Influence of Hot Forging Parameters on a Low Carbon Continuous Cooling Bainitic Steel Microstructure
Abstract
1. Introduction
2. Materials and Methods
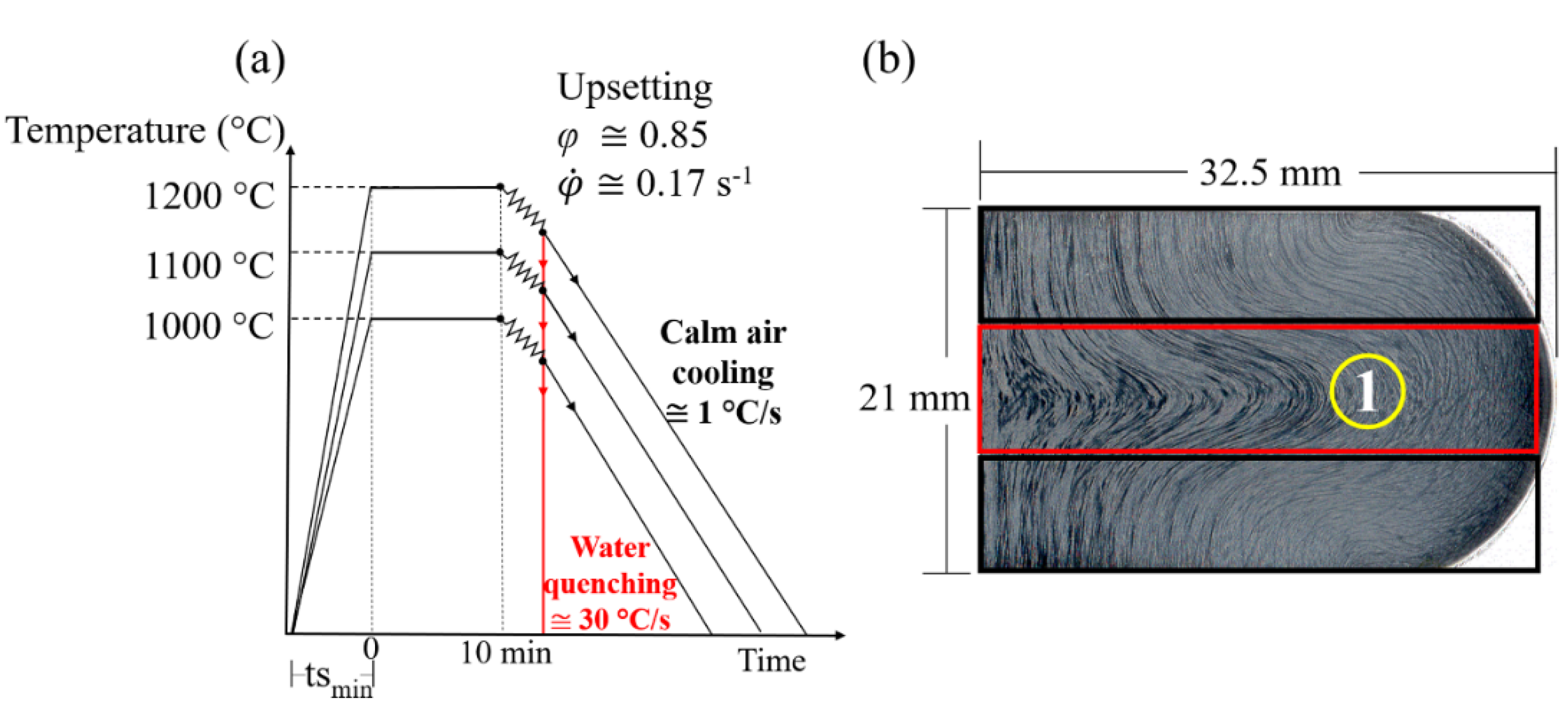
2.1. Grain Growth Evaluation on the Austenitizing Step for the Hot Forging
2.2. Hot Forging Experiments and Microstructure Evaluation
2.3. Critical Strain
2.4. Metallurgical Characterization
3. Results and Discussion
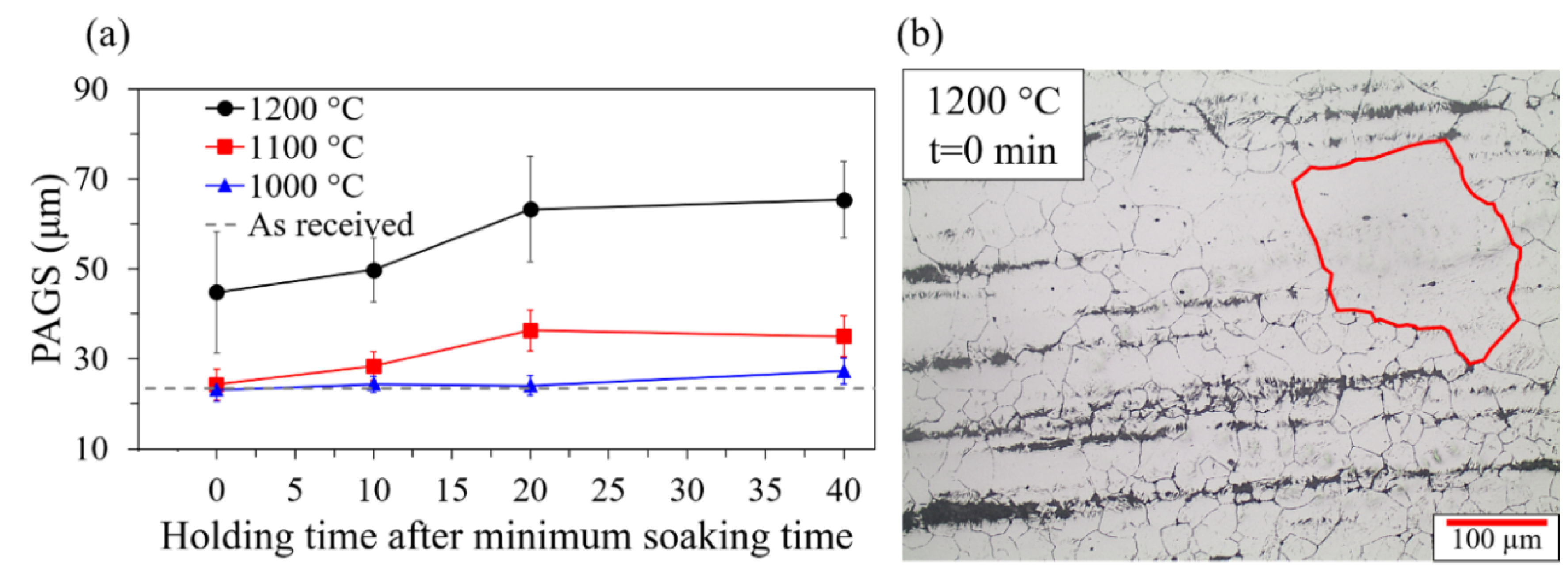
3.1. Grain Growth
3.2. Effect of the Hot Deformation Temperature
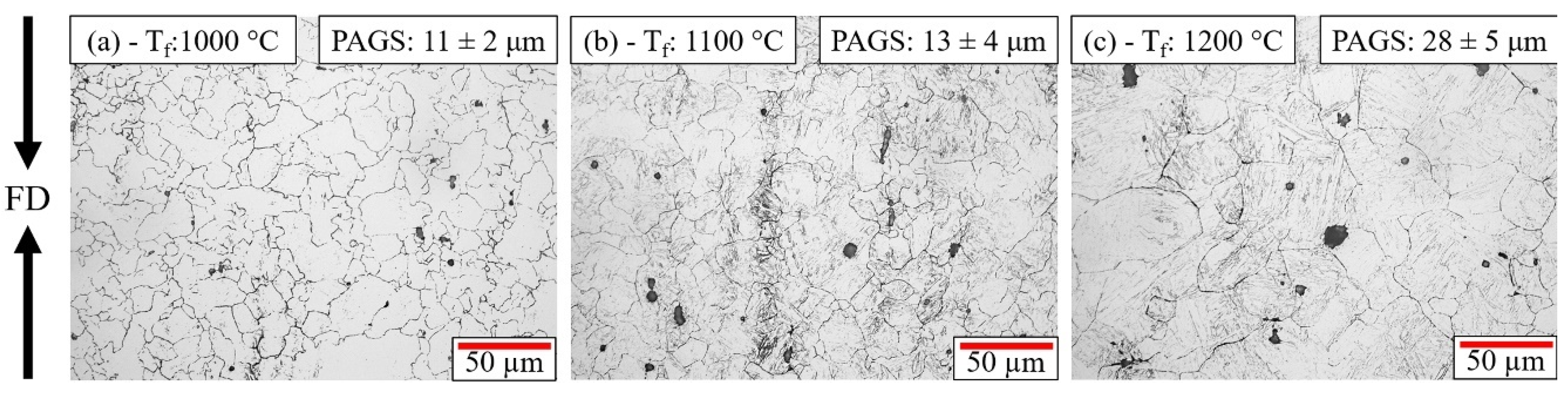
3.2.1. Austenitic Grain Refinement
3.2.2. Critical Strain
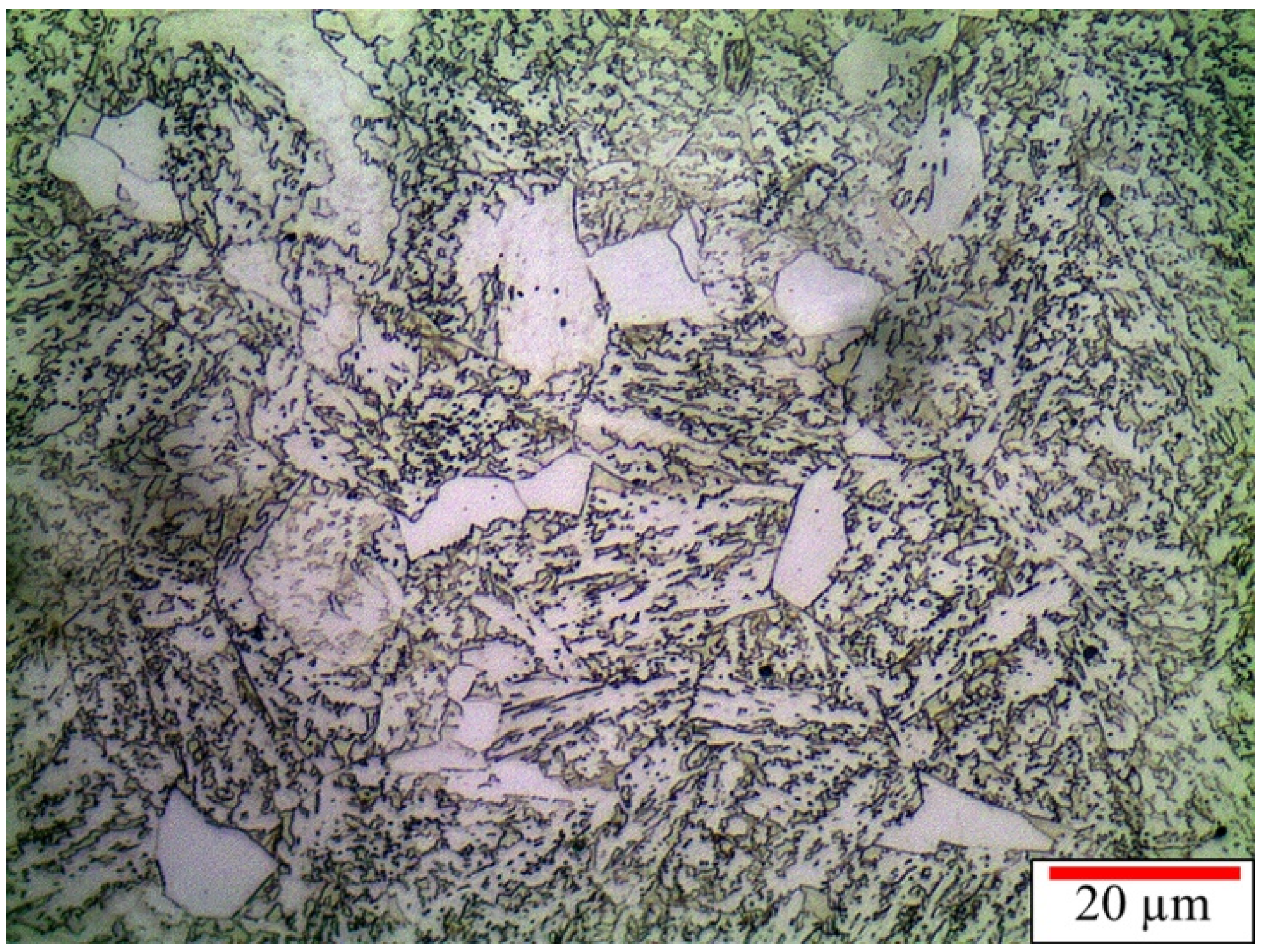
3.2.3. Continuously Cooled Microstructure
3.2.4. Summary of the Microstructure Characterization
4. Conclusions
- Heat treating experiments with different austenitizing temperatures and holding times were carried out to evaluate the prior austenite grain size. The prior austenite grain size results show that an austenitizing temperature of 1200 °C promoted a significant grain growth and the presence of abnormal grain growth, however, abnormal grain growth was not identified at 1100 and 1000 °C.
- The temperature also affected the prior austenite grain size after the hot forging, which affected the phase fractions in the final continuous cooling microstructure, especially the polygonal ferrite formation.
- X-ray diffraction analysis showed that the fraction of retained austenite was reduced as the forging temperature decreased due to the formation of higher quantities of polygonal ferrite. Moreover, the carbon content increased in austenite due to the growth of polygonal ferrite during cooling.
- Hot forging at 1000 °C promoted the formation of 30% of polygonal ferrite, resulting in a chemical heterogeneity of the remaining austenite, leading to martensite formation at the austenite regions with lower carbon and alloying elements.
