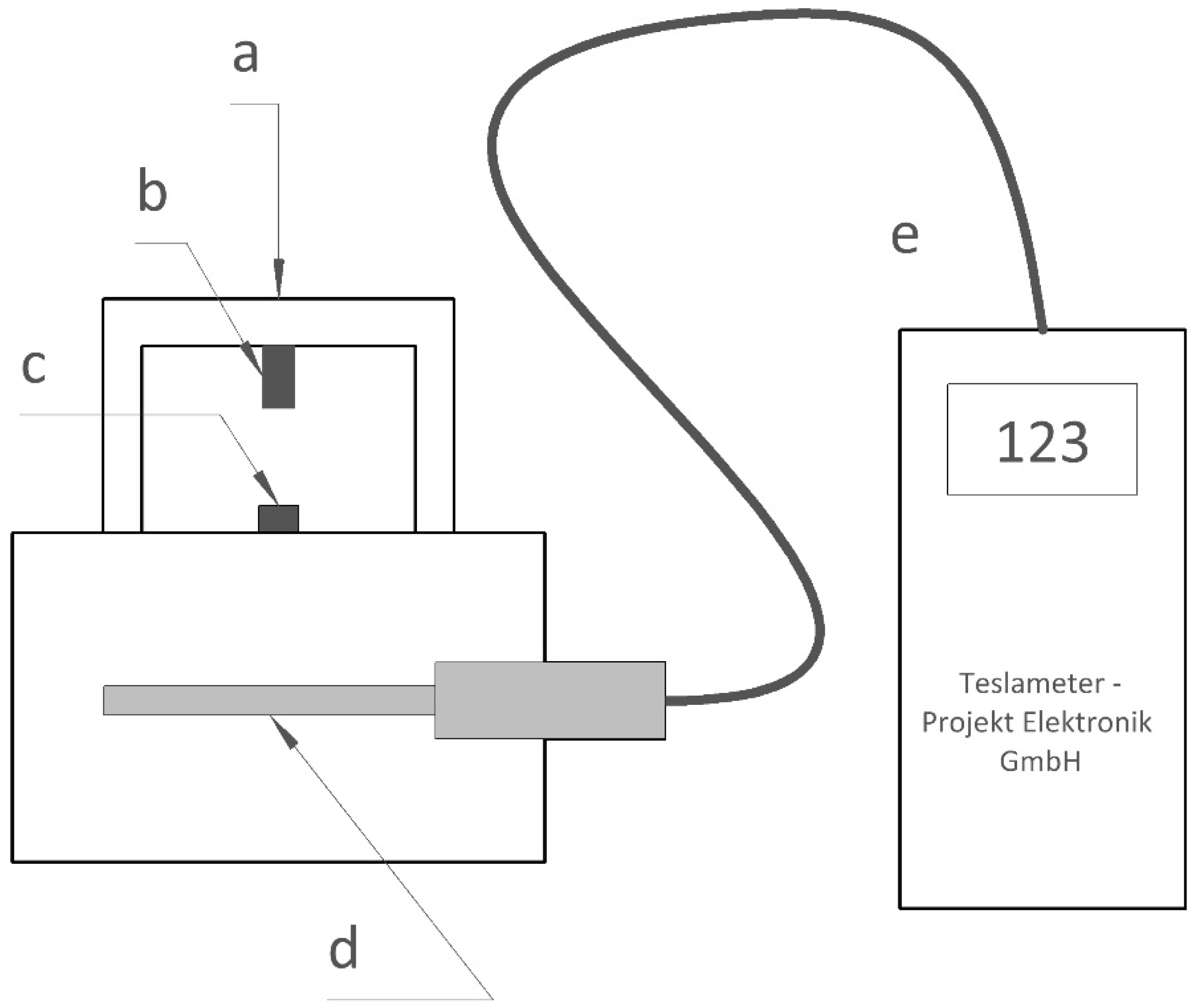
2.2.5. Observation of Changes in Magnetic Properties of PCD
3. Results and Discussion
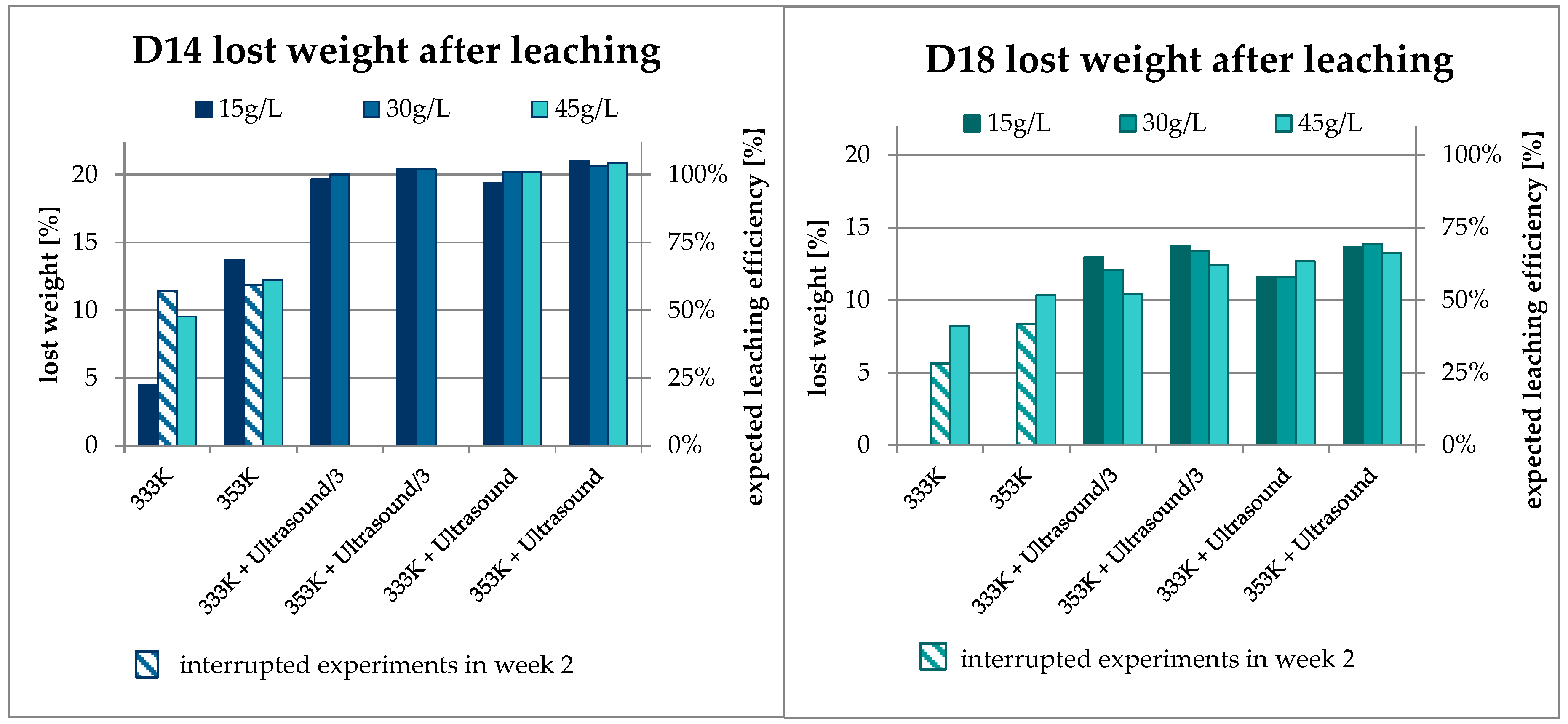
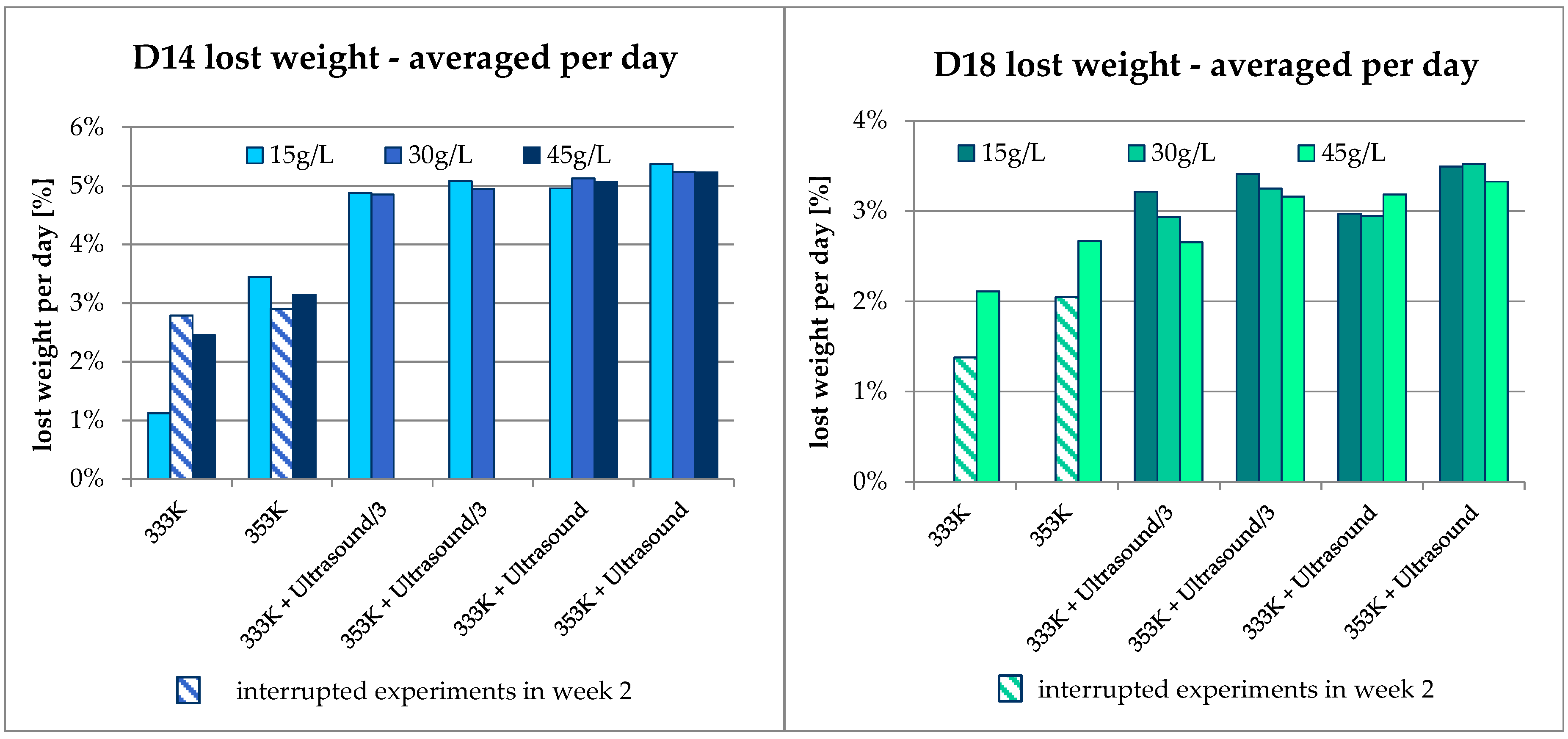
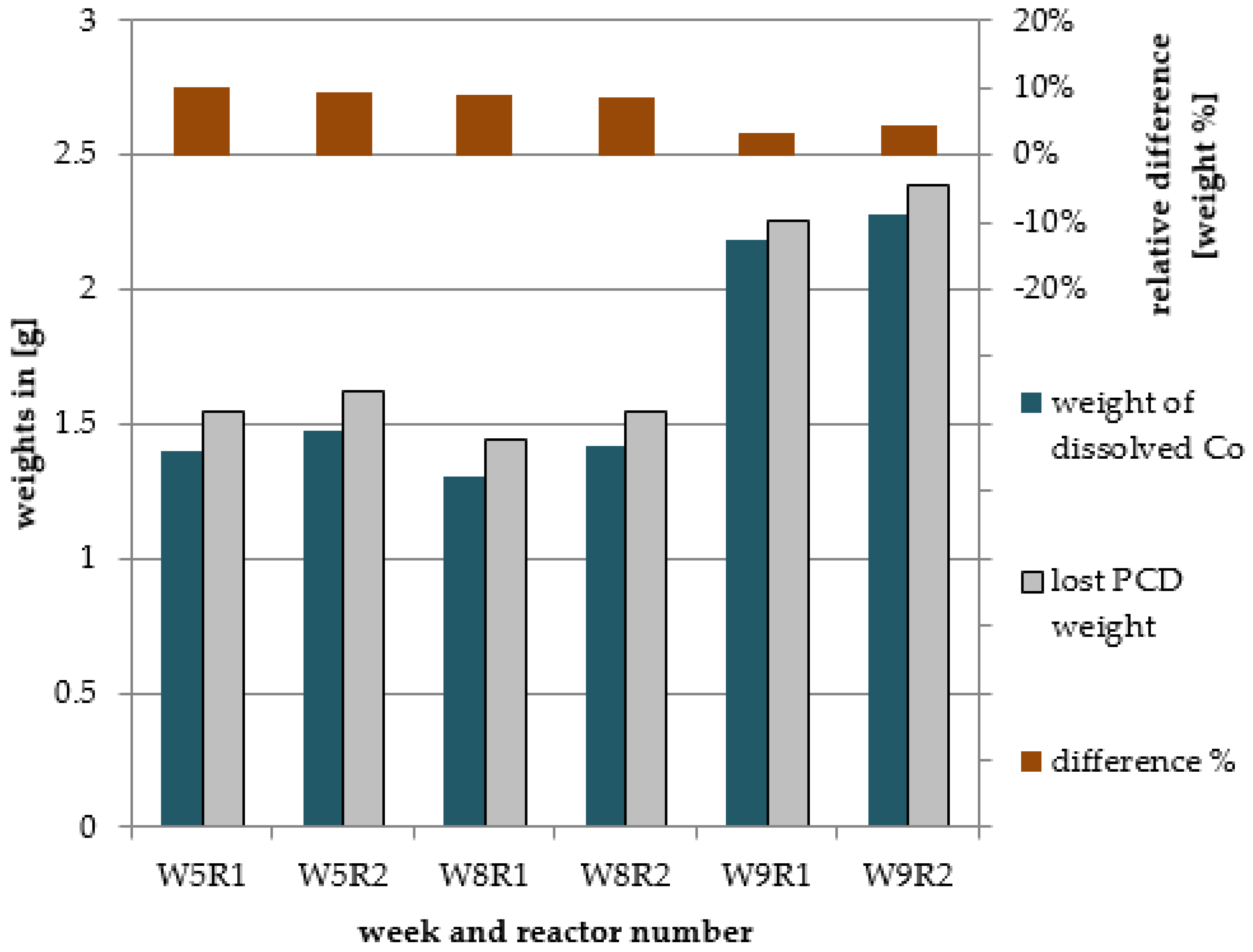
3.1. Mass Balance
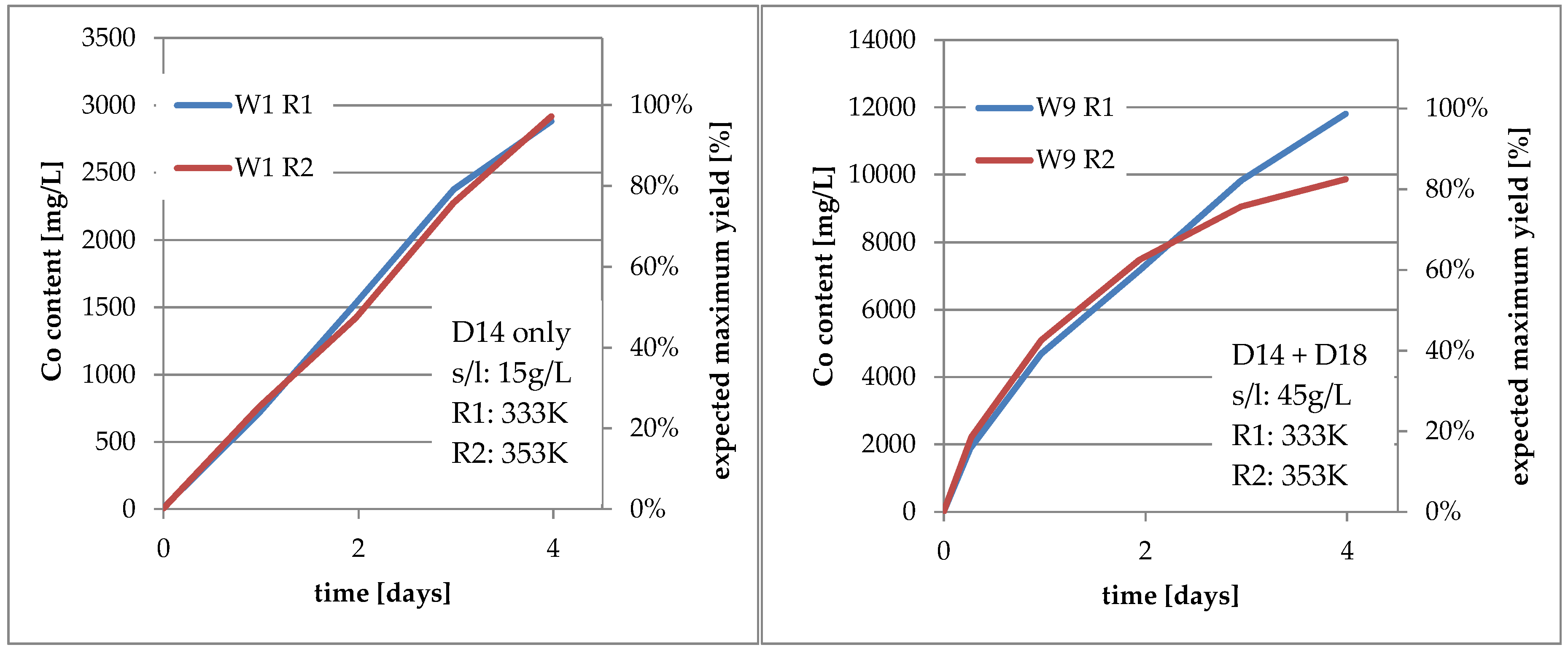
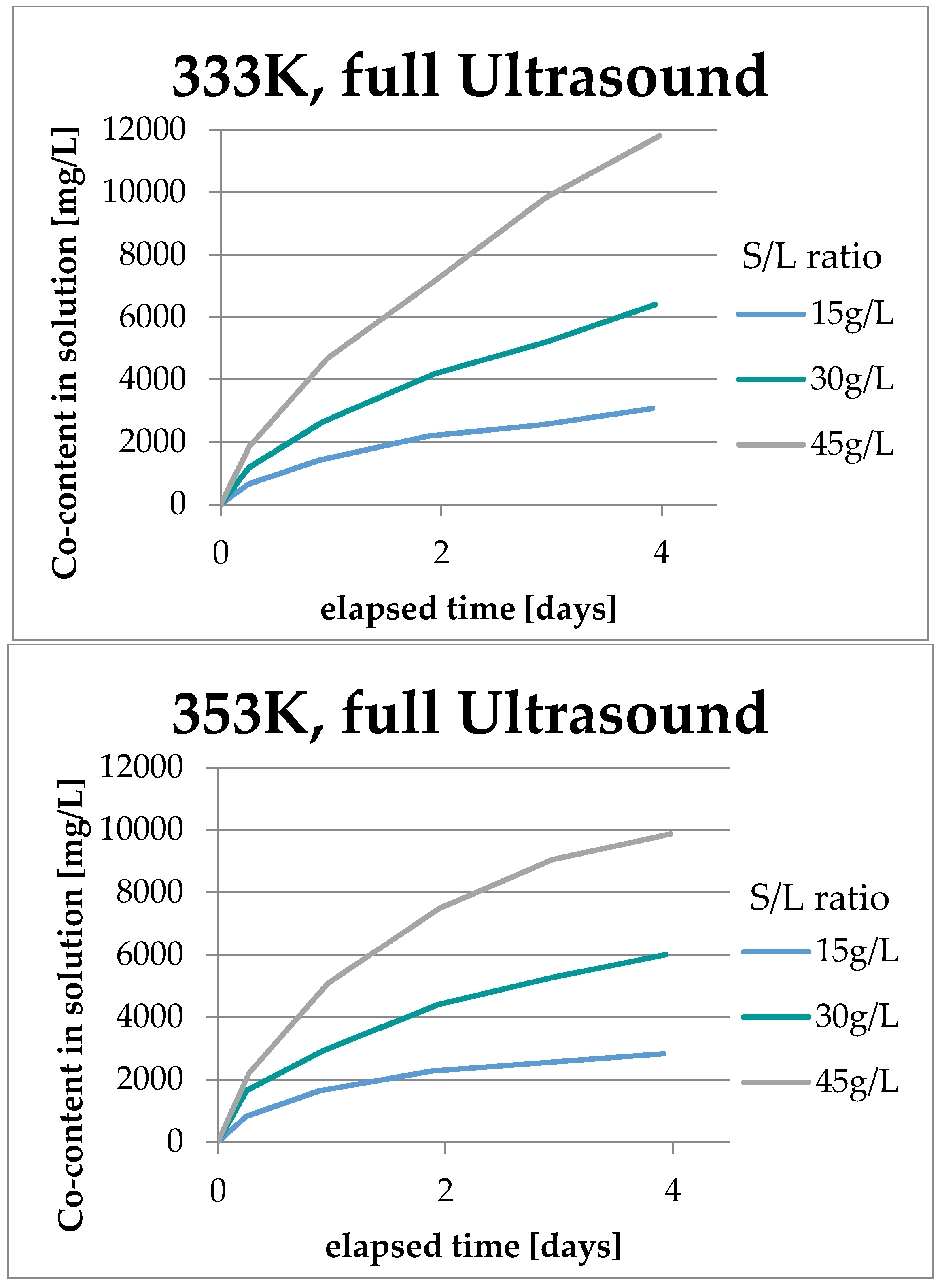
3.2. Results Regarding Process Optimization
3.3. Possible Recycling Routes for Cosolution in Order to Produce Cobalt Powder and Its Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moskalyk, R.R.; Alfantazi, A.M. Nickel laterite processing and electrowinning practice. Miner. Eng. 2002, 15, 593–605. [Google Scholar] [CrossRef]
- Olanipekun, E.O. Kinetics of leaching laterite. Int. J. Miner. Process. 2000, 60, 9–14. [Google Scholar] [CrossRef]
- Narasimhan, K.S.; Bhima Rao, R.; Das, B. Technical note—Characterisation and concentration of laterites. Miner. Eng. 1989, 2, 425–429. [Google Scholar] [CrossRef]
