Influence of B and Nb Additions and Heat Treatments on the Mechanical Properties of Cu-Ni-Co-Cr-Si Alloy for High Pressure Die Casting Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Heat Treatments
2.3. Sample Characterization
3. Results and Discussion
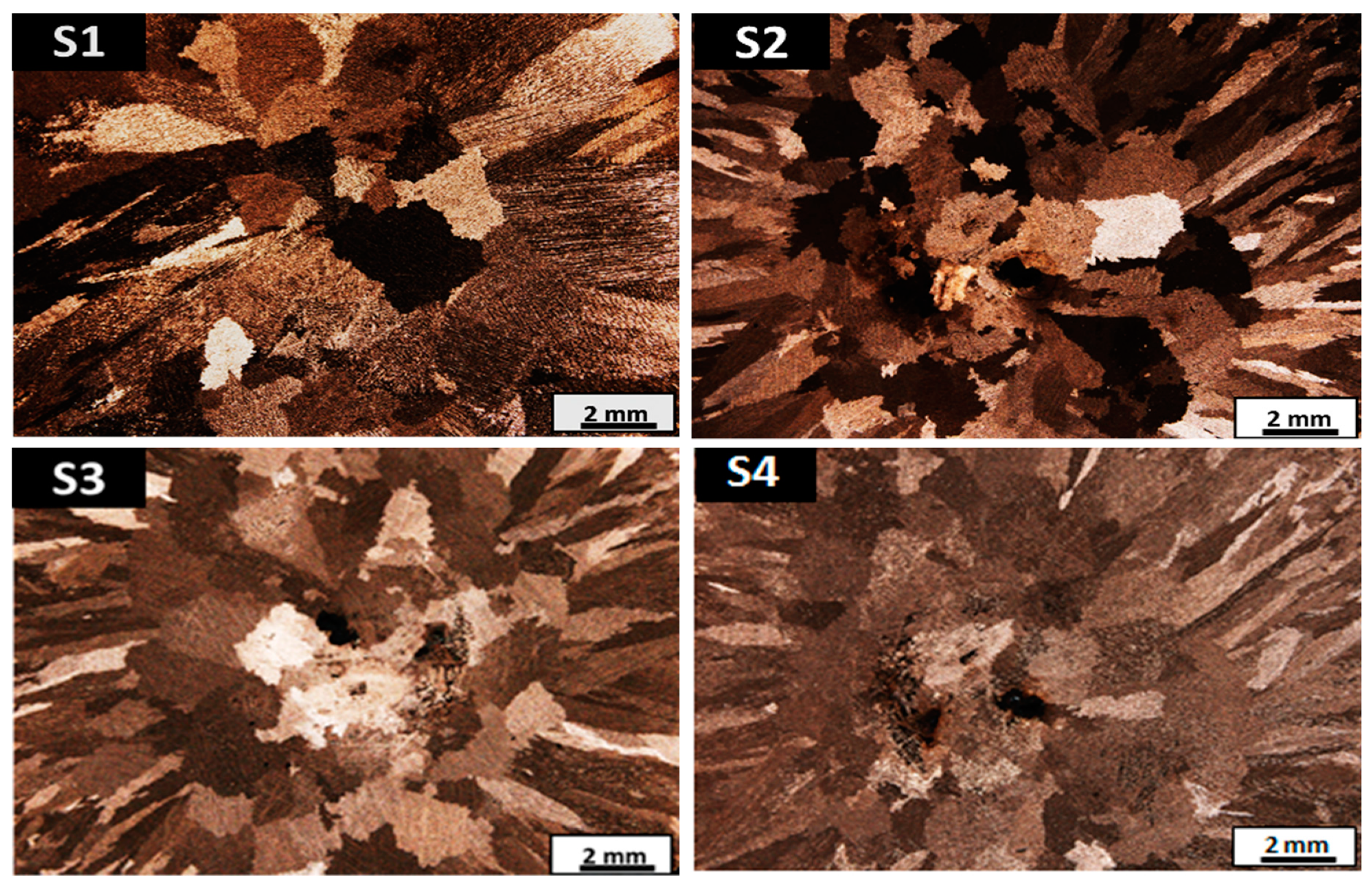