- The granular bainite formation and retained austenite fraction are favored in the coarser austenitic microstructure obtained at higher hot forging temperatures.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sourmail, T.; Smanio, V.; Ziegler, C.; Heuer, V.; Kuntz, M.; Caballero, F.G.; Garcia-Mateo, C.; Cornide, J.; Elvira, R.; Leiro, A.; et al. Novel Nanostructured Bainitic Steel Grades to Answer the Need for High-Performance Steel Components; European Commission: Brussels, Belgium, 2013. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Cornide, J.; Allain, S.; Puerta, J.; Crouvizer, M.; Mastrorillo, T.; Jantzen, L.; Vuorinen, E.; Lindgren, L.E.; et al. New Advanced Ultra High Strength Bainitic Steels: Ductility and Formability. Res. Fund Coal Steel Eur. Commision 2013, 1–124. [Google Scholar] [CrossRef]
- Cao, J.; Yan, J.; Zhang, J.; Yu, T. Effects of thermomechanical processing on microstructure and properties of bainitic work hardening steel. Mater. Sci. Eng. A 2015, 639, 192–197. [Google Scholar] [CrossRef]
- Liang, X.; Deardo, A.J. A study of the influence of thermomechanical controlled processing on the microstructure of bainite in high strength plate steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 5173–5184. [Google Scholar] [CrossRef]
- Zhao, L.; Qian, L.; Liu, S.; Zhou, Q.; Meng, J.; Zheng, C.; Zhang, F. Producing superfine low-carbon bainitic structure through a new combined thermo-mechanical process. J. Alloys Compd. 2016, 685, 300–303. [Google Scholar] [CrossRef]
- Sourmail, T. Bainite and Superbainite in Long Products and Forged Applications. HTM J. Heat Treat. Mater. 2017, 72, 371–378. [Google Scholar] [CrossRef]
- Bleck, W.; Bambach, M.; Wirths, V.; Stieben, A. Microalloyed Engineering Steels with Improved Performance—An Overview. HTM J. Heat Treat. Mater. 2017, 72, 346–354. [Google Scholar] [CrossRef]
- Fang, H.S.; Chun, F.E.; Zheng, Y.K.; Yang, Z.G.; Bai, B.Z. Creation of Air-Cooled Mn Series Bainitic Steels. J. Iron Steel Res. Int. 2008, 15, 1–9. [Google Scholar] [CrossRef]
- Hasler, S.; Roelofs, H.; Lembke, M.; Caballero, F.G. New air cooled steels with outstanding impact toughness. In Proceedings of the 3nd International Conferente On Steels in Cars and Trucks, Salzburg, Austria, 5–9 June 2011; p. 9. [Google Scholar]
