Application of Zr and Ti-Based Bulk Metallic Glasses for Orthopaedic and Dental Device Materials
Abstract
:1. Introduction
2. Classification of BMGs
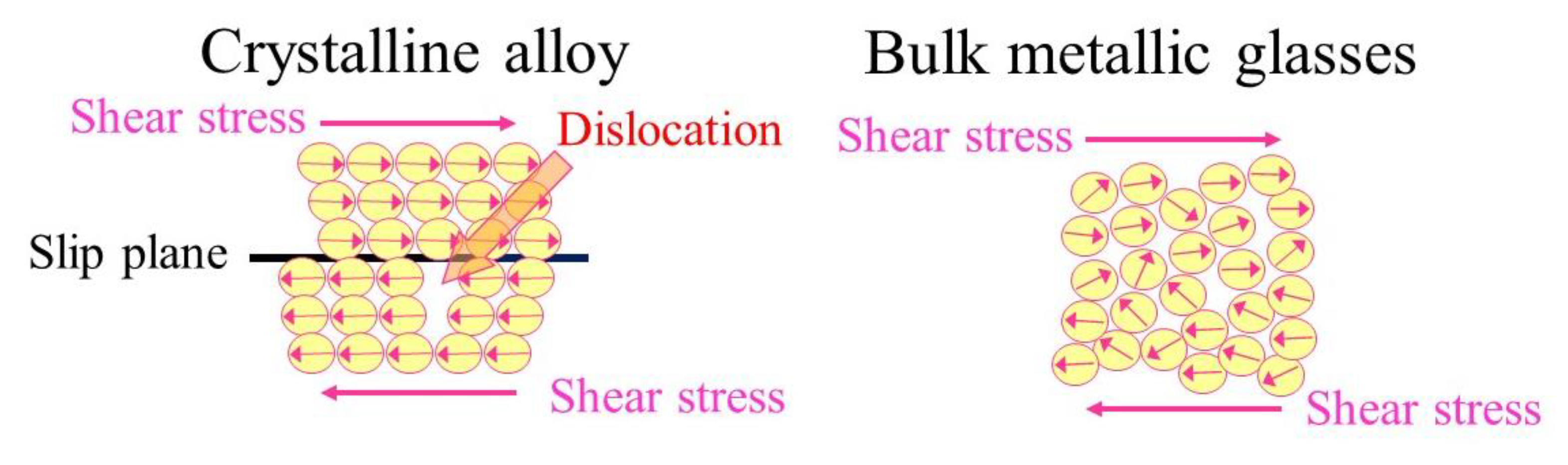
3. Mechanical Properties of Zr and Ti-Based BMGs
4. In Vitro Studies for Biomaterial Applications
4.1. Zr-Based BMGs
4.2. Ti-Based BMGs
5. In Vivo Studies for Biomaterial Applications
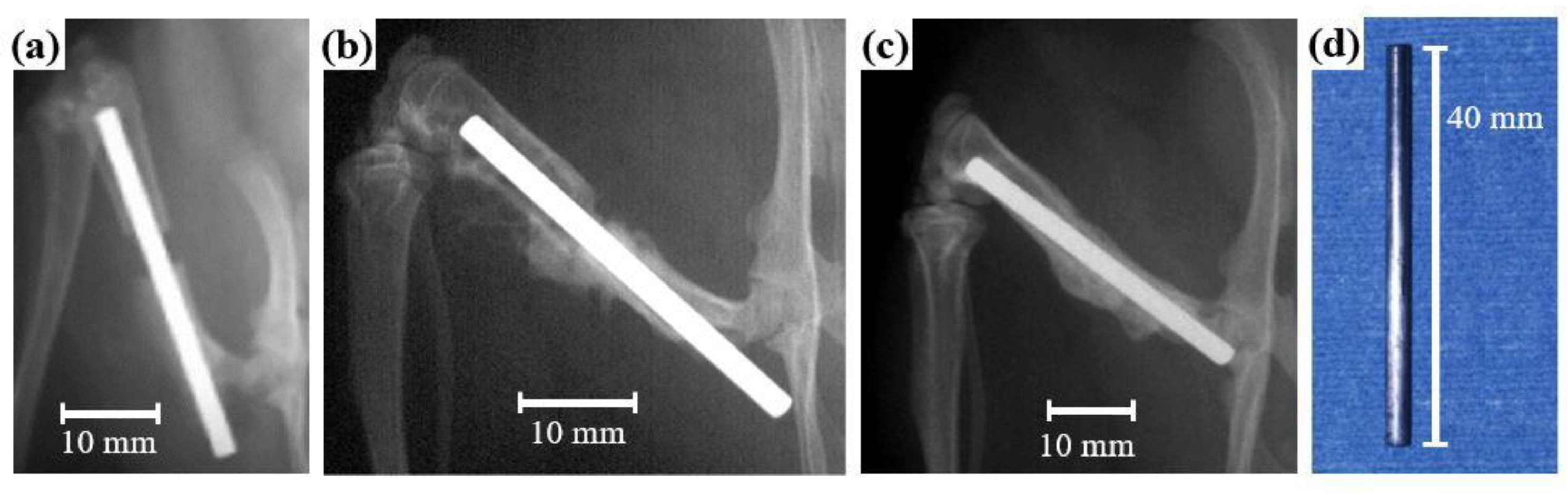
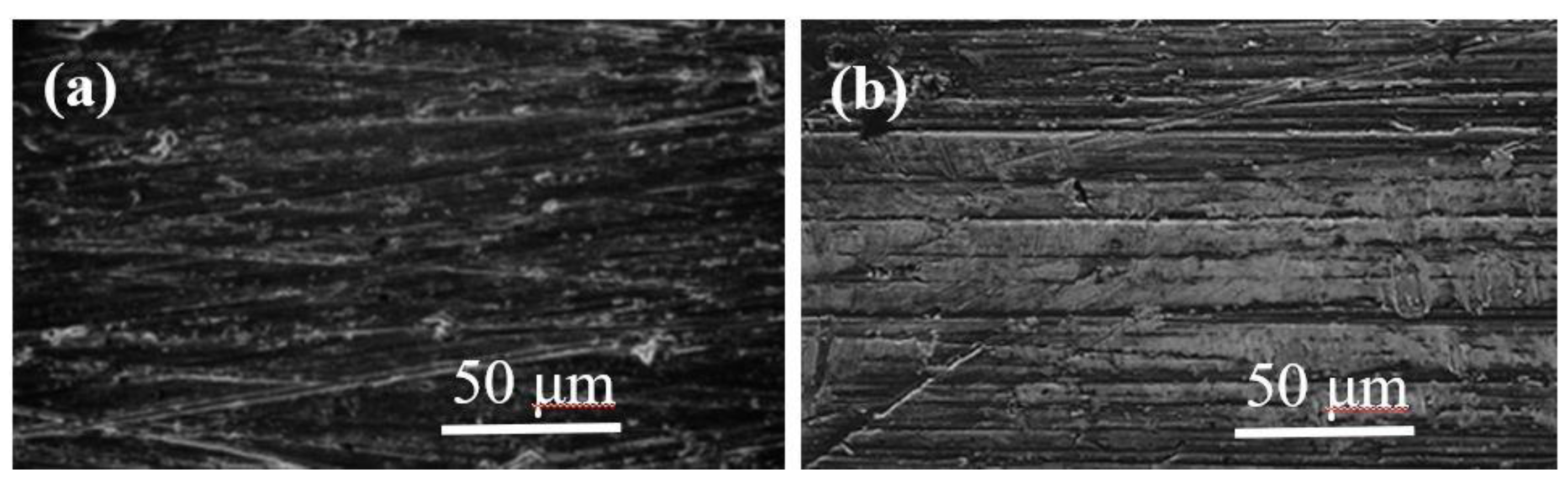
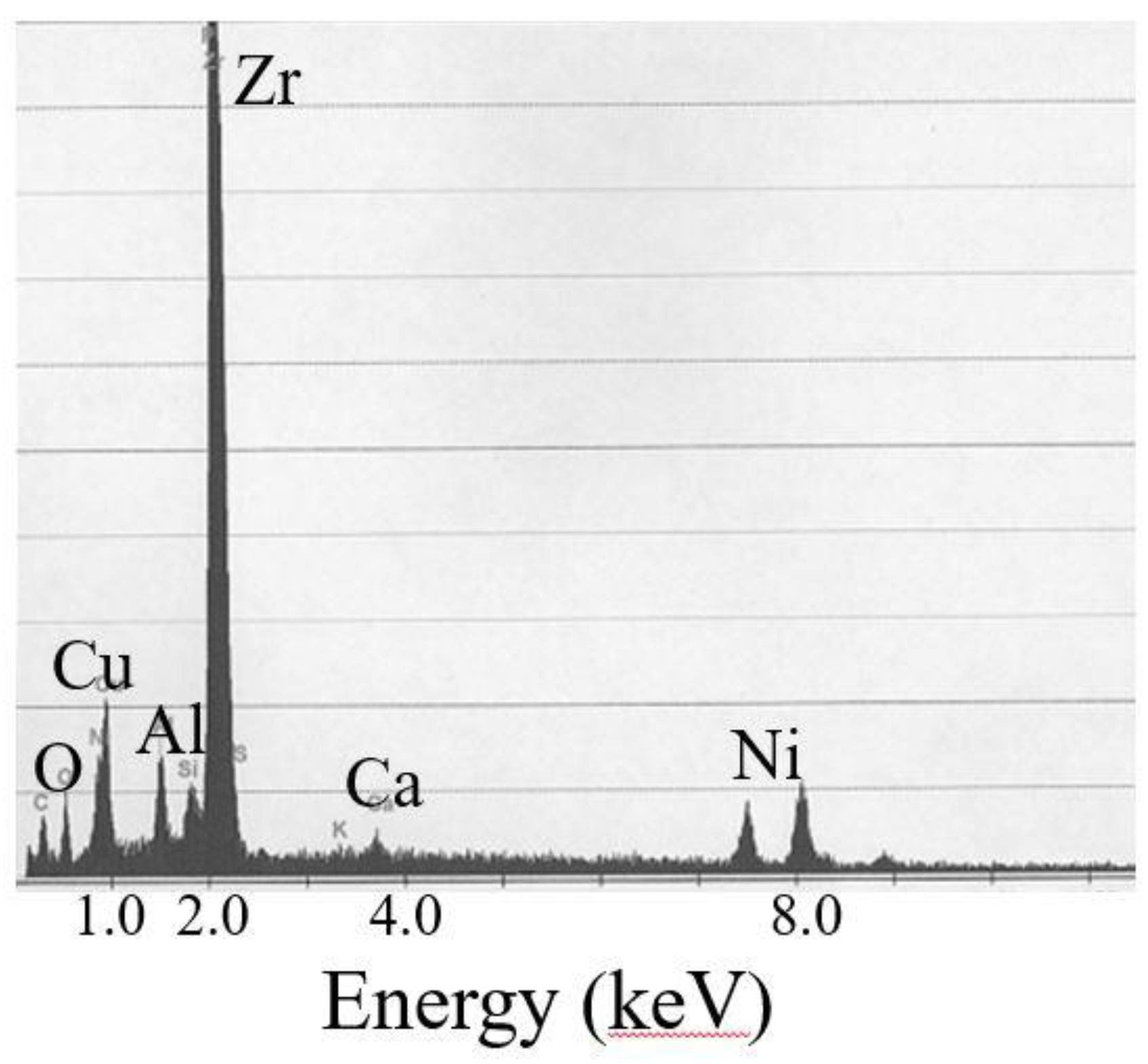
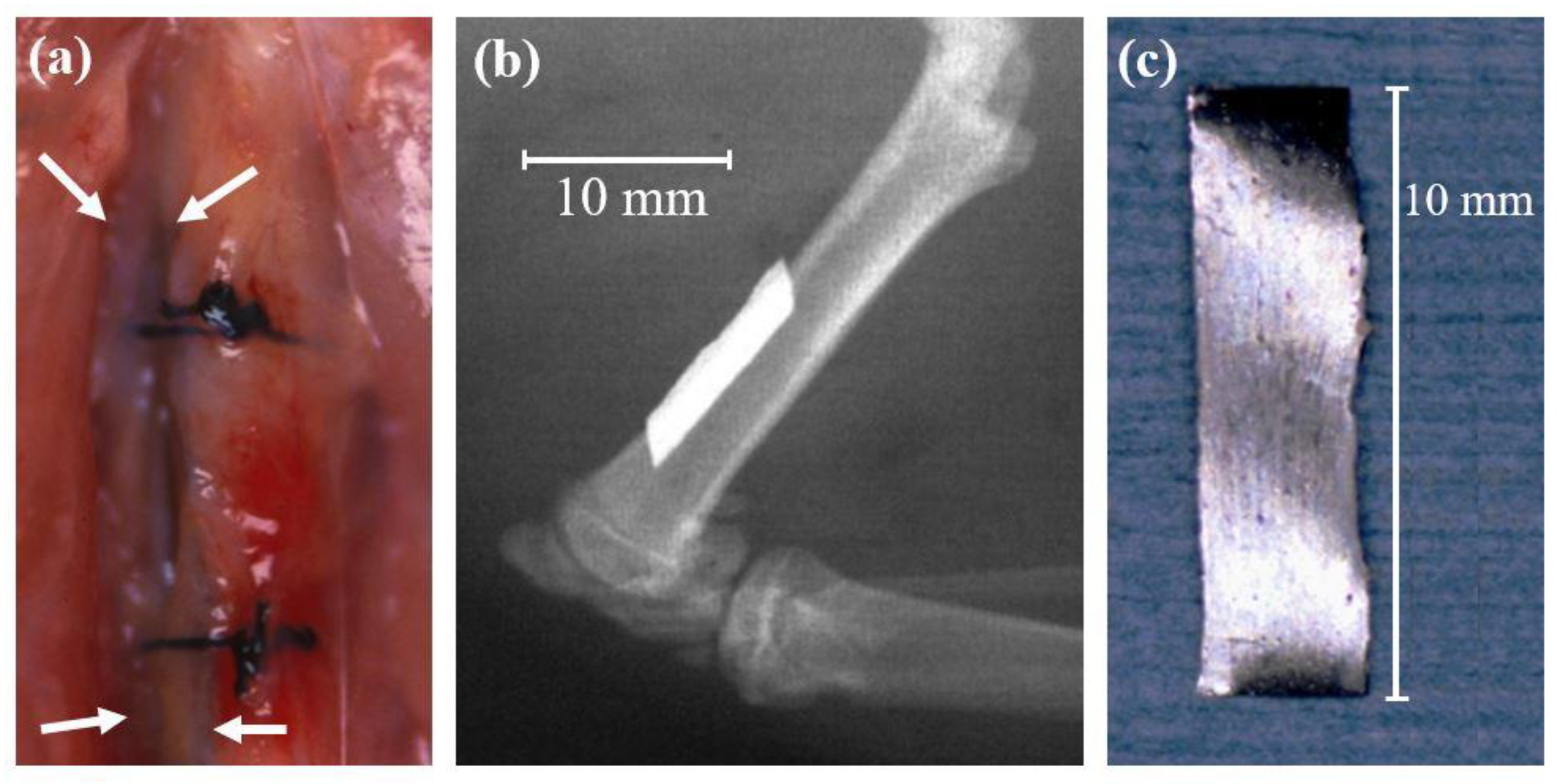
5.1. Animal Tests of Zr-Based BMGs
5.2. Animal Tests of Ti-Based BMGs
5.3. Animal Tests of Mg-Based, Sr-Substituted, and Nanopatterned Pt-Based BMGs
6. Anti-Corrosion Behavior and Biocompatibility of a BMGs Implant for Biomaterial Applications
7. Biomaterials for Dental Device Materials
8. Conclusions and Prospects for the Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antunes, R.A.; de Oliveira, M.C. Corrosion processes of physical vapor deposition-coated metallic implants. Crit. Rev. Biomed. Eng. 2009, 37, 425–460. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.A. Environmental effects on the life of bone-plate-type surgical implants. Rev. Environ. Health 1982, 4, 63–82. [Google Scholar]
- Molster, A.O. Biomechanical effects of intramedullary reaming and nailing on intact femora in rats. Clin. Orthop. 1986, 202, 278–285. [Google Scholar]
- Bradley, G.W.; McKenna, G.B.; Dunn, H.K.; Daniels, A.U.; Statton, W.O. Effects of flexural rigidity of plates on bone healing. J. Bone Joint Surg. Am. 1979, 61, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Akeson, W.H.; Coutts, R.D.; Rutherford, L.; Doty, D.; Jemmott, G.F.; Amiel, D. A comparison of cortical bone atrophy secondary to fixation with plates with large differences in bending stiffness. J. Bone Joint Surg. Am. 1976, 58, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Tonino, A.J.; Davidson, C.L.; Klopper, P.J.; Linclau, L.A. Protection from stress in bone and its effects. Experiments with stainless steel and plastic plates in dogs. J. Bone Joint Surg. Br. 1976, 58, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Uhthoff, H.K.; Dubuc, F.L. Bone structure changes in the dog under rigid internal fixation. Clin. Orthop. 