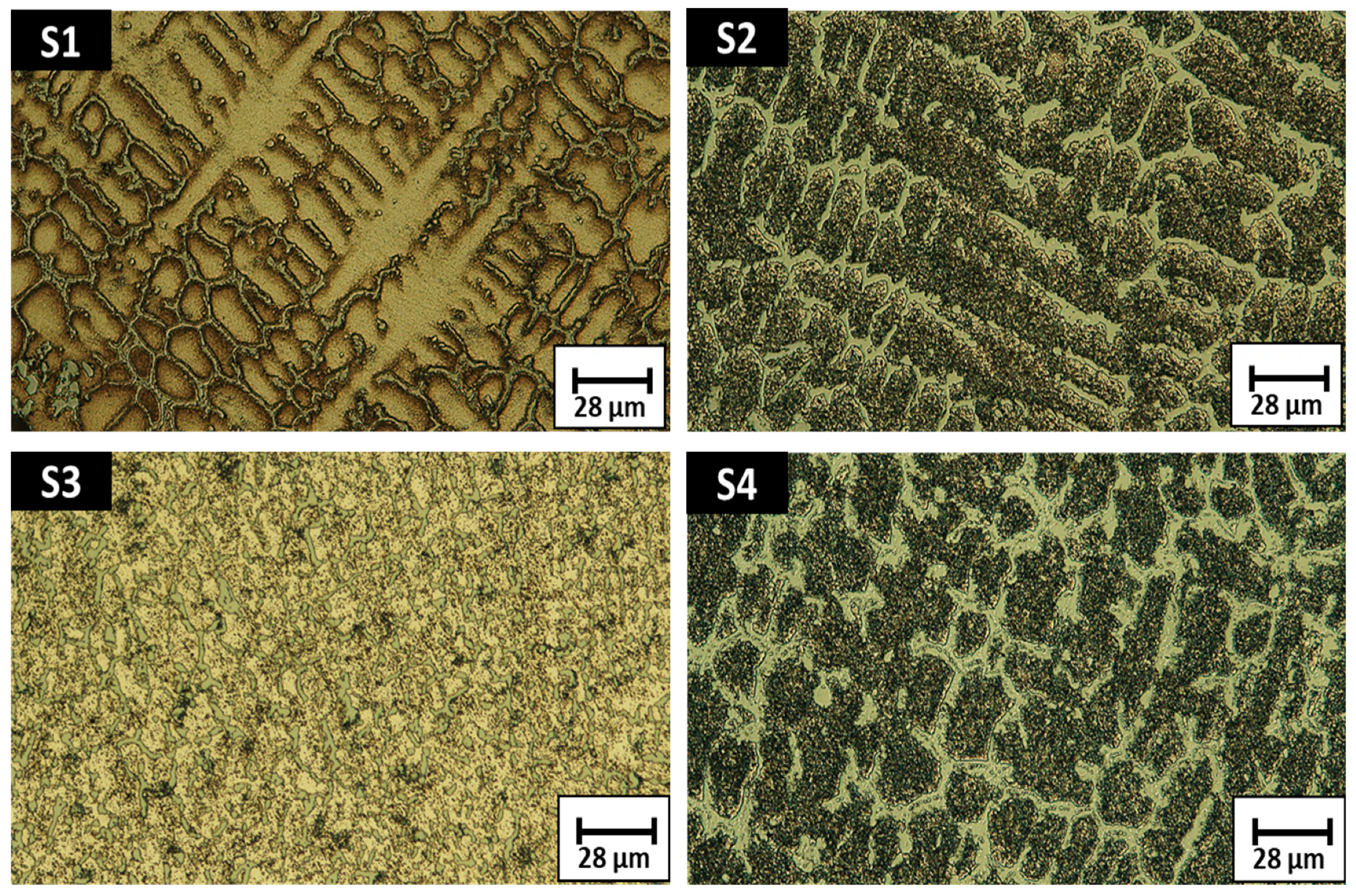
3.1. Macrostructure and Microstructure Analysis
3.2. Phase Analysis (XRD)
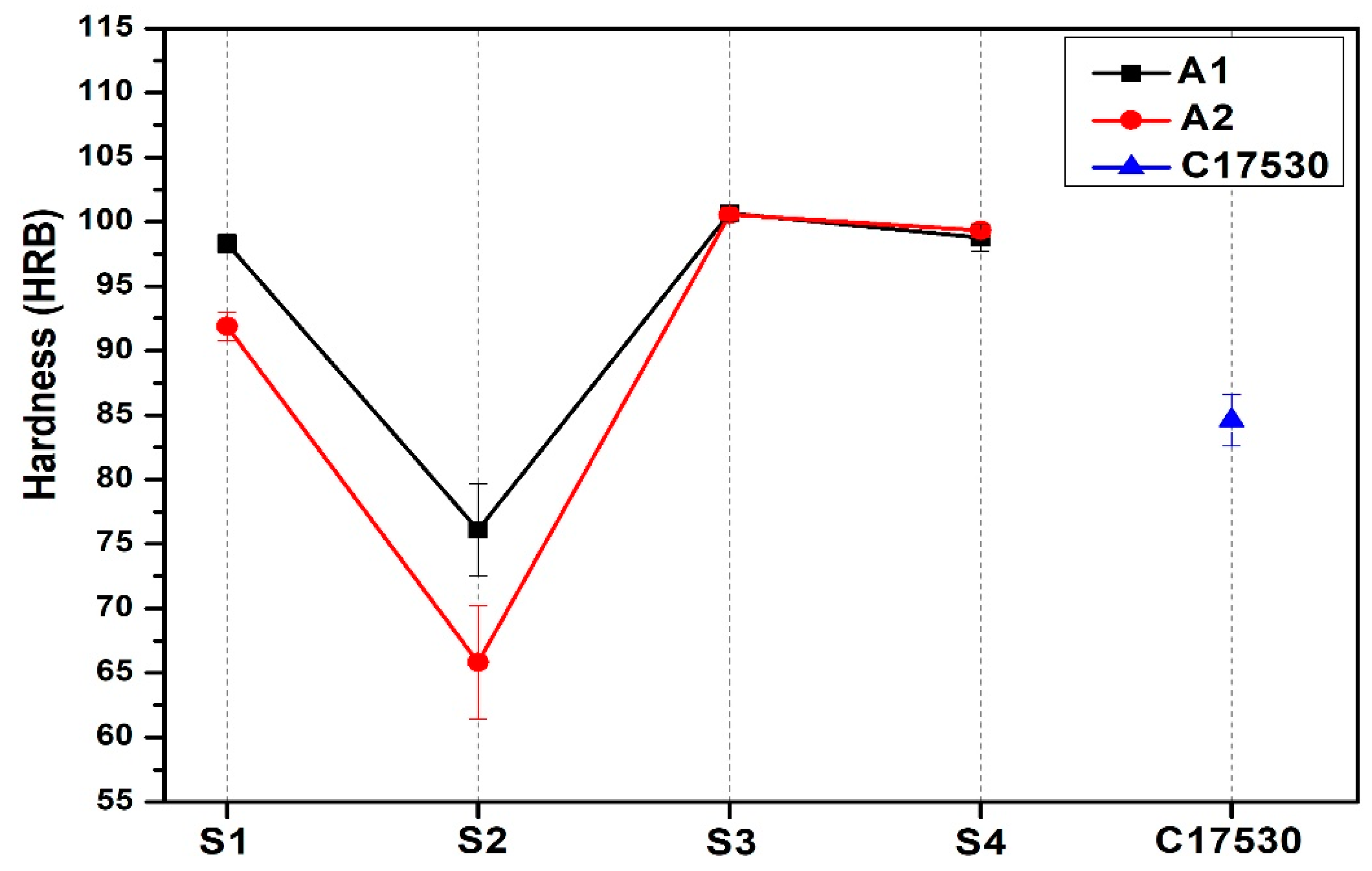
3.3. Hardness Test
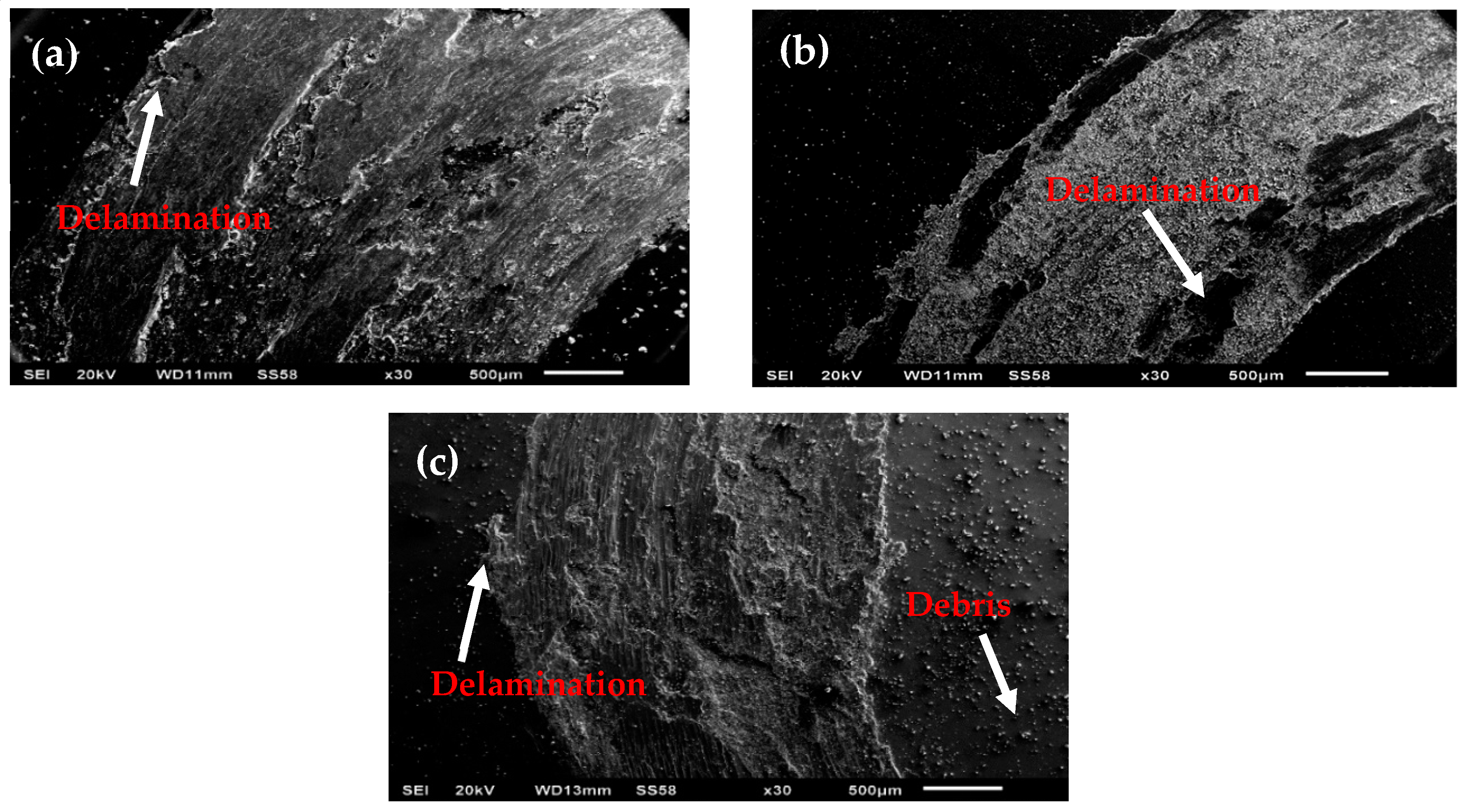
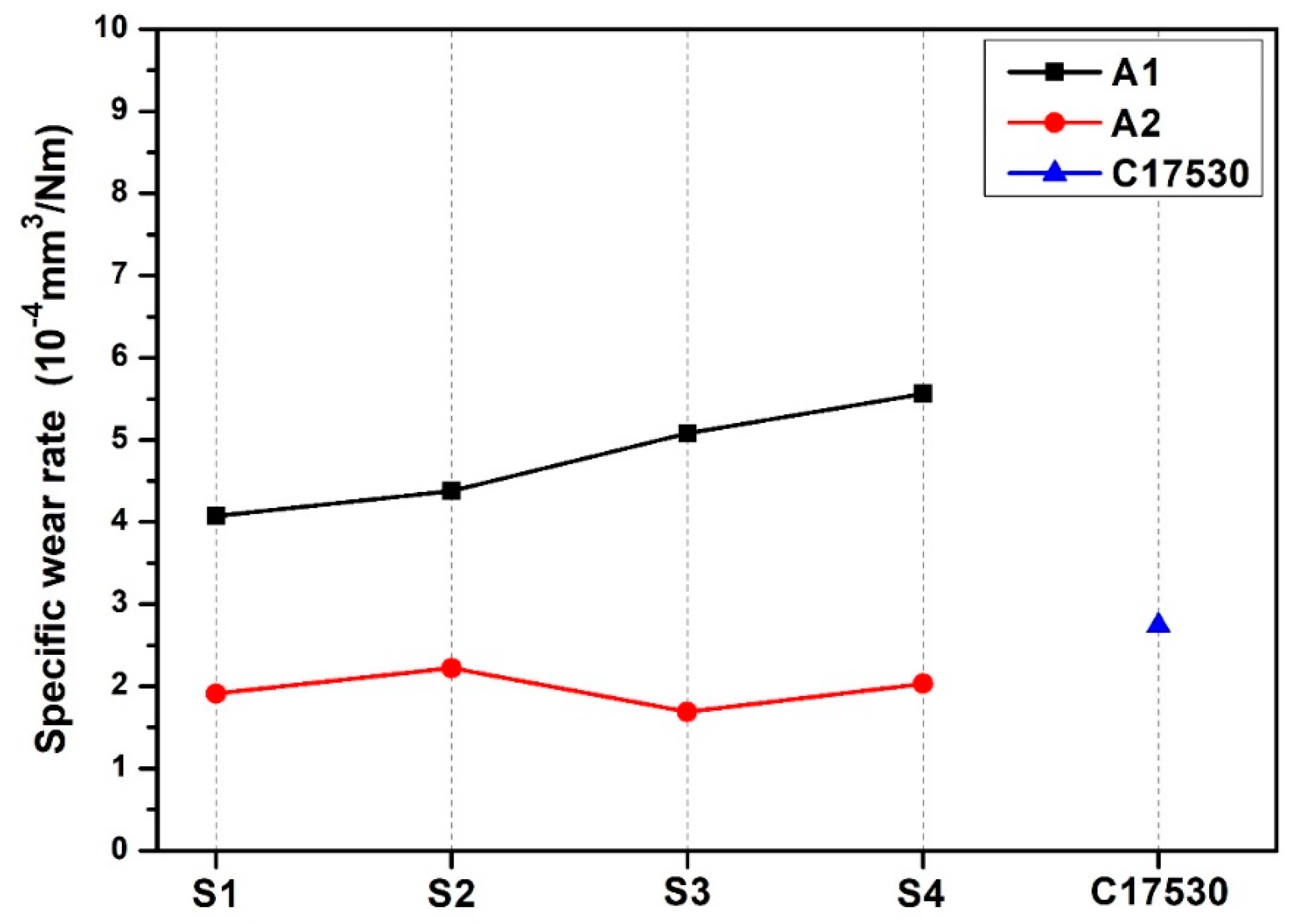
3.4. Analysis of Volume Loss and Friction on the Pin-On-Disk Machine