- ASM. Handbook. Volume 14: Forming and Forging; ASM International: Materials Park, OH, USA, 1993; Volume 14, ISBN 0-87170-007-7. [Google Scholar] [CrossRef]
- Fernández, J.; Illescas, S.; Guilemany, J.M. Effect of microalloying elements on the austenitic grain growth in a low carbon HSLA steel. Mater. Lett. 2007, 61, 2389–2392. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, F.; Zheng, C.; Zhang, M.; Lv, B.; Qu, L. Study on hot deformation behaviour and processing maps of low carbon bainitic steel. Mater. Des. 2015, 66, 258–266. [Google Scholar] [CrossRef]
- Rancel, L.; Gómez, M.; Medina, S.F. Influence of Microalloying Elements (Nb, V, Ti) on Yield Strength in Bainitic Steels. Steel Res. Int. 2008, 79, 947–953. [Google Scholar] [CrossRef]
- Zhao, H.; Wynne, B.P.; Palmiere, E.J. Effect of austenite grain size on the bainitic ferrite morphology and grain refinement of a pipeline steel after continuous cooling. Mater. Charact. 2017, 123, 128–136. [Google Scholar] [CrossRef]
- Li, X.; Xia, D.; Wang, X.; Wang, X.; Shang, C. Effect of austenite grain size and accelerated cooling start temperature on the transformation behaviors of multi-phase steel. Sci. China Technol. Sci. 2013, 56, 66–70. [Google Scholar] [CrossRef]
- Rancel, L.; Gómez, M.; Medina, S.F.; Gutierrez, I. Measurement of bainite packet size and its influence on cleavage fracture in a medium carbon bainitic steel. Mater. Sci. Eng. A 2011, 530, 21–27. [Google Scholar] [CrossRef]
- Capdevila, C.; Caballero, F.G. Carlos García de Andrés Austenite Grain Size Effects on Isothermal Allotriomorphic Ferrite Formation in 0.37C-1.45Mn-0.11V Microalloyed Steel. Mater. Trans. 2003, 44, 1087–1095. [Google Scholar] [CrossRef]
- Ferry, M.; Thompson, M.; Manohar, P.A. Decomposition of coarse grained austenite during accelerated cooling of C-Mn steels. ISIJ Int. 2002, 42, 86–93. [Google Scholar] [CrossRef][Green Version]
- Celada-Casero, C.; Sietsma, J.; Santofimia, M.J. The role of the austenite grain size in the martensitic transformation in low carbon steels. Mater. Des. 2019, 167. [Google Scholar] [CrossRef]