1971, 81, 165–170. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of supercooled liquid and opening-up of bulk glassy alloys. Proc. Jpn. Acad. Ser. B 1997, 73, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Zhang, T. Fabrication of Bulk Glassy Zr55Al10Ni5Cu30 Alloy of 30 mm in Diameter by a Suction Casting Method. Mater. Trans. 1996, 37, 185–187. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Nishiyama, N.; Kimura, H. Preparation and thermal stability of bulk amorphous Pd40Cu30Ni10P20 alloy cylinder of 72 mm in diameter. Mater. Trans. 1997, 38, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Inoue, A. Formation and mechanical strength of new Cu-based bulk glassy alloys with large supercooled liquid region. Mater. Trans. 2004, 45, 1210–1213. [Google Scholar] [CrossRef] [Green Version]
- Schroers, J.; Johnson, W.L. Highly processable bulk metallic glass-forming alloys in the Pt–Co–Ni–Cu–P system. Appl. Phys. Lett. 2004, 84, 3666–3668. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Xu, J.; Ma, E. High glass-forming ability correlated with fragility of Mg–Cu(Ag)–Gd alloys. J. Appl. Phys. 2007, 102, 113519-1–113519-5. [Google Scholar] [CrossRef]
- Li, R.; Pang, S.; Ma, C.; Zhang, T. Influence of similar atom substitution on glass formation in (La–Ce)–Al–Co bulk metallic glasses. Acta Mater. 2007, 55, 3719–3726. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, Y.; Mund, E.; Inoue, A.; Schultz, L. Production of Zr55Cu30Ni5Al10 glassy alloy rod of 30 mm in diameter by a cap-cast technique. Mater. Trans. 2007, 48, 3190–3192. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Nishiyama, N.; Yamamoto, T.; Inoue, A. Ni-Rich bulk metallic glasses with high glass-forming ability and good metallic properties. Mater. Trans. 2009, 50, 2441–2445. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Zhang, T.; Masumoto, T. Preparation of bulky amorphous Zr-Al-Ni-Cu alloys by copper mold casting and their thermal and mechanical properties. Mater. Trans. JIM 1995, 36, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.H. Roles of minor additions in formation and properties of bulk metallic glasses. Prog. Mater. Sci. 2007, 52, 540–596. [Google Scholar] [CrossRef]