4. Conclusions
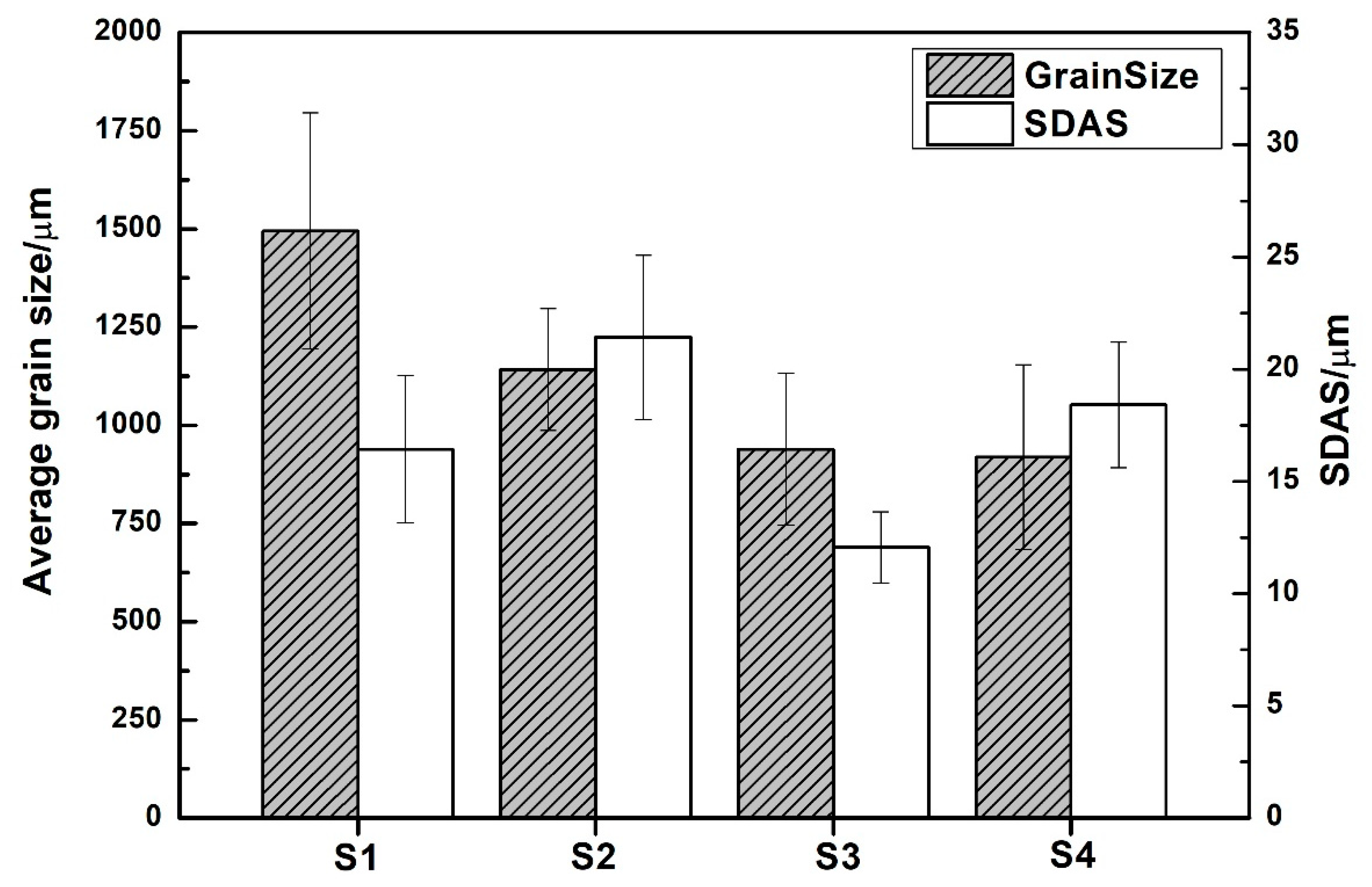
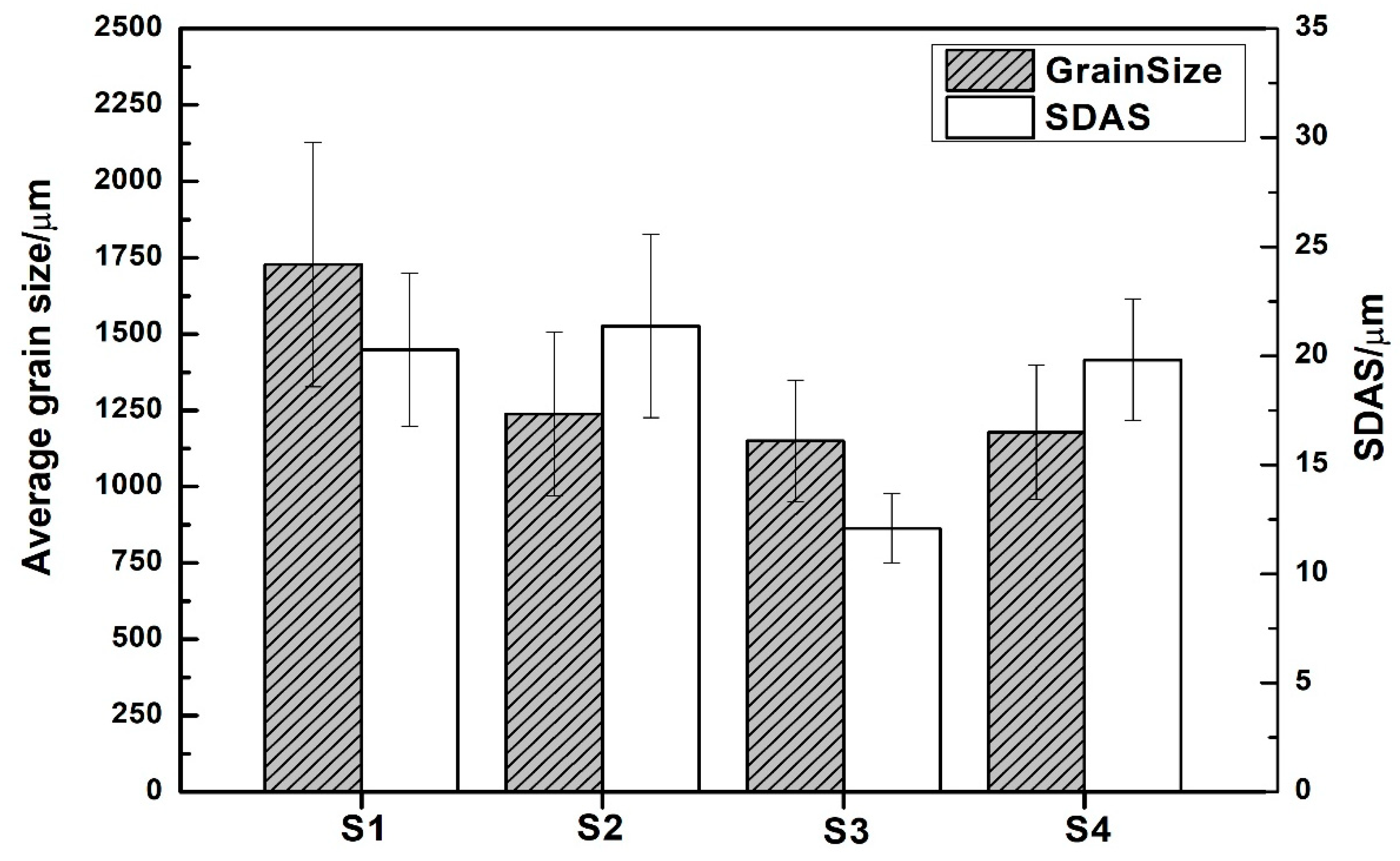
- There is an evolution of the microstructure due to B and Nb additions and heat treatments; however, this does not seem to have any effect on the SDAS.
- There is a change in grain size as heat treatments are applied; it decreases from 1500 to 900 μm for A1 alloy, and from 1700 to 1100 μm for A2 alloy.
- There are some precipitate phase growths in the grain boundary after conducting aging heat treatments: B1Cr1, CoNi, Cu0.9Ni0.1 and B1.43Ni4.29Si2 for A1 alloy, and CrSi2, Nb6Ni16Si7, Cu3Fe17 and CoNi for A2 alloy.
- B and Nb additions with the heat treatments increased HRB hardness, with A1S3 having the highest result of 100.7 ± 0.57 HRB, which is an increment with the base alloy, and higher than the commercial alloy C17530 85 ± 0.57 HRB.
- Specific wear rate was decreased, with B additions having the best result in the A1S1 condition with a specific wear rate of 4.07 × 10−4 mm3/Nm. With Nb additions, specific wear rate increased, with the A2S3 condition 1.69 × 10−4 mm3/Nm having the best result, while for the C17530 alloy, this was 2.74 × 10−4 mm3/Nm.