- Poliak, E.I.; Jonas, J.J. A one-parameter approach to determining the critical conditions for the initiation of dynamic recrystallization. Acta Mater. 1996, 44, 127–136. [Google Scholar] [CrossRef]
- McQueen, H.J.; Yue, S.; Ryan, N.D.; Fry, E. Hot working characteristics of steels in austenitic state. J. Mater. Process. Technol. 1995, 53, 293–310. [Google Scholar] [CrossRef]
- ASM International (Ed.) Volume 9: Metallography and Microstructure, 9th ed.; ASM International: Materials Park, OH, USA, 2004; Volume 9, ISBN 0871707063. [Google Scholar]
- ASTM Standard. E112-12:Standard Test Methods for Determining Average Grain Size; ASTM International: Materials Park, OH, USA, 2012. [Google Scholar] [CrossRef]
- Voort, G. Vander Introduction to quantitative metallography. Buehler TechNotes 2015, 1, 5. [Google Scholar]
- ASTM International. E384 Standard Test Method for Microindentation Hardness of Materials; ASTM International: Materials Park, OH, USA, 2017; pp. 1–40. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Leineweber, A.; Evans, J.S.O. Rietveld Refinement—Practical Powder Diffraction Pattern Analysis Using TOPAS; De Gruyter, Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; ISBN 9783110456219. [Google Scholar] [CrossRef]
- Razzak, M.A.; Perez, M.; Sourmail, T.; Cazottes, S.; Frotey, M. Preventing Abnormal Grain Growth of Austenite in Low Alloy Steels. ISIJ Int. 2014, 54, 1927–1934. [Google Scholar] [CrossRef]
- Totten, G.E.; Xie, L.; Funatani, K. Handbook of Mechanical Alloy Design; CRC Press: New York, NY, USA, 2003; ISBN 978-0-8247-4308-6. [Google Scholar] [CrossRef]
- Zener, C. Theory of Growth of Spherical Precipitates from Solid Solution. J. Appl. Phys. 1949, 20, 950. [Google Scholar] [CrossRef]
- Guo, L.; Roelofs, H.; Lembke, M.I.; Bhadeshia, H.K.D.H. Effect of manganese sulphide particle shape on the pinning of grain boundary. Mater. Sci. Technol. 2017, 33, 1013–1018. [Google Scholar] [CrossRef]
- Totten, G.E. Steel Heat Treatment; Taylor & Francis Group: Abingdon, UK, 2006; pp. 1–820. ISBN 978-0-8493-8455-4. [Google Scholar]
