The Influence of Vanadium Additions on Isothermally Formed Bainite Microstructures in Medium Carbon Steels Containing Retained Austenite
Abstract
1. Introduction
2. Materials and Methods
3. Results
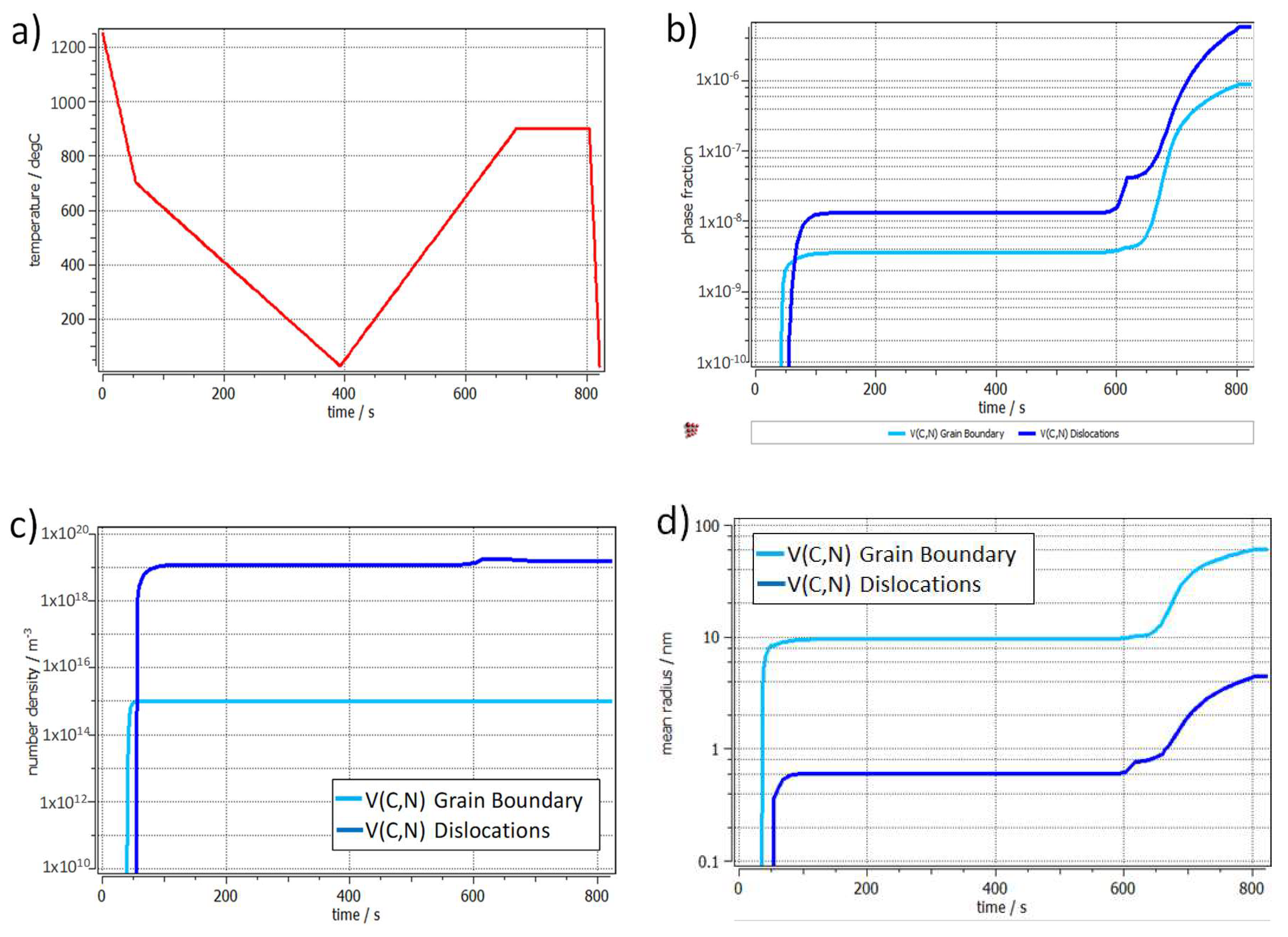
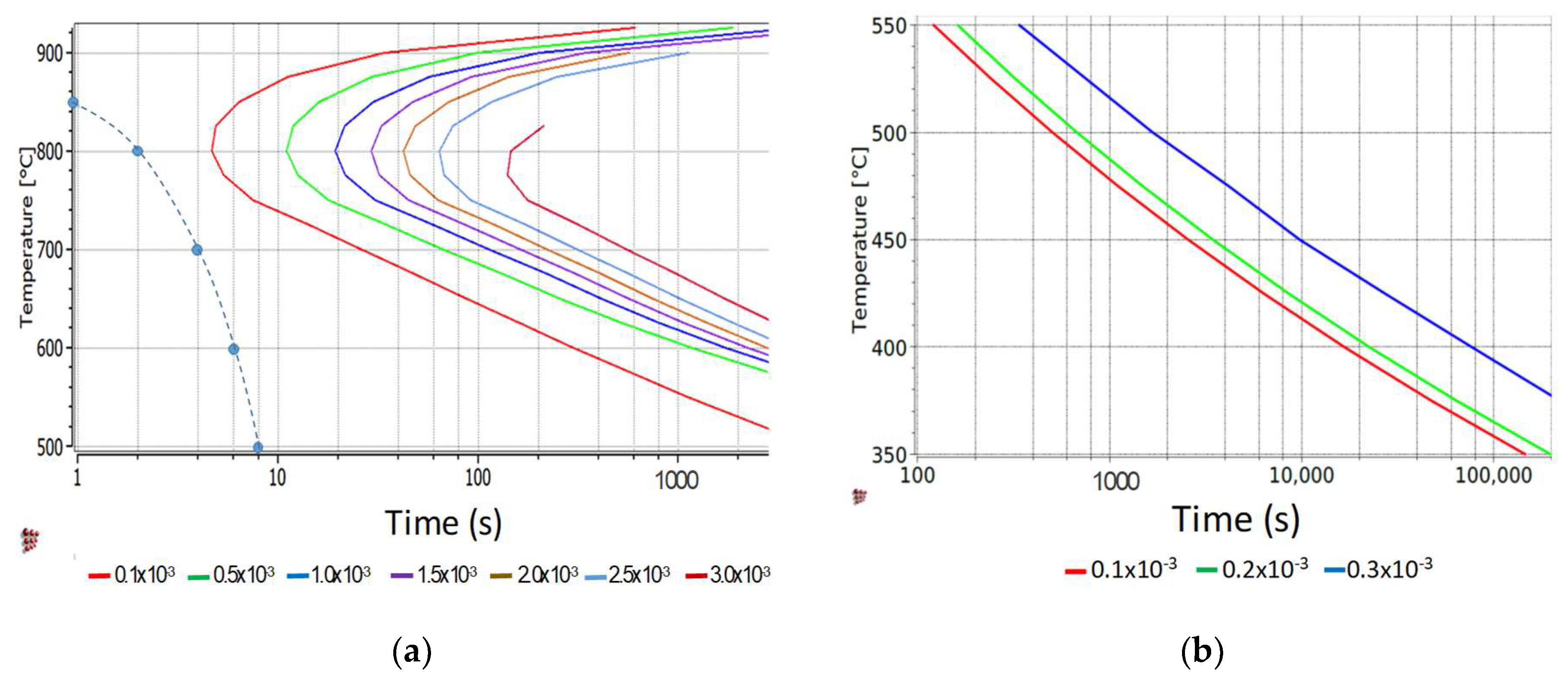
3.1. Definition of the Thermal Cycles
- The specimens should be completely austenitized with an austenite grain size as small as possible in order to maximize the number of diffracting crystallites and minimize texture effects in the HEXRD experiments.