- Anokhin, A.S.; Strel’nikova, S.S.; Andrianov, M.A.; Tkachenko, V.V.; Shipkov, A.N.; Kukueva, E.V.; Gol’dt, A.E.; Zaremba, O.T. Formation of the Structure and Phase Composition of Polycrystalline Diamond with Cobalt Infiltration in the System Diamond—Hard Alloy. Glass Ceram. 2019, 75, 475–478. [Google Scholar] [CrossRef]
- Wentorf, R.H.; DeVries, R.C.; Bundy, F.P. Sintered superhard materials. Science 1980, 208, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.A. The influence of microstructure on the mechanical properties of polycrystalline diamond: A literature review. Adv. Appl. Ceram. 2018, 117, 161–176. [Google Scholar] [CrossRef]
- Wang, S. Cobalt—Its recovery, recycling, and application. Metals 2006, 58, 47–50. [Google Scholar] [CrossRef]
- General Electric Research Laboratory. Man-made diamonds. Nature 1955, 176, 50–56. [Google Scholar]
- Rao, A.S.; Minango, R.; Nkhoma, J.; Singh, H.P. Process developments in the cobalt purification circuit at Chambishi RLE Cobalt Plant of ZCCM, Zambia. In Queneau International Symposium Extractive Metallurgy of Copper, Nickel and Cobalt: Fundamental Aspects; Paul, E., Ed.; The Minerals, Metals & Materials Society: Warrendale, PA, USA, 1993; Volume 1, p. 853. [Google Scholar]
- Wakenge, C.I.; Dijkzeul, D.; Vlassenroot, K. Regulating the old game of smuggling? Coltan mining, trade and reforms in the Democratic Republic of the Congo. J. Mod. Afr. Stud. 2018, 56, 497–522. [Google Scholar] [CrossRef]
- Slaczka, A.S. Effect of ultrasound on ammonium leaching of zinc from galmei ore. Ultrasonics 1986, 24, 53–55. [Google Scholar] [CrossRef]
- Oncel, M.S.; Ince, M.; Bayramoglu, M. Leaching of silver from solid waste using ultrasound assisted thiourea method. Ultrason. Sonochem. 2005, 12, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhang, E.-D.; Zhang, L.-B.; Peng, J.-H.; Zhou, J.-W.; Srinivasakannan, C.; Yang, C.-J. A comparison of ultrasound-augmented and conventional leaching of silver from sintering dust using acidic thiourea. Ultrason. Sonochem. 