- Li, M.; Li, J.; Qiu, D.; Zheng, Q.; Wang, G.; Zhang, M.X. Crystallographic study of grain refinement in low and medium carbon steels. Philos. Mag. 2016, 96, 1556–1578. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, H.; Masse, J.P.; Bouaziz, O.; Gachet, G. The effect of prior ferrite formation on bainite and martensite transformation kinetics in advanced high-strength steels. Acta Mater. 2013, 61, 6025–6036. [Google Scholar] [CrossRef]
- Humphreys, J.; Rohrer, G.S.; Rollett, A. Recrystallization and Related Annealing Phenomena. In Recrystallization and Related Annealing Phenomena, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–734. ISBN 978-0-08-098235-9. [Google Scholar]
- Derby, B. The dependence of grain size on stress during dynamic recrystallisation. Acta Metall. Mater. 1991, 39, 955–962. [Google Scholar] [CrossRef]
- Krauss, G. Steels: Processing, Structure, and Performance, 2nd ed.; ASM, I.H.C., Ed.; ASM International: Materials Park, OH, USA, 2005. [Google Scholar]
- Sietsma, J. 14—Nucleation and growth during the austenite-to-ferrite phase transformation in steels after plastic deformation. In Phase Transformations in Steels; Woodhead Publishing Limited: Sawstone, Cambridge, UK, 2012; Volume 1, pp. 505–526. ISBN 9781845699703. [Google Scholar] [CrossRef]
- Aranda, M.M.; Kim, B.; Rementeria, R.; Capdevila, C.; De Andrés, C.G. Effect of prior austenite grain size on pearlite transformation in A hypoeuctectoid Fe-C-Mn steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 1778–1786. [Google Scholar] [CrossRef]
- Lan, H.F.; Du, L.X.; Liu, X.H. Microstructure and mechanical properties of a low carbon bainitic steel. Steel Res. Int. 2013, 84, 352–361. [Google Scholar] [CrossRef]
- Quidort, D.; Brechet, Y.J.M. Isothermal growth kinetics of bainite in 0.5% C steels. Acta Mater. 2001, 49, 4161–4170. [Google Scholar] [CrossRef]
- Lambert, A.; Drillet, J.; Gourgues, A.F.; Sturel, T.; Pineau, A. Microstructure of martensite-austenite constituents in heat affected zones of high strength low alloy steel welds in relation to toughness properties. Sci. Technol. Weld. Join. 2000, 5, 168–173. [Google Scholar] [CrossRef]
- Caballero, F.G. Carbide-free bainite in steels. In Phase Transformations in Steels; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1, pp. 436–467. ISBN 9781845699703. [Google Scholar] [CrossRef]