- Vanadium precipitates formed either during hot rolling or during the reheating ramp should be redissolved to maximize the amount of vanadium and carbon in solution before the bainite transformation.
- Any cementite formed during reheating should be dissolved to maximize the interstitial carbon content in austenite before the bainite transformation.
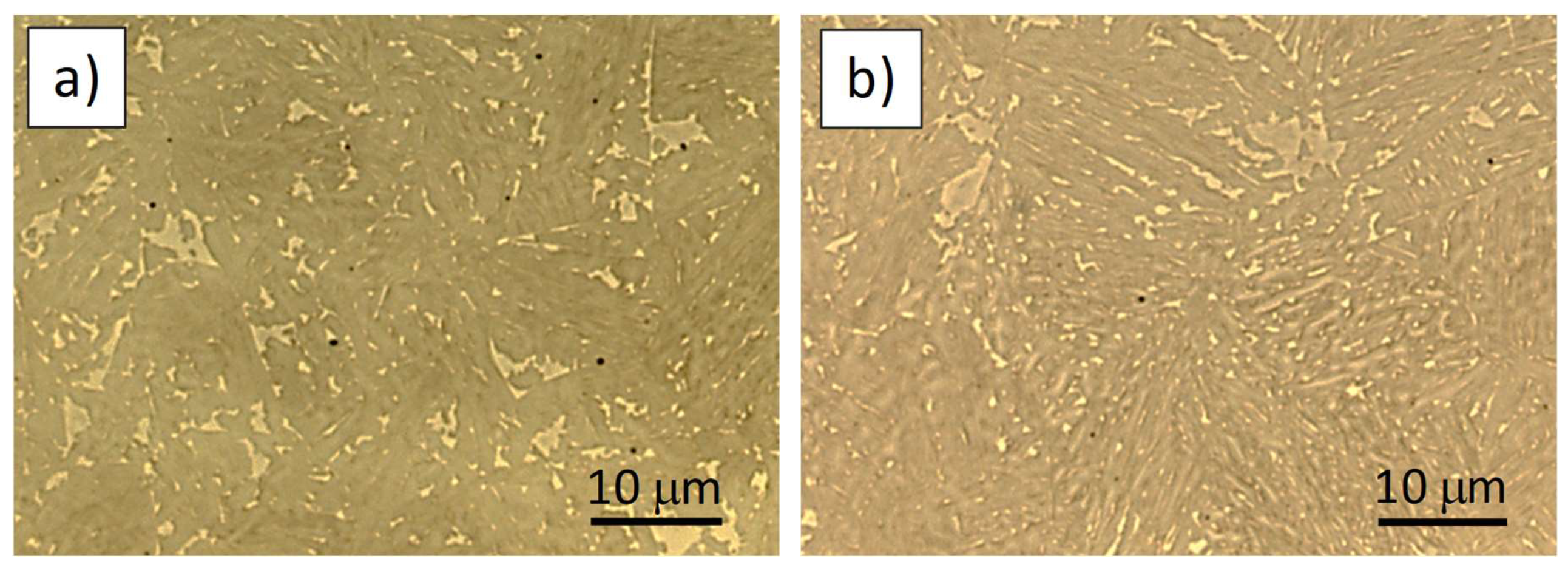
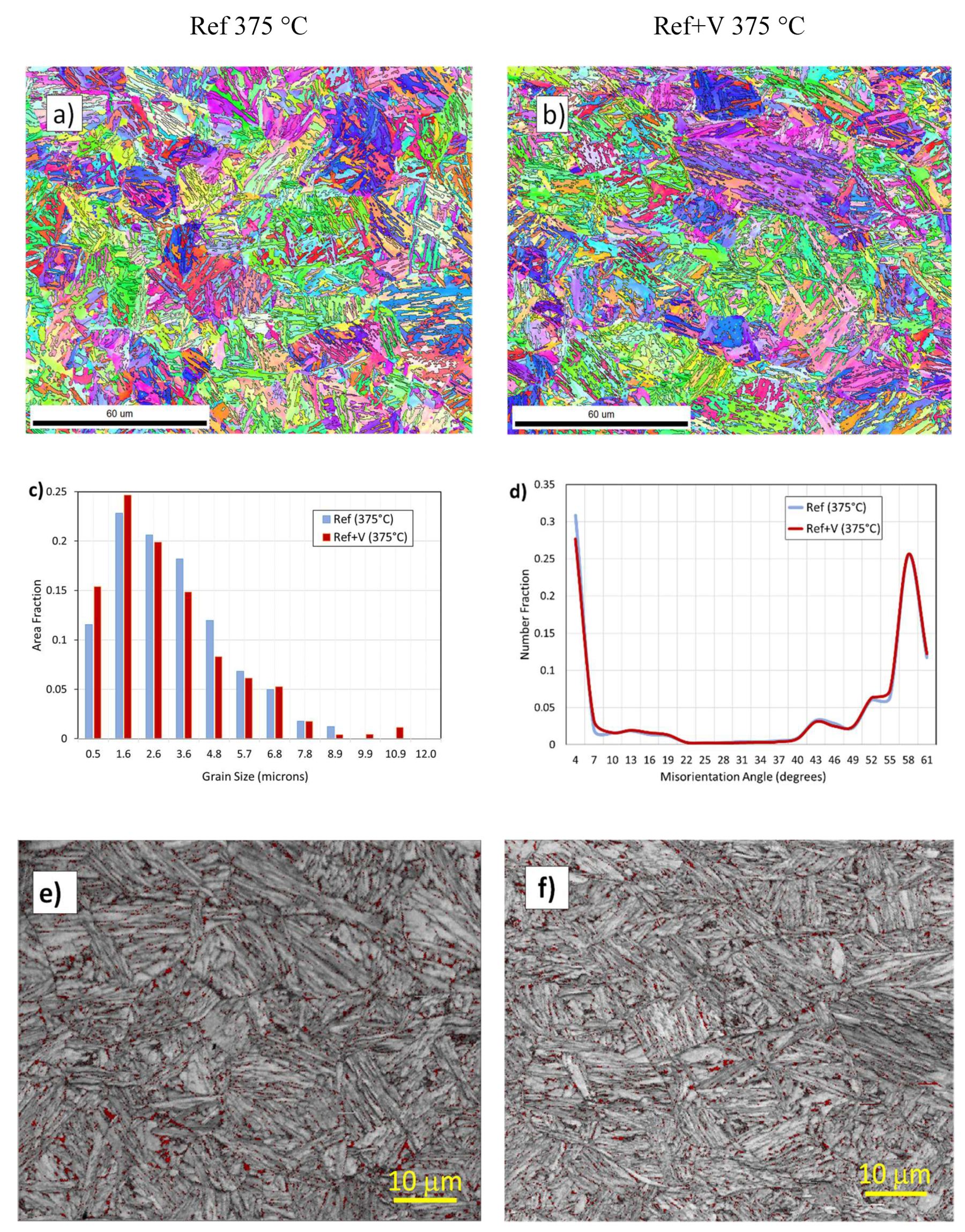
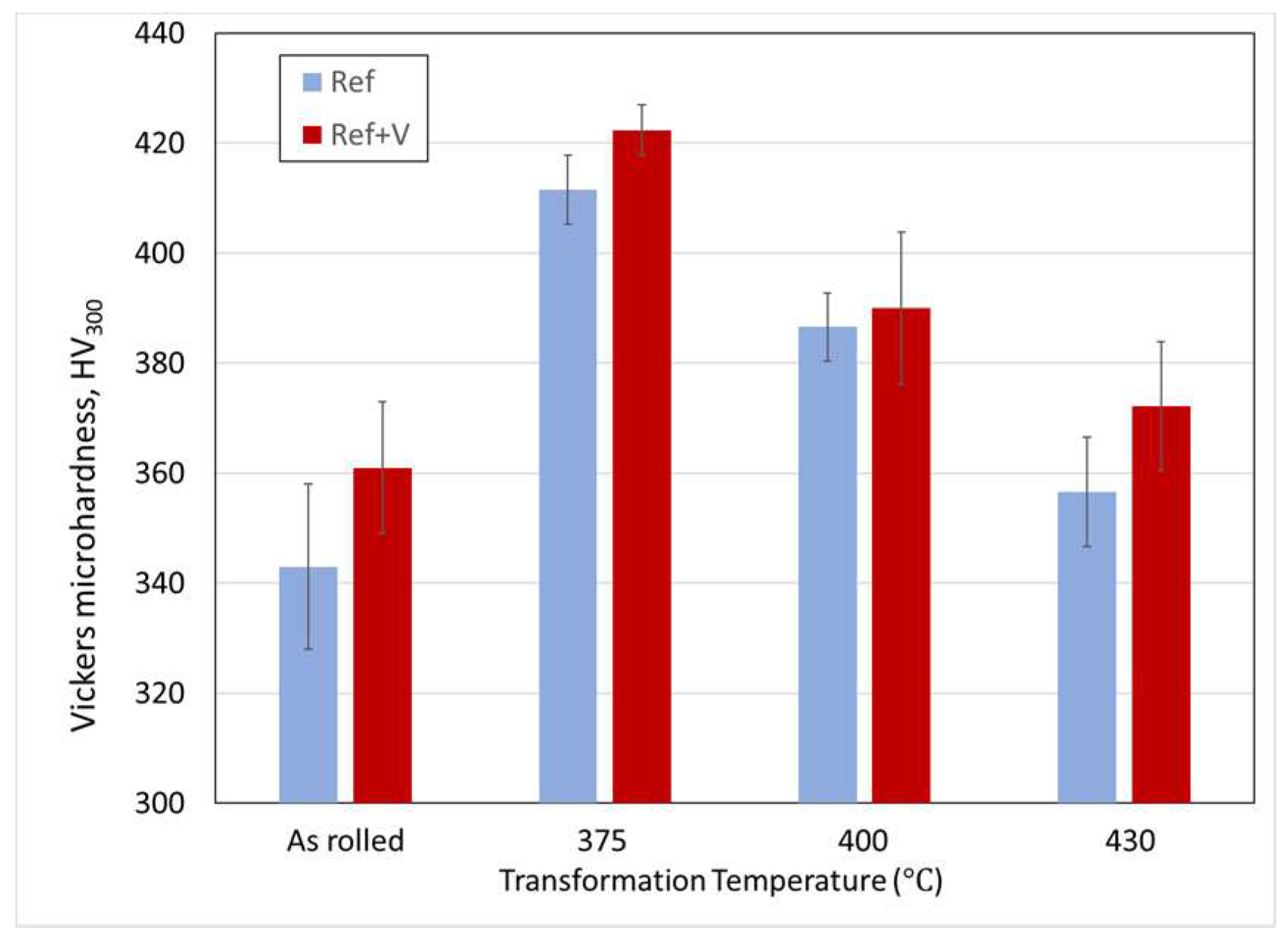
3.2. Microstructure and Mechanical Properties
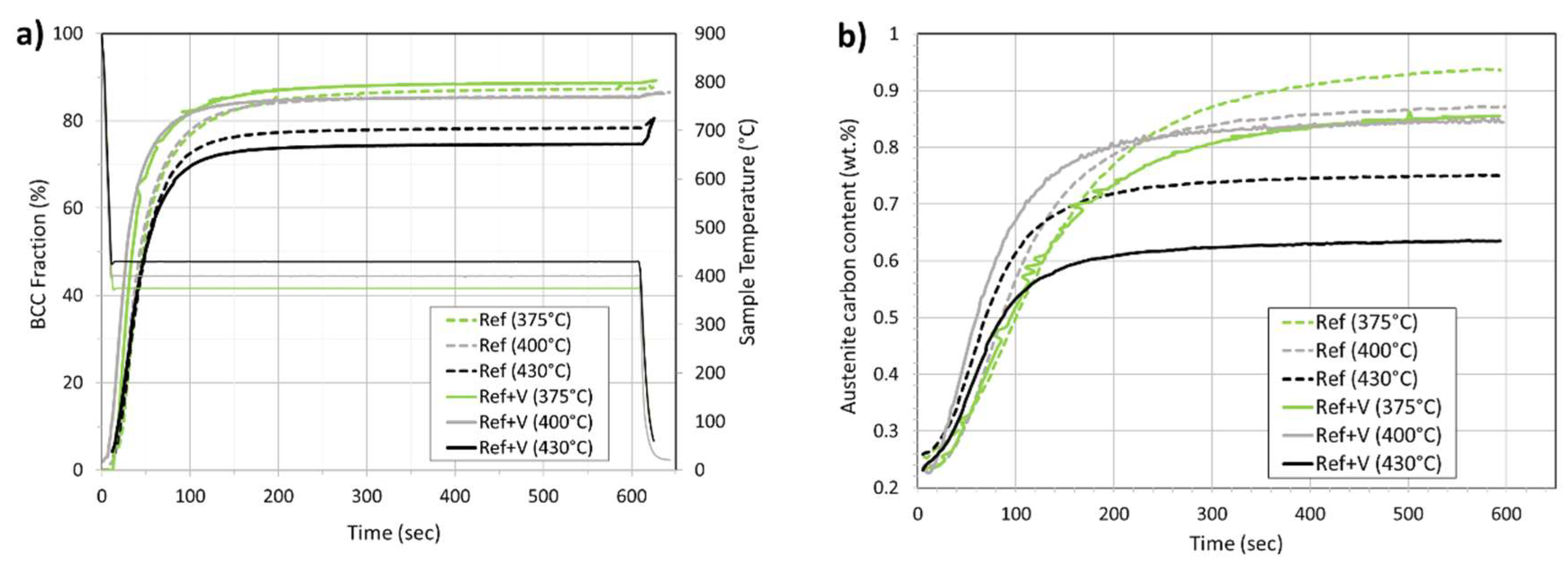
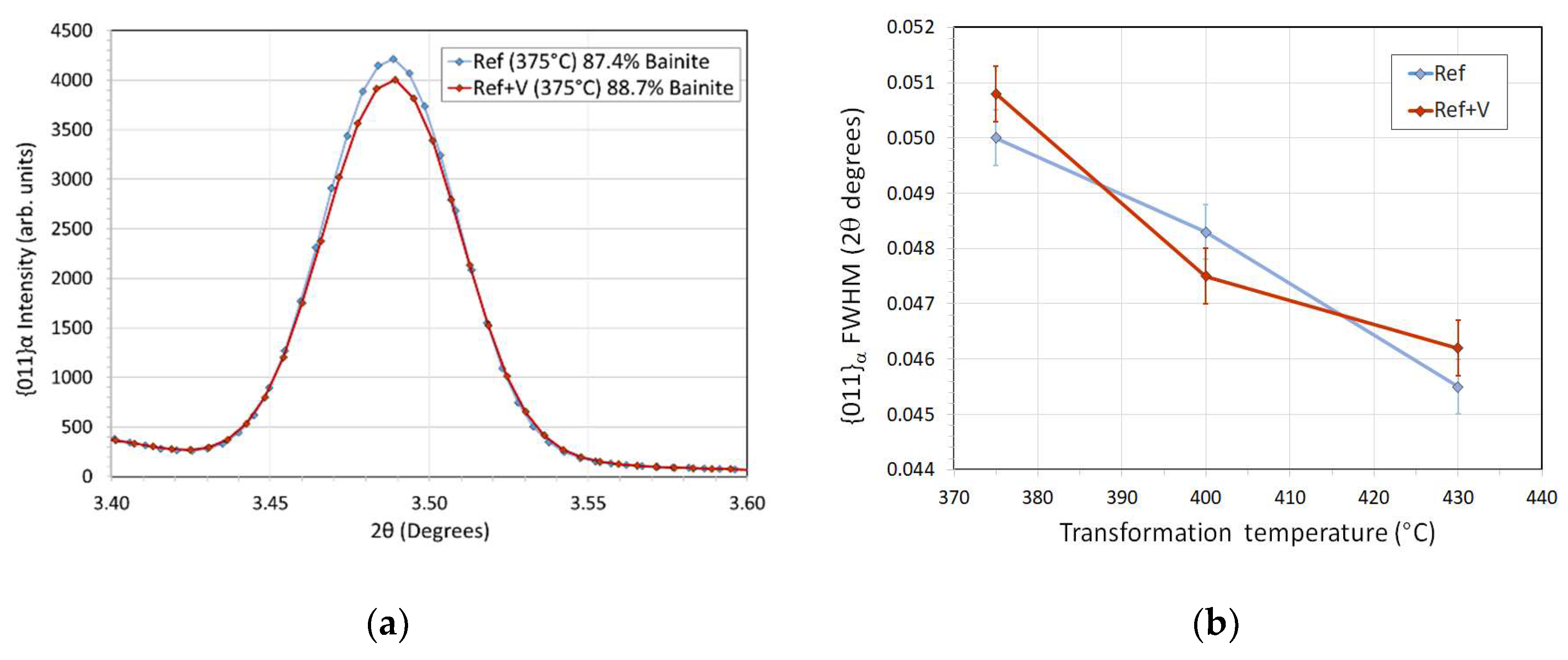