- Gilbert, C.J.; Ritchie, R.O.; Johnson, W.L. Fracture toughness and fatigue-crack propagation in a Zr-Ti-Ni-Cu-Be bulk metallic glass. Appl. Phys. Lett. 1997, 71, 476–478. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.J.; Zhang, T.; Kimura, H.; Asami, K.; Inoue, A. Corrosion behavior of Zr-(Nb-)Al-Ni-Cu glassy alloys. Mater. Trans. JIM 2000, 41, 1490–1494. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.G.; Choi, B.M.; Nieh, T.G.; Liu, C.T. Nano-scratch behavior of a bulk Zr-10Al-5Ti-17.9Cu-14.6Ni amorphous alloy. J. Mater. Res. 2000, 15, 913–922. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, C.L.; Zou, H.; Chan, K.C. The effect of the microalloying of Hf on the corrosion behavior of ZrCuNiAl bulk metallic glass. J. Alloys Compd. 2005, 399, 144–148. [Google Scholar] [CrossRef]
- Boyer, R.; Welsch, G.; Collings, E.W. Titanium Alloys. In Materials Properties Handbook; ASM International: Materials Park, OH, USA, 1994. [Google Scholar]
- Hanawa, T.; Yoneyama, T. Metals. In Biomaterials; Corona Publishing CO., LTD.: Tokyo, Japan, 2007. [Google Scholar]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Katsamanis, F.; Raftopoulos, D.D. Determination of mechanical properties of human femoral cortical bone by the Hopkinson bar stress technique. J. Biomech. 1990, 23, 1173–1184. [Google Scholar] [CrossRef]
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Kopperdahl, D.L.; Keaveny, T.M. Yield strain behavior of trabecular bone. J. Biomech. 1998, 31, 601–608. [Google Scholar] [CrossRef]
- Turner, C.H.; Wang, T.; Burr, D.B. Shear strength and fatigue properties of human cortical bone determined from pure shear tests. Calcif. Tissue Int. 2001, 69, 373–378. [Google Scholar] [CrossRef]
- Wang, G.Y.; Liaw, P.K.; Yokoyama, Y.; Inoue, A.; Liu, C.T. Fatigue behavior of Zr-based bulk-metallic glasses. Mater. Sci. Eng. A 2008, 494, 314–323. [Google Scholar] [CrossRef]