- Dyson, D.J. Holmes B effect of alloying additions on the lattice parameter of austenite. J. Iron Steel Inst. 1970, 208, 469–474. [Google Scholar]
- Xiong, X.C.; Chen, B.; Huang, M.X.; Wang, J.F.; Wang, L. The effect of morphology on the stability of retained austenite in a quenched and partitioned steel. Scr. Mater. 2013, 68, 321–324. [Google Scholar] [CrossRef]
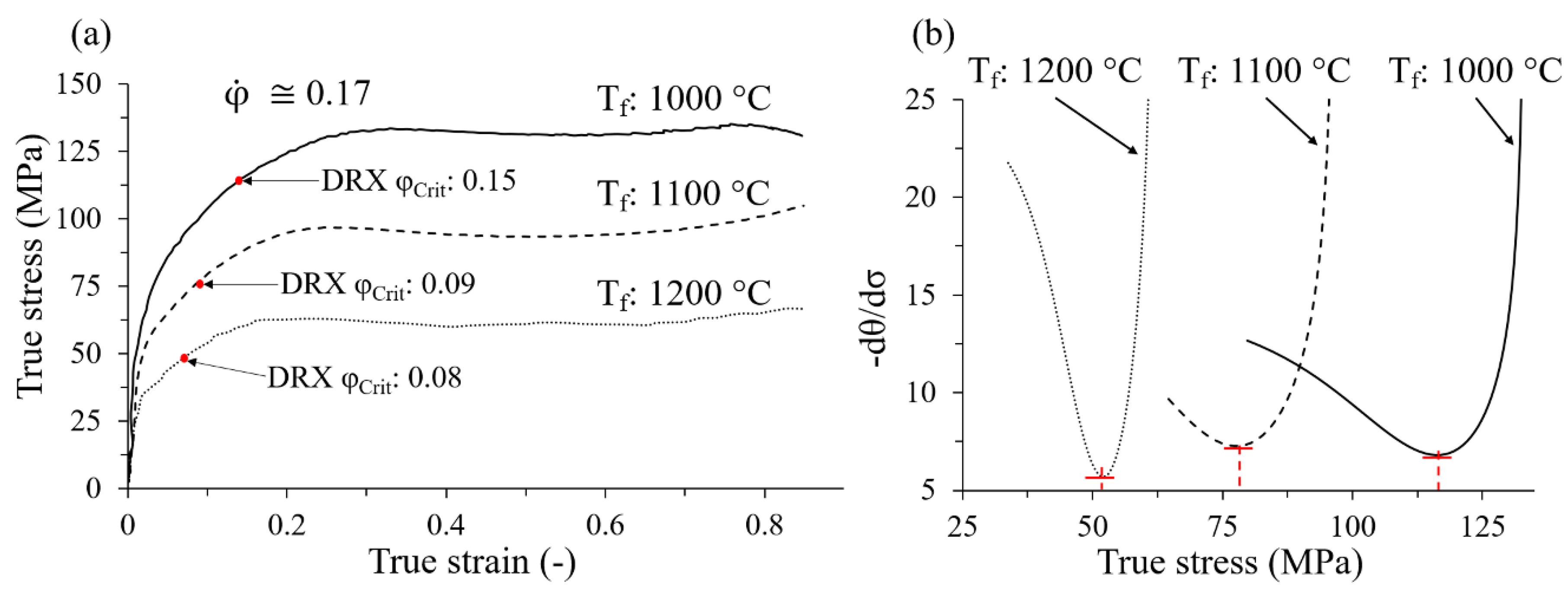
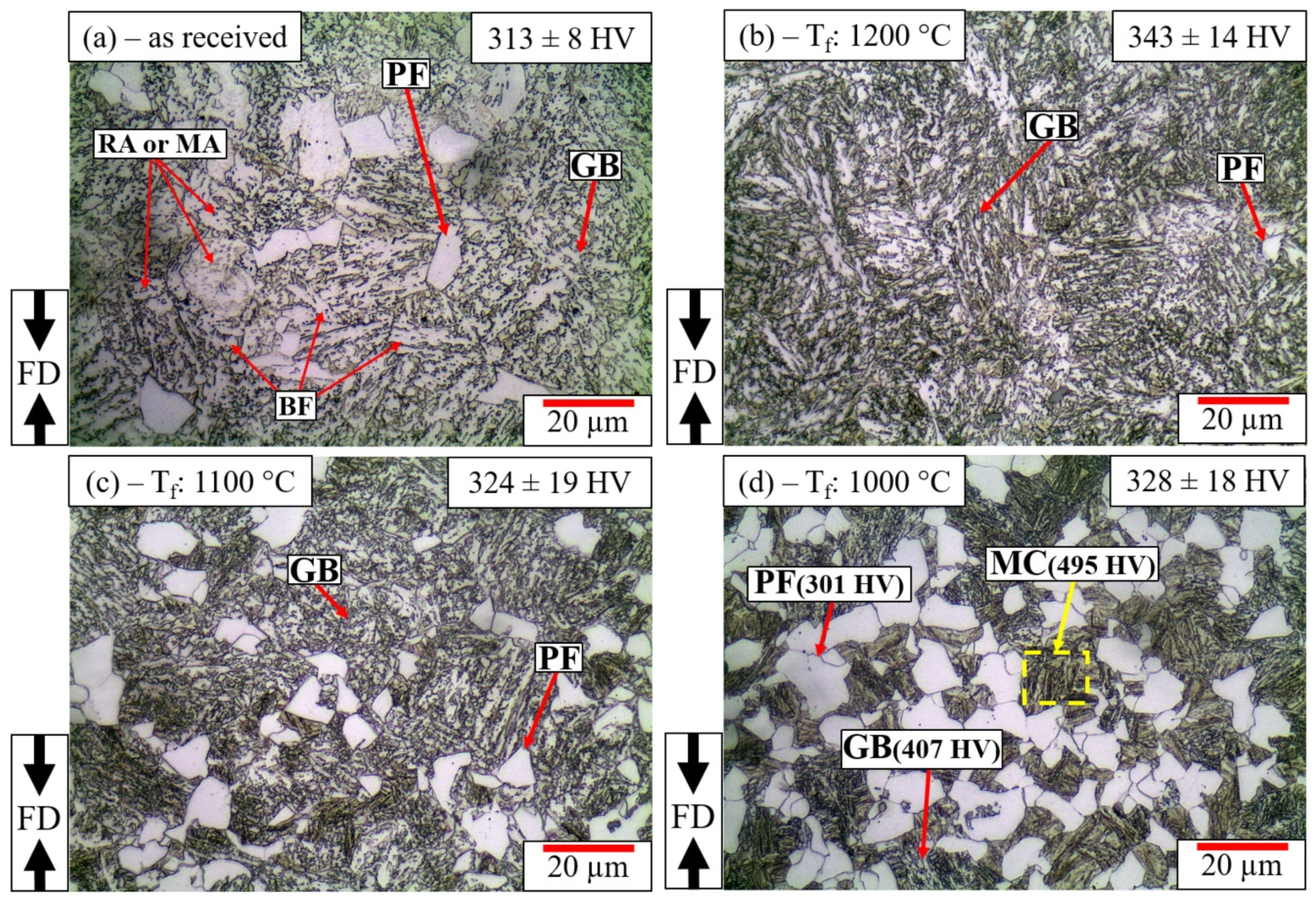
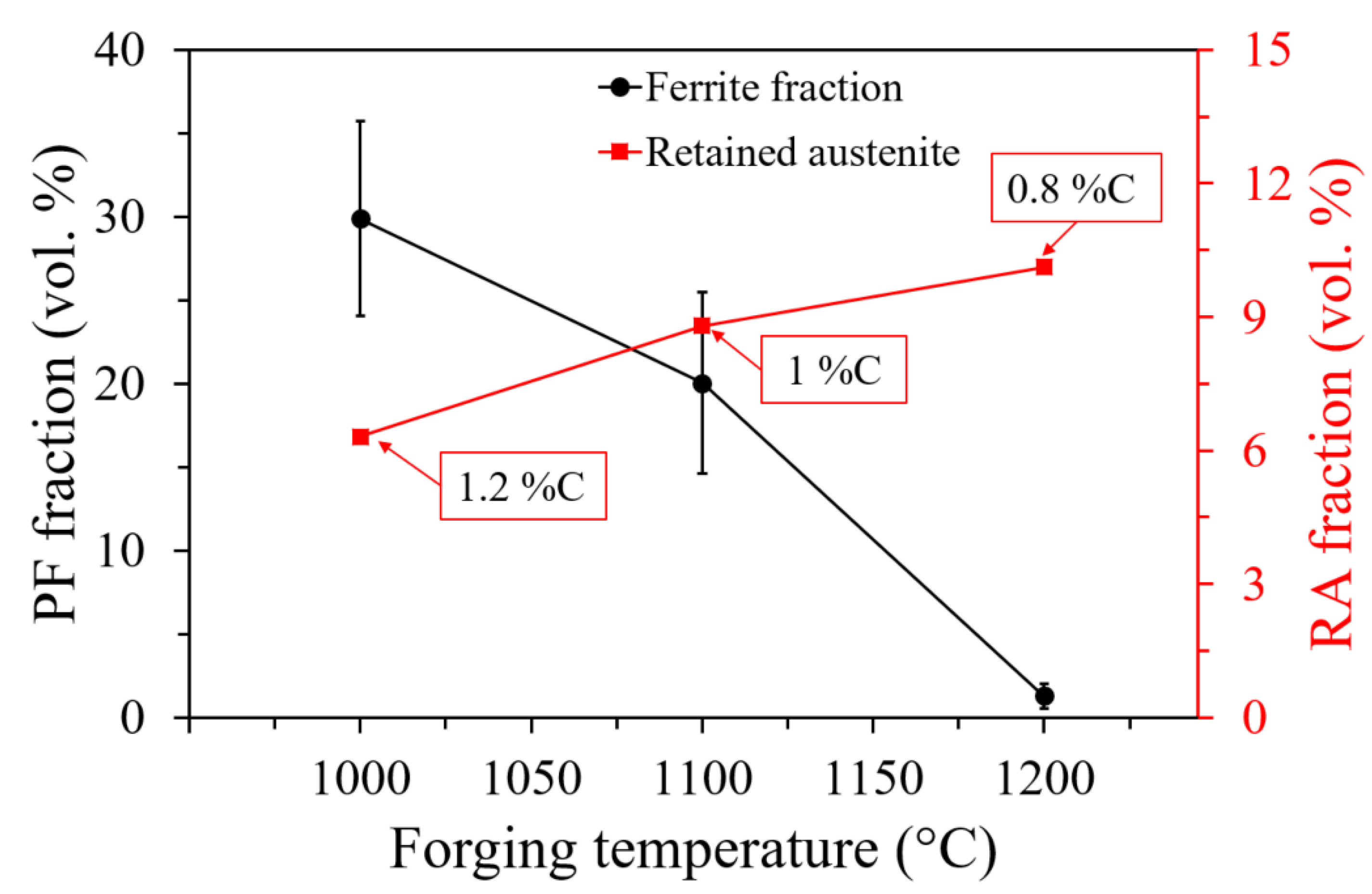
| C | Si | Mn | S | Ni | Cr | Cu | Mo | Al | Ti | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.18 | 1.19 | 1.42 | 0.015 | 0.063 | 1.17 | 0.10 | 0.27 | 0.005 | 0.004 | 0.01 | Balance |
| Forging Temperature | PAGS (μm) | Microstructure | PF Fraction (vol. %) | RA (vol. %) | CRA Content (ma. %) | Overall Hardness (HV 1) |
|---|---|---|---|---|---|---|
| As received | 24 ± 3.2 | GB + PF | 3.5 ± 1.8 | 10.5 | 0.8 | 313 ± 8 |
| 1000 °C | 11 ± 1.7 | PF + MC + GB | 30.0 ± 5.8 | 6.3 | 1.2 | 328 ± 18 |
| 1100 °C | 13 ± 3.0 | GB + PF | 20.0 ± 5.4 | 8.8 | 1.0 | 324 ± 19 |
| 1200 °C | 28 ± 5.3 | GB + PF | 1.3 ± 0.8 | 10.1 | 0.8 | 343 ± 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, A.C.d.F.; Bevilaqua, W.L.; Dias, V.W.; de Castro, P.J.; Epp, J.; Rocha, A.d.S. Influence of Hot Forging Parameters on a Low Carbon Continuous Cooling Bainitic Steel Microstructure. Metals 2020, 10, 601. https://doi.org/10.3390/met10050601
Silveira ACdF, Bevilaqua WL, Dias VW, de Castro PJ, Epp J, Rocha AdS. Influence of Hot Forging Parameters on a Low Carbon Continuous Cooling Bainitic Steel Microstructure. Metals. 2020; 10(5):601. https://doi.org/10.3390/met10050601
Chicago/Turabian StyleSilveira, Antonio Carlos de Figueiredo, William Lemos Bevilaqua, Vinicius Waechter Dias, Pedro José de Castro, Jeremy Epp, and Alexandre da Silva Rocha. 2020. "Influence of Hot Forging Parameters on a Low Carbon Continuous Cooling Bainitic Steel Microstructure" Metals 10, no. 5: 601. https://doi.org/10.3390/met10050601
APA StyleSilveira, A. C. d. F., Bevilaqua, W. L., Dias, V. W., de Castro, P. J., Epp, J., & Rocha, A. d. S. (2020). Influence of Hot Forging Parameters on a Low Carbon Continuous Cooling Bainitic Steel Microstructure. Metals, 10(5), 601. https://doi.org/10.3390/met10050601