5. Patent
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collini, L. Copper Alloys—Early Applications and Current Performance—Enhancing Processes; InTech: Rijeka, Croatia, 2012; Volume 11, pp. 12–31. [Google Scholar]
- Li, D.M.; Wang, Q.; Jiang, B.B.; Li, X.N.; Zhou, W.L.; Dong, C. Minor-alloyed Cu-Ni-Si alloys with high hardness and electric conductivity designed by a cluster formula approach. Prog. Nat. Sci. Mater. 2017, 27, 467–473. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chen, H.-G.; Gu, J.-W.; Hsu, C.-E.; Wu, C.-Y. Improvement in strength and thermal conductivity of powder metallurgy produced Cu-Ni-Si-Cr alloy by adjusting Ni/Si weight ratio and hot forging. J. Alloys Compd. 2015, 633, 59–64. [Google Scholar] [CrossRef]
- Lei, Q.; Li, S.Y.; Zhu, J.L.; Xiao, Z.; Zhang, F.F.; Li, Z. Microstructural evolution, phase transition, and physics properties of a high strength Cu-Ni-Si-Al alloy. Mater. Charact. 2019, 147, 315–323. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Xiao, T.; Pang, Y.; Xiang, Z.Q.; Qiu, W.T.; Xiao, Z. A new ultrahigh strength Cu-Ni-Si alloys. Intermetallics 2013, 42, 77–84. [Google Scholar] [CrossRef]
- Lee, E.; Han, S.; Euh, K.; Lim, S.; Kim, S. Effect of Ti addition on tensile properties of Cu-Ni-Si alloys. Met. Mater. Int. 2011, 17, 569–576. [Google Scholar] [CrossRef]
- Wang, H.S.; Chen, H.G.; Gu, J.W.; Hsu, C.E.; Wu, C.Y. Effect of heat treatment processes on the microstructures and properties of powder metallurgy produced Cu-Ni-Si-Cr alloy. Mater. Sci. Eng. A 2014, 619, 221–227. [Google Scholar] [CrossRef]
- Wang, W.; Kang, H.J.; Chen, Z.N.; Chen, Z.J.; Zou, C.L.; Li, R.G. Effects of Cr and Zr additions on microstructure and properties of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2016, 673, 378–390. [Google Scholar] [CrossRef]
- Han, S.Z.; Gu, J.H.; Lee, J.H.; Que, Z.P.; Shin, J.H.; Lim, S.H.; Kim, S.S. Effect of V addition on hardness and electrical conductivity in Cu-Ni-Si alloys. Met. Mater. Int. 2013, 19, 637–641. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.J.; Mi, X.J.; Peng, L.J.; Xie, H.F.; Kang, Y.L. Effect of Co addition on microstructure and properties of Cu-Ni-Si alloy. Adv. Mater. Process. 2018, 33, 353–360. [Google Scholar]
- Tsubakino, H.; Nozato, R.; Yamamoto, A. Precipitation sequence for simultaneous continuous and discontinuous modes in CuBe binary alloys. Mater. Sci. J. 2013, 9, 288–294. [Google Scholar]
- Ward, E.; Okun, A.; Ruder, A.; Fingerhut, M.; Steenland, K. A mortality study of workers at seven beryllium processing plants. Am. J. Ind. Med. 1992, 22, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Ward, E. Lung cancer incidence among patients with beryllium disease: A cohort mortality study. J. Natl. Cancer Inst. 1991, 83, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, W.; Ward, E.; Steenland, K.; Petersen, M.R. Lung cancer case-control study of beryllium disease. Am. J. Ind. Med. 2001, 39, 133–144. [Google Scholar] [CrossRef]
- Boffetta, P.; Fordyce, T.A.; Mandel, J.S. A mortality study of beryllium workers. Cancer Med. 2016, 512, 3596–3605. [Google Scholar] [CrossRef] [PubMed]
- Mayo, D.; Mariano, A.; Maffia Ernesto, G. Efecto del Solubilizado en la Estructura de la Aleación Cu-Cr-Zr; ProInTec I&D, Departamento de Mecánica, Facultad de Ingeniería, Universidad Nacional de la Plata (UNLP), 4 Jornadas ITE: Buenos Aires, Argentina, 2017; pp. 539–543. [Google Scholar]
- Meijering, J.L. Calculation of the nickel-chromium-copper phase diagram from binary data. Acta Metall. 1957, 5, 257–264. [Google Scholar] [CrossRef]
- González Martínez, R. Descomposición de Fases en Aleaciones Cu-Ni-Cr; Departamento de Ingeniería Metalúrgica, Instituto Politécnico Nacional: Ciudad de México, México, 2001. [Google Scholar]