- Maruyama, N.; Nakazawa, K.; Hanawa, T. Fatigue properties of Zr-based amorphous alloy in phosphate buffered saline solution. Mater. Trans. 2002, 43, 3118–3121. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liaw, P.K.; Yokoyama, Y.; Freels, M.; Inoue, A. Investigations of the factors that affected fatigue behavior of Zr-based bulk-metallic glasses. Adv. Eng. Mater. 2008, 10, 1030–1033. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, C.L.; Chen, Q.; Chan, K.C.; Zhang, S.M. Deformation behavior, corrosion resistance, and cytotoxicity of Ni-free Zr-based bulk metallic glasses. J. Biomed. Mater. Res. Part A 2008, 86, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yokoyama, Y.; Wu, W.; Liaw, P.K.; Pang, S.; Inoue, A.; Zhang, T.; He, W. Ni-free Zr-Cu-Al-Nb-Pd bulk metallic glasses with different Zr/Cu ratios for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.M.; Pang, H.F.; Zhao, Q.; Liu, L. A Ni-free ZrCuFeAlAg bulk metallic glass with potential for biomedical applications. Acta Biomater. 2013, 9, 7043–7053. [Google Scholar] [CrossRef]
- Inoue, A.; Wang, X.M.; Zhang, W. Developments and applications of bulk metallic glasses. Rev. Adv. Mater. Sci. 2008, 18, 1–9. [Google Scholar]
- Hiromoto, S.; Tsai, A.-P.; Sumita, M.; Hanawa, T. Effect of chloride ion on the polarization behavior of the Zr65Al7.5Ni10Cu17.5 amorphous alloy in phosphate buffered solution. Corr. Sci. 2000, 42, 1651–1660. [Google Scholar] [CrossRef]
- Hiromoto, S.; Tsai, A.-P.; Sumita, M.; Hanawa, T. Effect of surface finishing and dissolved oxygen on the polarization behavior of Zr65Al7.5Ni10Cu17.5 amorphous alloy in phosphate buffered solution. Corr. Sci. 2000, 42, 2167–2185. [Google Scholar] [CrossRef]
- Hiromoto, S.; Tsai, A.-P.; Sumita, M.; Hanawa, T. Effect of pH on the polarization behavior of Zr65Al7.5Ni10Cu17.5 amorphous alloy in a phosphate-buffered solution. Corr. Sci. 2000, 42, 2193–2200. [Google Scholar] [CrossRef]
- Hiromoto, S.; Asami, K.; Tsai, A.-P.; Sumita, M.; Hanawa, T. Surface composition and anodic polarization behavior of zirconium-based amorphous alloy with various alloying elements in a phosphate buffered saline solution. J. Electrochem. Soc. 2002, 149, B117–B122. [Google Scholar] [CrossRef]