3.3. Synchrotron XRD—Phase Fractions, Carbon Partitioning and Dislocation Densities.
Measuring Dislocation Densities
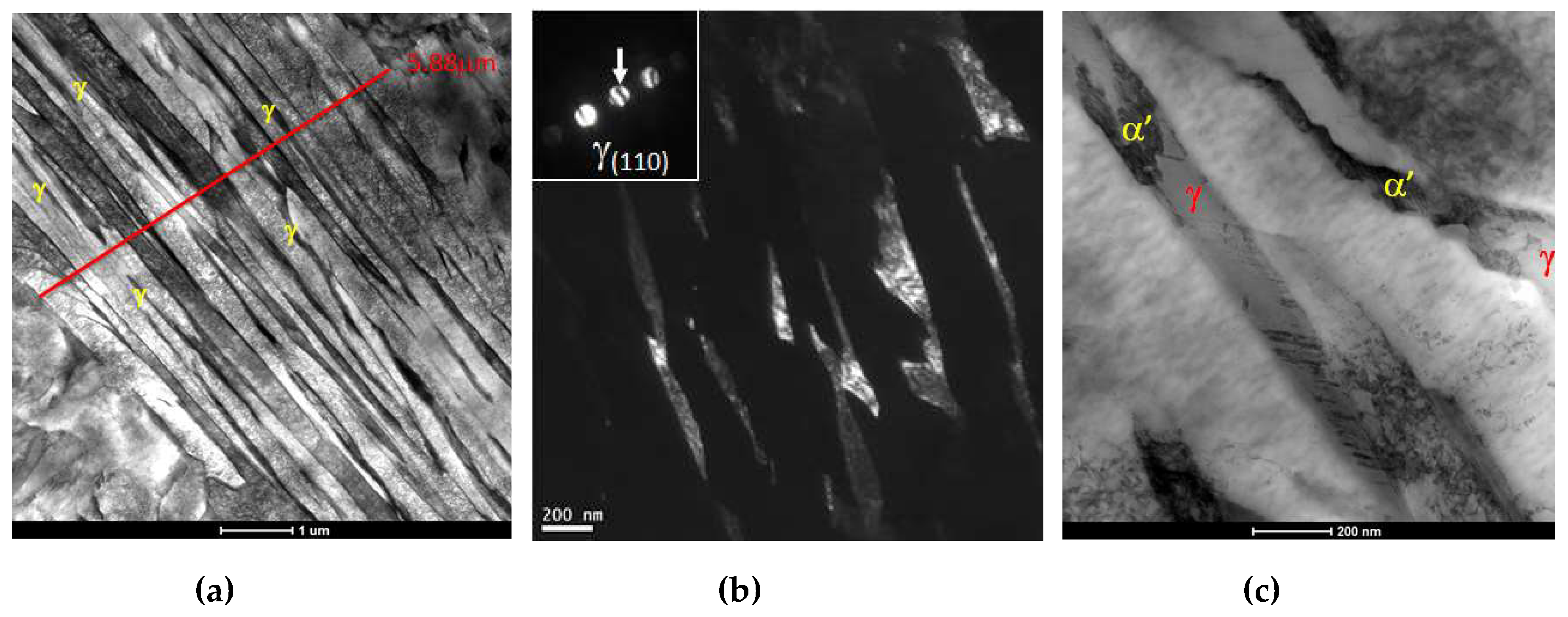
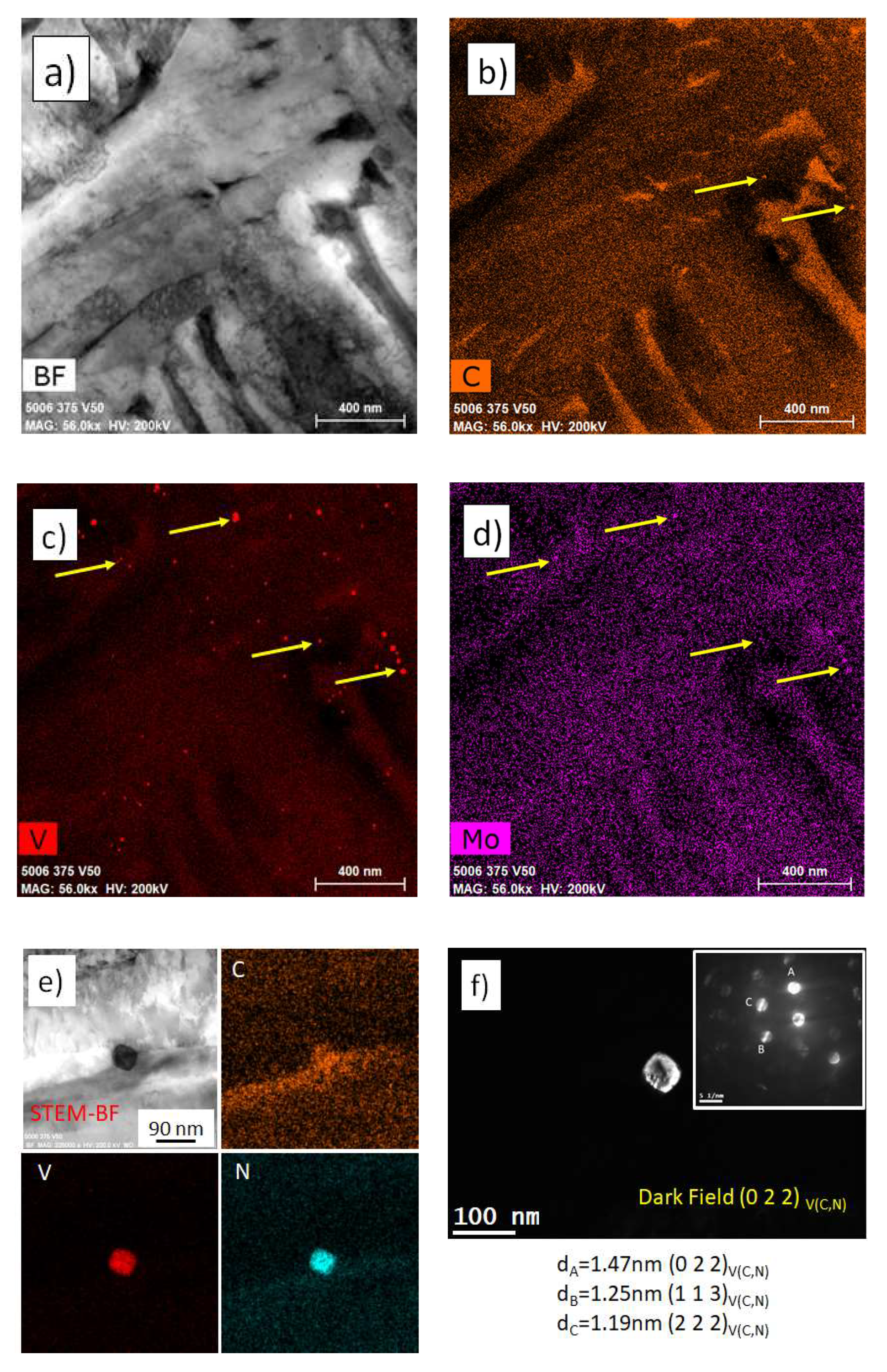
3.4. TEM—Microstructure
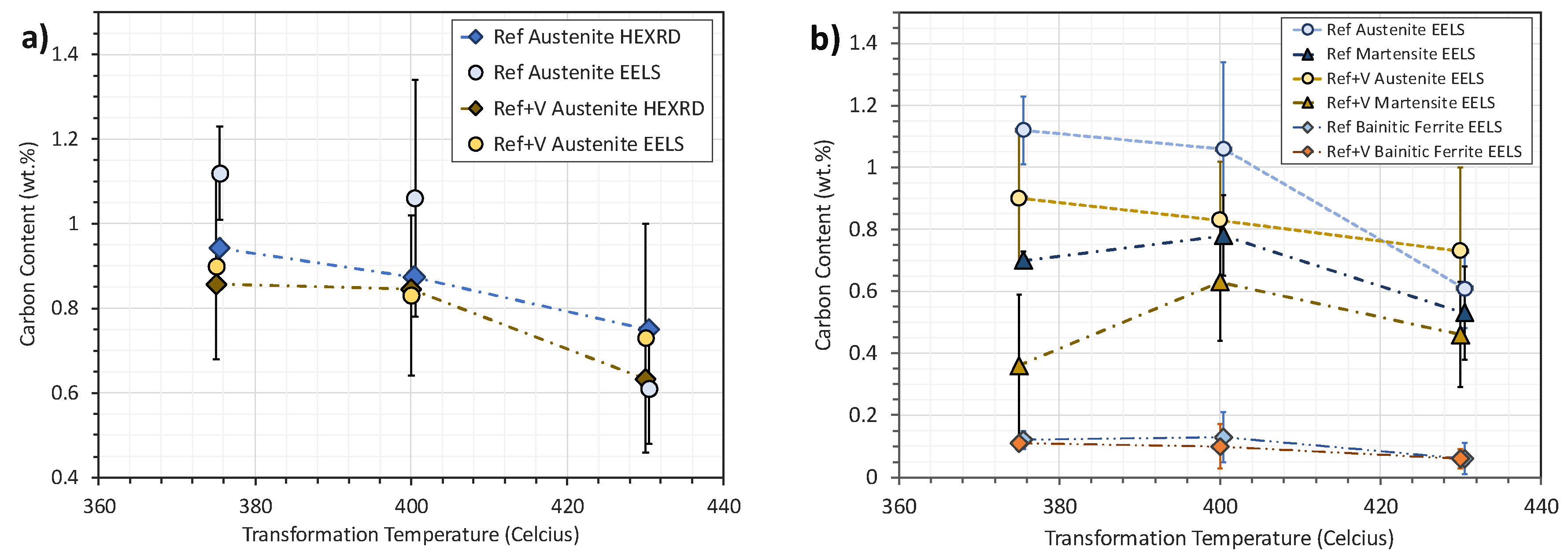
Measuring the Local Carbon Contents
4. Discussion
4.1. Strengthening from V Precipitation in Austenite and Ferrite
4.2. Strengthening from V Precipitation in Bainite
4.3. Size-Dependence Effects
4.4. Strengthening from Minority Phases
- Retained austenite in Ref+V contained less carbon, although the volume fraction of stable austenite was the same in both alloys.