2017, 34, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.R.; Gibson, D.C. Cavitation Bubbles near Boundaries. Ann. Rev. Fluid Mech. 1987, 19, 99–123. [Google Scholar] [CrossRef]
- Herbert, E.; Balibar, S.; Caupin, F. Cavitation pressure in water. Phys. Rev. 2006, 74, 041603. [Google Scholar] [CrossRef] [PubMed]
- OMFRS, L.R. On the pressure developed in a liquid during the collapse of a spherical cavity. Philos. Mag. 2009, 34, 94–98. [Google Scholar]
- Hixson, A.W.; Baum, S.J. Mass Transfer and Chemical Reaction in Liquid-solid Agitation. Ind. Eng. Chem. 1944, 36, 528–531. [Google Scholar] [CrossRef]
- Gürmen, S.; Stopić, S.; Friedrich, B. Synthesis of nanosized spherical cobalt powder by ultrasonic spray pyrolysis method. Mater. Res. Bull. 2006, 41, 1882–1890. [Google Scholar] [CrossRef]
- Gürmen, S.; Güven, A.; Ebin, B.; Stopic, S.; Friedrich, B. Synthesis of nanocrystalline spherical cobalt-iron (Co-Fe) alloy particles by ultrasonic spray pyrolysis and hydrogen reduction. J. Alloys Compd. 2009, 481, 600–604. [Google Scholar]