- Hiromoto, S.; Hanawa, T. Re-passivation current of amorphous Zr65Al7.5Ni10Cu17.5 alloy in a Hanks’ balanced solution. Electrochim. Acta 2002, 47, 1343–1349. [Google Scholar] [CrossRef]
- Hiromoto, S.; Tsai, A.-P.; Sumita, M.; Hanawa, T. Surface characterization of Zr-Al-(Ni, Cu) amorphous alloys immersed in a cell-culture medium. Mater. Trans. JIM 2002, 43, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Morrison, M.L.; Buchanan, R.A.; Leon, R.V.; Liu, C.T.; Green, B.A.; Liaw, P.K.; Horton, J.A. The electrochemical evaluation of a Zr-based bulk metallic glass in a phosphate-buffered saline electrolyte. J. Biomed. Mater. Res. Part A 2005, 74, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, E.E.; Buchanan, R.A. Faraday’s Law. In Fundamentals of Electrochemical Corrosion; ASM International: Materials Park, OH, USA, 2000; pp. 147–149. [Google Scholar]
- Wang, Y.B.; Zheng, Y.F.; Wei, S.C.; Li, M. In vitro study on Zr-based bulk metallic glasses as potential biomaterials. J. Biomed. Mater. Res. Part B 2011, 96, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Lockwood, P.E.; Schedle, A. Effect of silver, copper, mercury, and nickel ions on cellular proliferation during extended, low-dose exposures. J. Biomed. Mater. Res. 2000, 52, 360–364. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, C.L.; Huang, C.Y.; Yu, Y.; Huang, H.; Zhang, S.M. Biocompatibility of Ni-free Zr-based bulk metallic glasses. Intermetallics 2009, 17, 235–240. [Google Scholar] [CrossRef]
- Monfared, A.; Vali, H.; Faghihi, S. Biocorrosion and biocompatibility of Zr–Cu–Fe–Al bulk metallic glasses. Surf. Interface Anal. 2013, 45, 1714–1720. [Google Scholar] [CrossRef]
- Li, J.; Shi, L.L.; Zhu, Z.D.; He, Q.; Ai, H.J.; Xu, J. Zr61Ti2Cu25Al12 metallic glass for potential use in dental implants; Biocompatibility assessment by in vitro cellular responses. Mater. Sci. Eng. C 2013, 33, 2113–2121. [Google Scholar] [CrossRef]