- Both OM and EBSD data show that MA islands in Ref+V were refined at the two lowest transformation temperatures.
- Thermal martensite in Ref+V contained less carbon, especially at lower transformation temperatures.
- At higher transformation temperatures (430 °C) more thermal martensite formed in Ref+V.
- At 375 °C the retained austenite in Ref+V was significantly stabilized by size refinement. This should result in a higher work hardening coefficient and hence higher hardness, mitigated by the fact that any strain induced martensite will contain less carbon.
- At 400 °C minority phase strengthening was at a minimum and only weak precipitation strengthening was apparent.
- At 430 °C there was no size effect operating so the austenite stability should actually reverse. However, this was compensated by more thermal martensite formation in Ref+V at this transformation temperature.
5. Conclusions
- Vanadium did not change the final fractions of bainite or of retained austenite.
- There was no significant effect on the bainite reaction rate.
- The presence of bainitic ferrite tetragonality hindered the quantitative dislocation density analysis. Qualitatively, there was no detectable effect of vanadium on the dislocation density in bainitic ferrite.
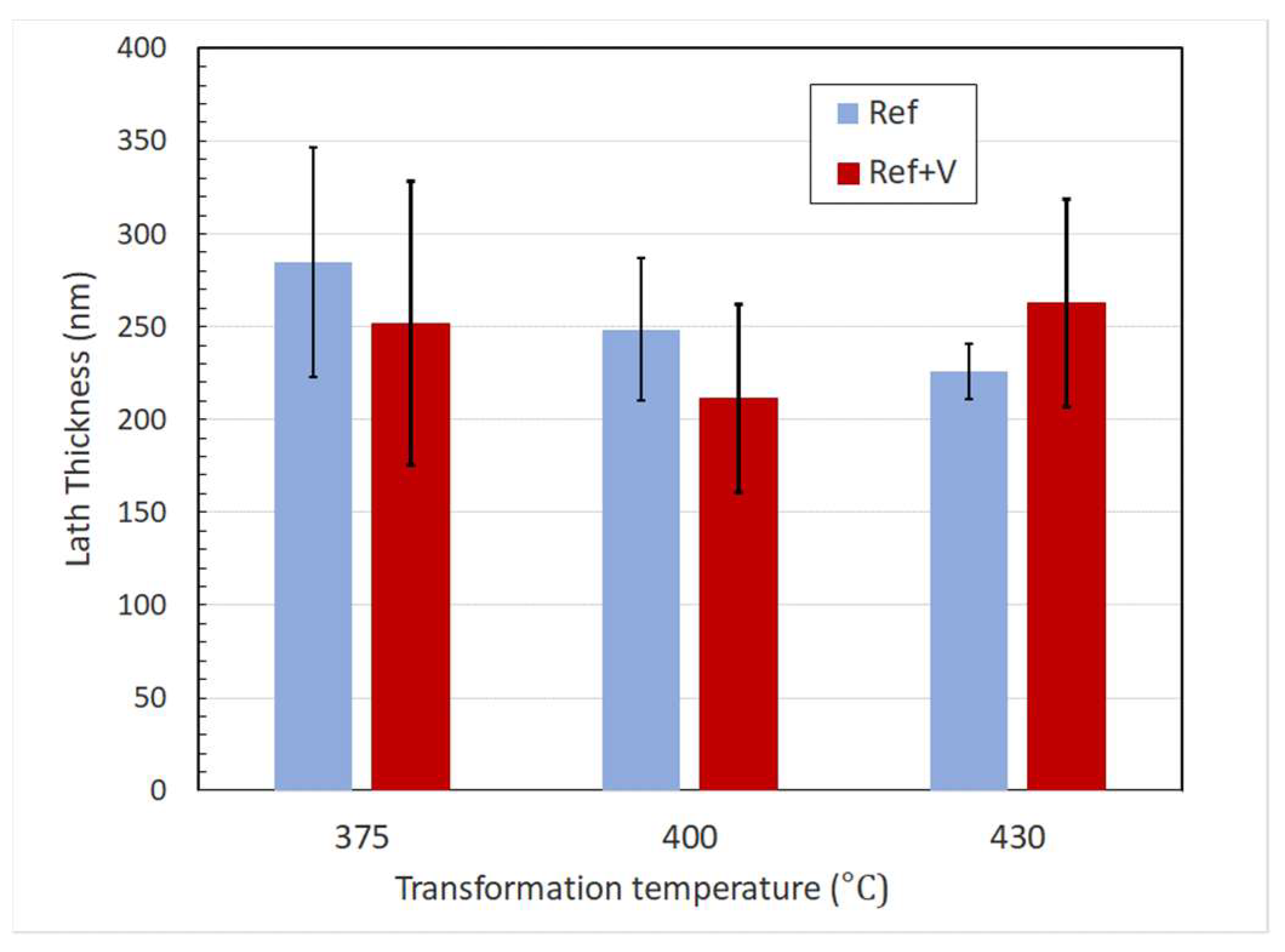
- No clear influence of vanadium on bainite lath widths could be observed.
- No significant vanadium precipitation occurred in bainite.