- Feloy, L.; Maffia Ernesto, G.; Tovio, D.; González, A. Influence of Temperature on the Hardening of the Cu-Ni-Si-Cr Alloy; Mechanics Department, LIMF, Faculty of Engineering, National University of La Plata (UNLP): Buenos Aires, Argentina, 2016; pp. 1–7. [Google Scholar]
- John, F.K.; Quentin, F. Ingerson, Method of Heat-Treating Copper Base Alloy. U.S. Patent No. 3,072,508, 8 January 1963. [Google Scholar]
- Michael, G. Copper Alloy and Process of Producing and Treating the Same. U.S. Patent No. 1,658,186, 7 February 1928. [Google Scholar]
- Walter, W.E.; Quentin, F. Ingenson, Copper-Nickel-Silicon-Chromium Alloys Having Improved Electrical Conductivity, Amped-Pittsburgh Corporation. U.S. Patent No. 4,191,601, 4 March 1980. [Google Scholar]
- Mejía, J.M.C. Desarrollo de una Aleación de Cobre Libre de Berilio Utilizado en la Industria de hpdc en Cabezales para Pistones Hidráulicos en la Inyección de Aluminio; Universidad Autónoma de Nuevo León: Monterrey, Nuevo León, México, 2018. [Google Scholar]
- Rafael Nunes, J.H. Adams and Mitchell Ammons, Properties and selection: Nonferrous Alloys and Special Purpose Materials; Copyright materials, ASM International Metals Handbook Committee: Kinsman Rd, OH, USA, 1992; Volume 2, pp. 828–832. [Google Scholar]
- Quentin, F. Ingerson, Copper-Nickel-Silicon and Chromium alloy, Amoco Metal Manufacturing. U.S. Patent No. 5028391, 2 July 1991. [Google Scholar]
- Davis, J.R. Copper and Copper Alloys; ASM Handbook International: Kinsman Road, OH, USA, 2001; pp. 31–52. [Google Scholar]
- Norma ASTM E407–07, A. Standard practice for Microetching Metals and Alloys; Copyright ASTM International: West Conshohocken, PA, USA, 2012; pp. 9–10. [Google Scholar]
- Norma ASTM E18–15. Rockwell Hardness and Surface Rockwell Hardness Test; Copyright ASTM International: West Conshohocken, PA, USA, 2015; pp. 3–8. [Google Scholar]
- Norma ASTM G99–95. Standard Test Method for Wear Testing with a Pin on Disk Apparatus; Copyright ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Lozovoi, A.Y.; Paxton, A.T. Boron in copper: A perfect misfit in the bulk and cohesion enhancer at a grain boundary. Phys. Rev. B 2008, 77, 1–14. [Google Scholar] [CrossRef]



| Alloy | Ni | Co | Cr | Si | Fe | B | Nb | Cu |
|---|---|---|---|---|---|---|---|---|
| A1 | 9 | 1 | 1.6 | 2 | 1.3 | 0.25 | - | Balance |
| A2 | 9 | 1 | 1.6 | 2 | 0.1 | - | 0.20 | Balance |
| Sample | AC/HT | Temperature (°C) | Time (Min) | Analysis Condition |
|---|---|---|---|---|
| S1 | AC | 1300 | - | AC |
| S2 | TS | 925 | 120 | AC + TS |
| S3 | TA1 | 450 | 100 | AC + TS+ TA1 |
| S4 | TA2 | 550 | 180 | AC + TS + TA1 + TA2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Salgado, D.A.; Juárez-Hernández, A.; Medina-Ortíz, F.; Lara Banda, M.; Hernández-Rodríguez, M.A.L. Influence of B and Nb Additions and Heat Treatments on the Mechanical Properties of Cu-Ni-Co-Cr-Si Alloy for High Pressure Die Casting Applications. Metals 2020, 10, 602. https://doi.org/10.3390/met10050602
Avila-Salgado DA, Juárez-Hernández A, Medina-Ortíz F, Lara Banda M, Hernández-Rodríguez MAL. Influence of B and Nb Additions and Heat Treatments on the Mechanical Properties of Cu-Ni-Co-Cr-Si Alloy for High Pressure Die Casting Applications. Metals. 2020; 10(5):602. https://doi.org/10.3390/met10050602
Chicago/Turabian StyleAvila-Salgado, Denis Ariel, Arturo Juárez-Hernández, Fermín Medina-Ortíz, María Lara Banda, and Marco Antonio Loudovic Hernández-Rodríguez. 2020. "Influence of B and Nb Additions and Heat Treatments on the Mechanical Properties of Cu-Ni-Co-Cr-Si Alloy for High Pressure Die Casting Applications" Metals 10, no. 5: 602. https://doi.org/10.3390/met10050602
APA StyleAvila-Salgado, D. A., Juárez-Hernández, A., Medina-Ortíz, F., Lara Banda, M., & Hernández-Rodríguez, M. A. L. (2020). Influence of B and Nb Additions and Heat Treatments on the Mechanical Properties of Cu-Ni-Co-Cr-Si Alloy for High Pressure Die Casting Applications. Metals, 10(5), 602. https://doi.org/10.3390/met10050602