- Fornell, J.; Van Steenberge, N.; Varea, A.; Rossinyol, E.; Pellicer, E.; Suriñach, S.; Baró, M.D.; Sort, J. Enhanced mechanical properties and in vitro corrosion behavior of amorphous and devitrified Ti40Zr10Cu38Pd12 metallic glass. J. Mech. Behav. Biomed. Mater. 2011, 4, 1709–1717. [Google Scholar] [CrossRef]
- Blanquer, A.; Pellicer, E.; Hynowska, A.; Barrios, L.; Ibáñez, E.; Baró, M.D.; Sort, J.; Nogués, C. In vitro biocompatibility assessment of Ti40Cu38Zr10Pd12 bulk metallic glass. J. Mater. Sci. Mater. Med. 2014, 25, 163–172. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, H.F.; Cheng, Y.; Zheng, Y.F.; Ruan, L.Q. In vitro and in vivo studies on Ti-based bulk metallic glass as potential dental implant material. Mater. Sci. Eng. C 2013, 33, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Hiromoto, S. In vivo evaluation of Zr-based bulk metallic glass alloy intramedullary nails in rat femora. J. Mater. Sci. Mater. Med. 2014, 25, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, Y.; Fan, H.; Wang, Y.; Ning, Z.; Liu, F.; Feng, D.; Jin, X.; Shen, J.; Sun, J.; et al. In vitro and in vivo biocompatibility of an Ag-bearing Zr-based bulk metallic glass for potential medical use. J. Non-Cryst. Solids 2015, 419, 82–91. [Google Scholar] [CrossRef]
- Imai, K.; Hiromoto, S. In vivo evaluation of bulk metallic glasses for osteosynthesis devices. Materials 2016, 9, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, K. In vivo investigation of Zr-based bulk metallic glasses sub-periosteally implanted on the bone surface. J. Mater. Sci. Chem. Eng. 2016, 4, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Kokubun, R.; Wang, W.; Zhu, S.; Xie, G.; Ichinose, S.; Itoh, S.; Takakuda, K. In vivo evaluation of a Ti-based bulk metallic glass alloy bar. Bio-Med. Mater. Eng. 2015, 26, 9–17. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.H.; Huang, Y.S.; Huang, C.H.; Huang, J.C.; Jang, J.S.C.; Lin, Y.S. In-vivo investigations and cytotoxicity tests on Ti/Zr-based metallic glasses with various Cu contents. Mater. Sci. Eng. C 2017, 77, 308–317. [Google Scholar] [CrossRef]
- Wong, C.C.; Wong, P.C.; Tsai, P.H.; Jang, J.S.; Cheng, C.K.; Chen, H.H.; Chen, C.H. Biocompatibility and osteogenic capacity of Mg-Zn-Ca bulk metallic glass for rabbit tendon-bone interference fixation. Int. J. Mol. Sci. 2019, 20, 2191. [Google Scholar] [CrossRef] [Green Version]