- Baghalha, M.; Gh, H.K.; Mortaheb, H.R. Kinetics of platinum extraction from spent reforming catalysts in aqua-regia solutions. Hydrometallurgy 2009, 95, 247–253. [Google Scholar] [CrossRef]
- Gogoleva, E.M. The leaching kinetics of brannerite ore in sulfate solutions with iron (III). J Radioanal. Nucl. Chem. 2012, 293, 185–191. [Google Scholar] [CrossRef]
- Wen, C.Y. Noncatalytic heterogeneous solid-fluid reaction models. Ind. Eng. Chem. 1968, 60, 34–54. [Google Scholar] [CrossRef]
- Massucci, M.; Clegg, S.L.; Brimblecombe, P. Equilibrium Partial Pressures, Thermodynamic Properties of Aqueous and Solid Phases, and Cl2 Production from Aqueous HCl and HNO3 and Their Mixtures. J. Phys. Chem. A 1999, 103, 4209–4226. [Google Scholar] [CrossRef]
- Kiessling, F.; Gürmen, S.; Stopic, S.; Friedrich, B. Advances in Synthesis of Metallic Powders Using Ultrasound Assisted Leaching Process from Polycrystalline Diamond Blanks—Thermochemistry and Kinetics of Ultrasound Leached Process—(Second Part). Metals. in press.