- No direct evidence of bainitic ferrite carbon supersaturation levels showing a vanadium dependence was found. Indirect calculations (from carbon mass balance) suggest that Cb was slightly raised.
- At low transformation temperatures, vanadium additions provided blocky MA and RA refinement.
- Retained austenite carbon content was decreased. This is not believed to be due to competition with vanadium precipitation.
- At low transformation temperatures (375 °C), retained austenite stability was enhanced due to a strong size effect.
- More thermal martensite forms during cooling, especially after transformation at 430 °C.
- Vanadium additions resulted in a modest increase in strength levels.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fazeli, F.; Amirkhiz, B.S.; Scott, C.P.; Arafin, M.; Collins, L. Kinetics and microstructural change of low-carbon bainite due to vanadium microalloying. Mater. Sci. Eng. A 2018, 720, 248–256. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Morales-Rivas, L.; Caballero, F.G.; Milbourn, D.; Sourmail, T. Vanadium Effect on a Medium Carbon Forging Steel. Metals 2016, 6, 130. [Google Scholar] [CrossRef]
- Sourmail, T.; Garcia-Mateo, C.; Caballero, F.G.; Cazottes, S.; Epicier, T.; Danoix, F.; Milbourn, D. The Influence of Vanadium on Ferrite and Bainite Formation in a Medium Carbon Steel. Metall. Mater. Trans. A 2017, 48, 3985–3996. [Google Scholar] [CrossRef]
- Wang, Z.; Hui, W.; Chen, Z.; Zhang, Y.; Zhao, X. Effect of vanadium on microstructure and mechanical properties of bainitic forging steel. Mater. Sci. Eng. A 2020, 771, 138653. [Google Scholar] [CrossRef]
- Scott, C.P.; Fazeli, F.; Amirkhiz, B.S.; Pushkareva, I.; Allain, S.Y.P. Structure-properties relationship of ultra-fine grained V-microalloyed dual phase steels. Mater. Sci. Eng. A 2017, 703, 293–303. [Google Scholar] [CrossRef]
- Rijkenberg, R.A.; Blowey, A.; Bellina, P.; Wooffindin, C. Advanced High Stretch-Flange Formability Steels for Chassis & Suspension Applications. In Proceedings of the 4th International Conference on Steels in Cars and Trucks (SCT2014), Braunschweig, Germany, 15–19 June 2014; pp. 426–433. [Google Scholar]
- Ioannidou, C.; Arechabaleta, Z.; Navarro-Lopez, A.; Rijkenberg, R.A.; Dalgliesh, R.M.; Kolling, S.; Bliznuk, V.; Pappas, C.; Sietsma, J.; Van Well, A.A.; et al. Interaction of precipitation with austenite-to-ferrite phase transformation in vanadium micro-alloyed steels. Acta Mater. 2019, 181, 10–24. [Google Scholar] [CrossRef]
- Scott, C.P.; Remy, B.; Collet, J.-L.; Cael, A.; Bao, C.; Danoix, F.; Malard, B.; Curfs, C. Precipitation strengthening in high manganese austenitic TWIP steels. Int. J. Mater. Res. 2011, 102, 538–549. [Google Scholar] [CrossRef]
- Gwon, H.; Kima, J.-K.; Shina, S.; Choa, L.; De Cooman, B.C. The effect of vanadium micro-alloying on the microstructure and the tensile behavior of TWIP steel. Mater. Sci. Eng. A 2017, 696, 416–428. [Google Scholar] [CrossRef]
- Lagneborg, R.; Siwecki, T.; Zajac, S.; Hutchinson, B. The Role of Vanadium in Microalloyed steels. Scand. J. Metall. 1999, 28, 186–241. [Google Scholar]
- Siwecki, T.; Eliasson, J.; Lagneborg, R.; Hutchinson, B. Vanadium microalloyed bainitic hot strip steels. ISIJ Int. 2010, 50, 760–767. [Google Scholar] [CrossRef]
- Matsumura, O.; Sakuma, Y.; Takechi, H. Enhancement of Elongation by Retained Austenite in Intercritical Annealed 0.4C-1.5Si-0.8Mn Steel. Trans. ISIJ 1987, 27, 570–579. [Google Scholar] [CrossRef]
- Perrard, F.; Scott, C. Vanadium Precipitation during Intercritical Annealing in Cold Rolled TRIP Steels. ISIJ Int. 2007, 47, 1168–1177. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Gaudez, S.; Geandier, G.; Hell, J.-C.; Gouné, M.; Danoix, F.; Soler, M.; Aoued, S.; Poulon-Quintin, A. Internal stresses and carbon enrichment in austenite of Quenching and Partitioning steels from high energy X-ray diffraction experiments. Mater. Sci. Eng. A 2018, 710, 245–250. [Google Scholar] [CrossRef]
- Available online: http://www.esrf.eu/computing/scientific/FIT2D/ (accessed on 18 March 2020).
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Cheng, L.; Bottger, A.; de Keijser, T.H.; Mittemeijer, E.J. Lattice parameters of iron-carbon and iron-nitrogen martensites and austenites. Scr. Metall. Mater. 1990, 24, 509–514. [Google Scholar] [CrossRef]
- Scott, C.P.; Drillet, J. A study of the carbon distribution in retained austenite. Scr. Mater. 2007, 56, 489–492. [Google Scholar] [CrossRef]
- Available online: https://www.matcalc-engineering.com/index.php/matcalc-software/matcalc-6 (accessed on 18 March 2020).
- Fazeli, F.; Scott, C.P.; Amirkhiz, B.S.; Pushkareva, I. Challenges with Design and Processing of Carbide-free Bainitic AHSS Sheets. In Proceedings of the International Symposium on New Developments in Advanced High-Strength Sheet Steels, Keystone, CO, USA, 30 May 2017; pp. 135–145. [Google Scholar]
- Venugopalan, D.; Kirkaldy, J. New Relations for Predicting the Mechanical Properties of Quenched and Tempered Low Alloy Steels. In Proceedings of the Hardenability Concepts with Application to Steel, Chicago, IL, USA, 26–28 October 1977; pp. 249–268. [Google Scholar]
- Andrews, K.W. Empirical Formulae for the Calculation of Some Transformation temperatures. JISI 1965, 203, 721–727. [Google Scholar]
- Zhu, K.; Bouaziz, O.; Oberbillig, C.; Huang, M. An approach to define the effective lath size controlling yield strength of bainite. Mater. Sci. Eng. A 2010, 527, 6614–6619. [Google Scholar] [CrossRef]
- Pavlina, E.J.; Van Tyne, C.J. Correlation of Yield Strength and Tensile Strength with Hardness for Steels. J. Mater. Eng. Perform. 2008, 17, 888–893. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels; Maney Publishing: Leeds, UK, 2015. [Google Scholar]
- Takebayashi, S.; Kunieda, T.; Yoshinaga, N.; Ushioda, K.; Ogata, S. Comparison of the Dislocation Density in Martensitic Steels Evaluated by Some X-ray Diffraction Methods. ISIJ Int. 2010, 50, 875–882. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminum and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- He, S.H.; He, B.B.; Zhu, K.Y.; Ding, R.; Chen, H.; Huang, M.X. Revealing the role of dislocations on the stability of retained austenite in a tempered bainite. Scr. Mater. 2019, 168, 23–27. [Google Scholar] [CrossRef]
- Hulme-Smith, C.N.; Peet, M.J.; Lonardelli, I.; Dippel, A.C.; Bhadeshia, H.K.D.H. Further evidence of tetragonality in bainitic ferrite. Mater. Sci. Technol. 2015, 31, 254–256. [Google Scholar] [CrossRef]
- Gladman, T. The Physical Metallurgy of Microalloyed Steels; The Institute of Materials: London, UK, 1997. [Google Scholar]
- Available online: https://www.matcalc.at/wiki/doku.php?id=tutorials:t18 (accessed on 18 March 2020).