- Basu, B.; Sabareeswaran, A.; Shenoy, S.J. Biocompatibility property of 100% strontium-substituted SiO2-Al2O3-P2O5-CaO-CaF2 glass ceramics over 26 weeks implantation in rabbit model: Histology and micro-Computed Tomography analysis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1168–1179. [Google Scholar] [CrossRef]
- Shayan, M.; Padmanabhan, J.; Morris, A.H.; Cheung, B.; Smith, R.; Schroers, J.; Kyriakides, T.R. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization. Acta Biomater. 2018, 75, 427–438. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.D.; Molon, R.S.; Lima, B.P.; Lux, R.; Shi, W.; Junior, M.J.; Spolidorio, D.M.; Vergani, C.E.; Assis Mollo Junior, F. Impact of physical chemical characteristics of abutment implant surfaces on bacteria adhesion. J. Oral Implantol. 2016, 42, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Maruthamuthu, S.; Rajan, S.T. Biocompatibility evaluation of sputtered zirconium-based thin film metallic glass-coated steels. Int. J. Nanomed. 2015, 10, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanka, R.S.; Bendavid, A; Subramanian, B. Cytocompatibility assessment of Ti-Nb-Zr-Si thin film metallic glasses with enhanced osteoblast differentiation for biomedical applications. Colloids Surf. B Biointerfaces 2019, 173, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ghidelli, M.; Gravier, S.; Blandin, J.J.; Djemia, P.; Mompiou, F.; Abadias, G.; Raskin, J.P.; Pardoen, T. Extrinsic mechanical size effects in thin ZrNi metallic glass films. Acta Mater. 2015, 90, 232–241. [Google Scholar] [CrossRef]
- Ghidelli, M.; Idrissi, H.; Gravier, S.; Blandin, J.J.; Raskin, J.P.; Schryvers, D.; Pardoen, T. Homogeneous flow and size dependent mechanical behavior in highly ductile Zr65Ni35 metallic glass films. Acta Mater. 2017, 131, 246–259. [Google Scholar] [CrossRef]
| Group | Representatives |
|---|---|
| I | Zr-Al-Ni, Zr-Al-Cu, Ln-Al-Ni, Ln-Al-Cu Zr-Al-Ni-Cu, Ln-Al-Ni-Cu, Zr-Ti-Al-Ni-Cu Zr-Ga-Ni, Ln-Ga-Ni, Ln-Ga-Cu |