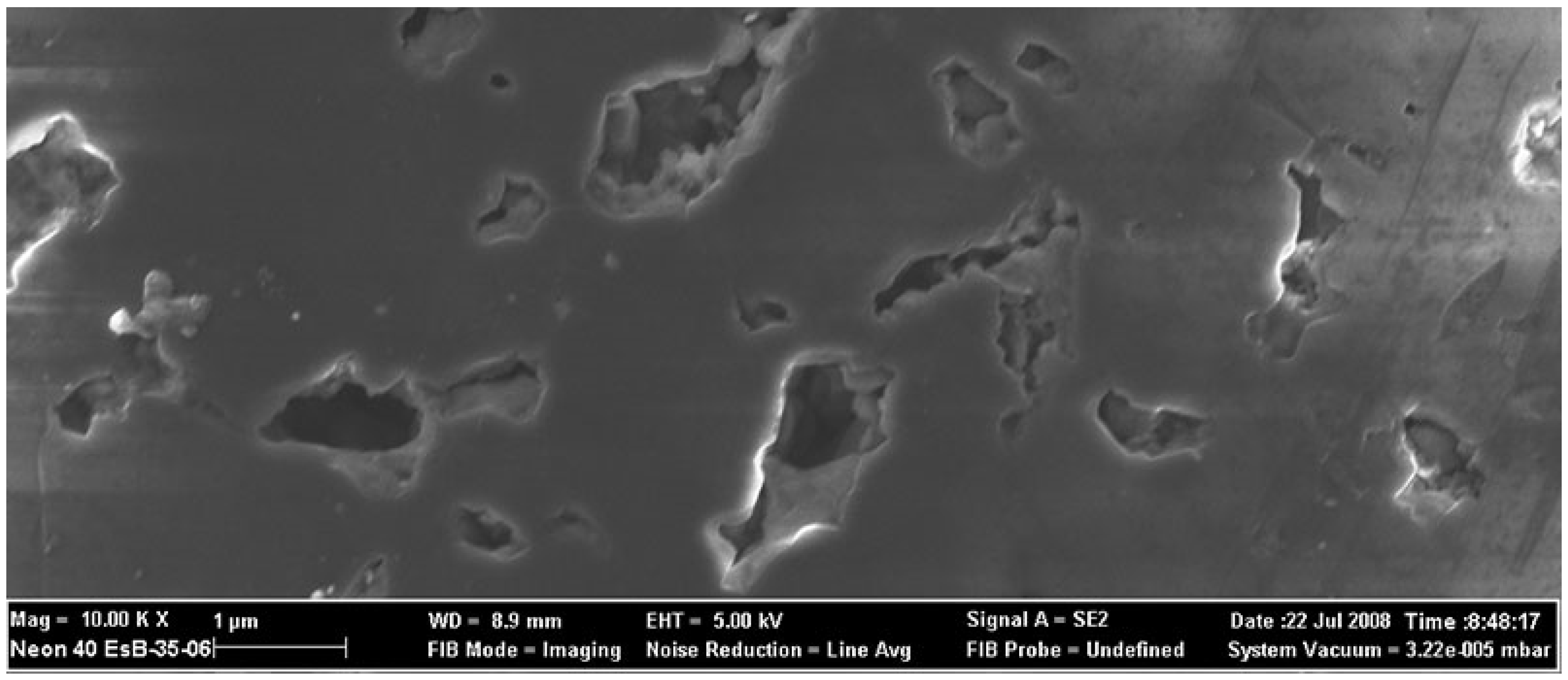



| Content (Weight %) | C | O | Fe | Co |
|---|---|---|---|---|
| Spectrum 1 | 86.81 | 12.94 | - | 0.25 |
| Spectrum 2 | 88.31 | 10.42 | 0.21 | 1.05 |
| Spectrum 3 | 89.35 | 10.32 | - | 0.34 |
| Spectrum 4 | 87.41 | 10.78 | 0.14 | 1.67 |
| Max. | 89.35 | 12.94 | 0.21 | 1.67 |
| Min. | 86.81 | 10.32 | 0.00 | 0.25 |
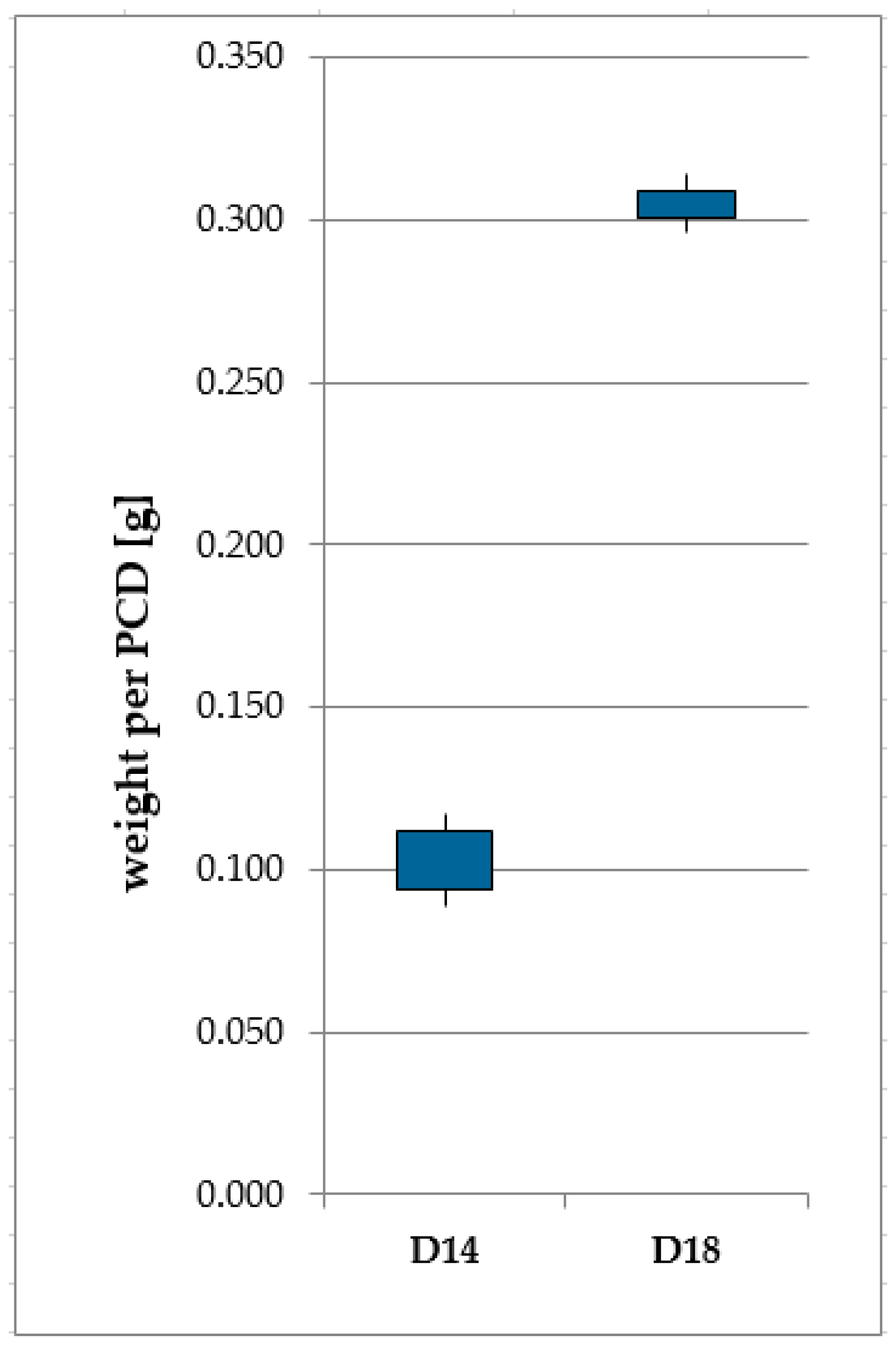
| Blank Type | Symbol | Diameter [mm] | Height [mm] | Weight [g] | Volume [mm3] | Surface Area [mm2] | Surface/Volume [mm−1] |
|---|---|---|---|---|---|---|---|
| Mant® MSD-06-005 | DO6 | 2.97 ± 0.01 | 1.10 ± 0.02 | 0.029 | 7.62 ± 0.18 | 24.09 ± 0.28 | 3.16 ± 0.11 |
| Mant® MSD-14-005 | D14 | 4.05 ± 0.09 | 2.00 ± 0.04 | 0.099 | 25.71 ± 1.40 | 51.14 ± 1.97 | 1.99 (+0.20|−0.18) |
| Mant® MSD-15-005 | D15 | 5.2 | 2.5 | 0.241 | 53.093 | 83.315 | 1.569 |
| Mant® MSD-18-005 | D18 | 5.22 ± 0.02 | 3.50 ± 0.02 | 0.299 | 74.90 ± 0.77 | 100.22 ± 0.68 | 1.338 ± 0.023 |