| Steel | C | Mn | Si | Mo | V | Al | N |
|---|---|---|---|---|---|---|---|
| Ref | 0.22 | 2.2 | 1.8 | 0.2 | - | 0.01 | 0.0028 |
| Ref+V | 0.22 | 2.2 | 1.8 | 0.2 | 0.15 | 0.01 | 0.0027 |
| Steel | Ae1 (calc) | Ac1 (exp) | Ae3 (calc) | Ac3 (exp) | Tsol V(CN) | Bs (calc) [21] | Ms (calc) [22] |
|---|---|---|---|---|---|---|---|
| Ref | 707 °C | 700 °C | 831 °C | 933 °C | - | 423 °C | 375 °C |
| Ref+V | 713 °C | 748 °C | 840 °C | 975 °C | 1067 °C | 423 °C | 375 °C |
| Alloy | Average Cooling Rate | Transformation Temperature | Bs (5%) | Time for 95% Completion | HEXRD Final Bainite Fraction | HEXRD RA Fraction (25 °C) | HEXRD Martensite Fraction | EBSD RA Fraction | EBSD MA Fraction | OM MA Fraction |
|---|---|---|---|---|---|---|---|---|---|---|
| Ref | 44 °C/s | 375 °C | 375 °C | 160 s | 87% | 12% | 0.3% | 5.4% | 1.5% | 5.1% |
| Ref | 50 °C/s | 400 °C | 400 °C | 129 s | 86% | 14% | 0.9% | 5.3% | 3.4% | 6.9% |
| Ref | 45 °C/s | 430 °C | 430 °C | 117 s | 78% | 19% | 2.5% | NA | NA | 11.3% |
| Ref+V | 42 °C/s | 375 °C | 381 °C | 127 s | 89% | 11% | 0.6% | 4.8% | 5.4% | 2.3% |
| Ref+V | 50 °C/s | 400 °C | 504 °C | 94 s | 85% | 14% | 0.9% | 4.8% | 6.8% | 6.9% |
| Ref+V | 45 °C/s | 430 °C | 432 °C | 114 s | 75% | 19% | 5.9% | NA | NA | 10.7% |
| Alloy | Transformation Temperature | Vickers Hardness | EBSD Effective Grain Size q < 5° | TEM Mean Lath Width | EBSD Mean MA Area | OM Mean MA Area |
|---|---|---|---|---|---|---|
| Ref | 375 °C | 412 HV | 1.01 mm | 0.285 mm | 0.53 mm2 | 0.22 mm2 |
| Ref | 400 °C | 387 HV | 0.96 mm | 0.249 mm | 0.60 mm2 | 0.22 mm2 |
| Ref | 430 °C | 357 HV | NA | 0.226 mm | NA | 0.44 mm2 |
| Ref+V | 375 °C | 422 HV | 0.90 mm | 0.252 mm | 0.38 mm2 | 0.09 mm2 |
| Ref+V | 400 °C | 390 HV | 1.04 mm | 0.211 mm | 0.49 mm2 | 0.16 mm2 |
| Ref+V | 430 °C | 372 HV | NA | 0.263 mm | NA | 0.44 mm2 |
| Alloy | Transformation Temperature | Cg HEXRD (wt. %) | Cg EELS (wt. %) | Ca′ EELS (wt. %) | Cb Calculated from Mass Balance (wt. %) | Cb Measured from EELS (wt. %) |
|---|---|---|---|---|---|---|
| Ref | 375 °C | 0.94 | 1.12 | 0.7 | 0.12 | 0.12 |
| Ref | 400 °C | 0.87 | 1.06 | 0.78 | 0.11 | 0.13 |
| Ref | 430 °C | 0.75 | 0.61 | 0.53 | 0.08 | 0.06 |
| Ref+V | 375 °C | 0.86 | 0.9 | 0.36 | 0.14 | 0.11 |
| Ref+V | 400 °C | 0.85 | 0.83 | 0.63 | 0.11 | 0.1 |
| Ref+V | 430 °C | 0.63 | 0.73 | 0.46 | 0.09 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushkareva, I.; Shalchi-Amirkhiz, B.; Allain, S.Y.P.; Geandier, G.; Fazeli, F.; Sztanko, M.; Scott, C. The Influence of Vanadium Additions on Isothermally Formed Bainite Microstructures in Medium Carbon Steels Containing Retained Austenite. Metals 2020, 10, 392. https://doi.org/10.3390/met10030392
Pushkareva I, Shalchi-Amirkhiz B, Allain SYP, Geandier G, Fazeli F, Sztanko M, Scott C. The Influence of Vanadium Additions on Isothermally Formed Bainite Microstructures in Medium Carbon Steels Containing Retained Austenite. Metals. 2020; 10(3):392. https://doi.org/10.3390/met10030392
Chicago/Turabian StylePushkareva, Irina, Babak Shalchi-Amirkhiz, Sébastien Yves Pierre Allain, Guillaume Geandier, Fateh Fazeli, Matthew Sztanko, and Colin Scott. 2020. "The Influence of Vanadium Additions on Isothermally Formed Bainite Microstructures in Medium Carbon Steels Containing Retained Austenite" Metals 10, no. 3: 392. https://doi.org/10.3390/met10030392
APA StylePushkareva, I., Shalchi-Amirkhiz, B., Allain, S. Y. P., Geandier, G., Fazeli, F., Sztanko, M., & Scott, C. (2020). The Influence of Vanadium Additions on Isothermally Formed Bainite Microstructures in Medium Carbon Steels Containing Retained Austenite. Metals, 10(3), 392. https://doi.org/10.3390/met10030392