| II | Fe-Zr-B, Fe-Hf-B Fe-Zr-Hf-B, Fe-Co-Ln-B Co-Zr-Nb-B, Co-Fe-Ta-B |
| III | Fe-(Al, Ga)-Metalloid |
| IV | Mg-Ln-Ni, Mg-Ln-Cu Zr-Ti-Be-Ni-Cu, Ti-Cu-Ni-Sn-Be, Ti-Cu-Ni-Sn-Be-Zr |
| V | Pd-Ni-P, Pd-Cu-Ni-P, Pt-Ni-P |
| VI | Cu-Zr-Ti, Ni-Nb-Ta, Ni-Nb-Sn Ti-Zr-Cu-Ni, Ti-Ni-Cu-Sn, Ti-Cu-Ni-Mo-Fe |
| VII | Ca-Mg-Cu, Ca-Mg-Zn |
| Mechanical Properties | Yield Strength (MPa) | Fracture Strength (MPa) | Young’s Modulus (GPa) | Hardness (MPa) |
|---|---|---|---|---|
| 316L stainless steel [25,26,27] | >175 | 190–690 | 200–203 | 3580 |
| Ti-6Al-4V alloy [24,26,27] | 853 | 950 | 108–116 | 3138 |
| Zr65Al7.5Ni10Cu17.5 BMGs [18] | - | 1500–1700 | 70–80 | - |
| Zr60Nb5Cu22.5Pd5Al7.5 BMGs [35] | 1378 | 1724 | 70–85 | - |
| Zr60Nb5Cu20Fe5Al10 BMGs [35] | 1393 | 1795 | 70–85 | - |
| Zr50Cu35Al7Nb5Pd3 BMGs [36] | 1806 | - | 88 | 5060 |
| Zr55Cu30Al7Nb5Pd3 BMGs [36] | 1664 | - | 86 | 4790 |
| Zr60.14Cu22.31Fe4.85Al9.7Ag3 BMGs [37] | - | 1720 | 82 | 4200 |
| Human bone (femur) [28,29,30,31] | 80 | 120 | 15–20 | - |
| Mechanical Properties | Yield Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|
| 316L stainless steel | >175 | 200–203 |
| Ti-6Al-4V alloy | 853 | 108–116 |
| Pure titanium | 800 ± 50 | 100 ± 7 |
| Ti-based BMGs | 2000 ± 78 | 80 ± 12 |
| Human bone (femur) | 80 | 15–20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, K.; Zhou, X.; Liu, X. Application of Zr and Ti-Based Bulk Metallic Glasses for Orthopaedic and Dental Device Materials. Metals 2020, 10, 203. https://doi.org/10.3390/met10020203
Imai K, Zhou X, Liu X. Application of Zr and Ti-Based Bulk Metallic Glasses for Orthopaedic and Dental Device Materials. Metals. 2020; 10(2):203. https://doi.org/10.3390/met10020203
Chicago/Turabian StyleImai, Kazuhiro, Xiao Zhou, and Xiaoxuan Liu. 2020. "Application of Zr and Ti-Based Bulk Metallic Glasses for Orthopaedic and Dental Device Materials" Metals 10, no. 2: 203. https://doi.org/10.3390/met10020203
APA StyleImai, K., Zhou, X., & Liu, X. (2020). Application of Zr and Ti-Based Bulk Metallic Glasses for Orthopaedic and Dental Device Materials. Metals, 10(2), 203. https://doi.org/10.3390/met10020203