| ID | TBath [K] | PCD Type | Leaching Time [h/d] in the Presence of Ultrasound | S/L [g/L] |
|---|---|---|---|---|
| W1R1 | 333 | D14 | 0 | 15 |
| W1R2 | 353 | D14 | 0 | 15 |
| W2R1 | 333 | D14 + D18 | 0 | 30 |
| W2R2 | 353 | D14 + D18 | 0 | 30 |
| W3R1 | 333 | D14 + D18 | 0 | 45 |
| W3R2 | 353 | D14 + D18 | 0 | 45 |
| W4R1 | 333 | D14 + D18 | 8 h/d | 15 |
| W4R2 | 353 | D14 + D18 | 8 h/d | 15 |
| W5R1 | 333 | D14 + D18 | 8 h/d | 30 |
| W5R2 | 353 | D14 + D18 | 8 h/d | 30 |
| W6R1 | 333 | D15 + D18 | 8 h/d | 45 |
| W6R2 | 353 | D15 + D18 | 8 h/d | 45 |
| W7R1 | 333 | D14 + D18 | 24 h/d | 15 |
| W7R2 | 353 | D14 + D18 | 24 h/d | 15 |
| W8R1 | 333 | D14 + D18 | 24 h/d | 30 |
| W8R2 | 353 | D14 + D18 | 24 h/d | 30 |
| W9R1 | 333 | D14 + D18 | 24 h/d | 45 |
| W9R2 | 353 | D14 + D18 | 24 h/d | 45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kießling, F.; Stopic, S.; Gürmen, S.; Friedrich, B. Recovery of Diamond and Cobalt Powder from Polycrystalline Drawing Die Blanks via Ultrasound-Assisted Leaching Process—Part 1: Process Design and Efficiencies. Metals 2020, 10, 731. https://doi.org/10.3390/met10060731
Kießling F, Stopic S, Gürmen S, Friedrich B. Recovery of Diamond and Cobalt Powder from Polycrystalline Drawing Die Blanks via Ultrasound-Assisted Leaching Process—Part 1: Process Design and Efficiencies. Metals. 2020; 10(6):731. https://doi.org/10.3390/met10060731
Chicago/Turabian StyleKießling, Ferdinand, Srecko Stopic, Sebahattin Gürmen, and Bernd Friedrich. 2020. "Recovery of Diamond and Cobalt Powder from Polycrystalline Drawing Die Blanks via Ultrasound-Assisted Leaching Process—Part 1: Process Design and Efficiencies" Metals 10, no. 6: 731. https://doi.org/10.3390/met10060731
APA StyleKießling, F., Stopic, S., Gürmen, S., & Friedrich, B. (2020). Recovery of Diamond and Cobalt Powder from Polycrystalline Drawing Die Blanks via Ultrasound-Assisted Leaching Process—Part 1: Process Design and Efficiencies. Metals, 10(6), 731. https://doi.org/10.3390/met10060731







