3.1. Surface Analysis
Figure 1 shows SEM images of the as-received specimen of 6061-T6 aluminum alloy surface. Small precipitates (labeled as A–C) may be seen (
Figure 1b). In accordance with EDS, presented in
Table 1, these precipitates correspond to particles rich in Fe and could be considered as elements of the following phases: Al
3Fe, Al–Si–Mn–Fe, and α-Al(Fe, Mn, Cu) [
9,
10,
36]. The elemental composition of the surface layer is indicated by zone D.
Figure 2a presents the SEM image of the film formed on the surface of the sample after 5 days of immersion in seawater from the Cozumel Channel under laminar flow. It can be clearly seen that the passive layer begins to break down around the alloying elements (
Figure 2b). Probably, this layer is less protective in the vicinity of the intermetallic particles, causing the formation of local electrochemical cells with the Al matrix. EDS analysis (
Table 2) confirmed the presence of particles mentioned above. According to EDS (
Table 2), zone A is characterised by its higher content of Al, O, and Cl, which could relate to corrosion products. According to previous studies, at pH > 8.5, Al(H
2O)
63+ cations appear, while in the range of pH 4.5–8.5, Al(OH)
3 predominates [
37,
38]. In chloride solutions, aluminum metal ionises rapidly to the Al
3+ ion, which also hydrolyzes very rapidly (owing to the negative potential value) [
39]. Both of these Al cations can react with chloride ions and form AlCl
3 soluble in water (31.77 wt.%) [
40]; this is converted later to a relatively stable species of basic aluminum chloride (AlCl
3·H
2O) and transformed slowly to Al(OH)
3 and finally to Al
2O
3∙H
2O, an important corrosion product for the repassivation process of the aluminum surface [
39].
Based on EDS analysis, in zone B, in addition to oxygen and aluminum, elements of calcium and carbon were present, both possibly as a part of a CaCO3 precipitate originating from seawater. Meanwhile, the layer in zone C maintained a composition similar to that of the alloy, which indicates that the corrosion process was still beginning on the surfaces of similar areas.
After removal of the layer of corrosion products (
Figure 2b), an area damaged by pitting and cracking was observed on the alloy surface. However, some precipitates remained on the surface of AA6061-T6, which according to EDS (
Table 2) correspond to cathodic particles rich in Fe, α-Al(Fe,Mn,Cu)Si, that promoted the preferential dissolution of the aluminum matrix (local alkalisation) [
10].
Figure 3 compares SEM images of the aluminum alloy 6061-T6 after exposure at 30 days in laminar flow (
Figure 3a,c) and stationary flow (
Figure 3b,d). In these micrographs, the products formed on the surfaces of the alloy (
Figure 3a,b) can be seen and compared; EDS analysis is summarised in
Table 3. In laminar flow (
Figure 3a), the segregation of particles rich in Cu (particles A) with the presence of O and Al could be considered as the phases of Al
2Cu, AlMgSiCu (Q-phase), and Al
7Cu
2Fe, both relatively resistant to corrosion because they are nobler than the aluminum matrix [
4,
13]. Q-phase acts as a cathode and does not contribute to intergranular corrosion when it is not connected to any other Cu particle. Otherwise, the Q-phase as cathode promotes the development of intergranular corrosion, initiated in the presence of chloride ions (NaCl). In the corrosion layer, particles without Cu appeared (particles B), rich in Fe, Al, and Mg, which could be attributed to the following phases: Al–Si–Mn–Fe and Al–Mg–Si, reported for aluminum alloy series 6xxx [
36]. Zone C of the layer formed under stationary flow (
Figure 3b) presented a similar composition of the alloy (
Table 3), however, with the oxide layer of Al
2O
3·H
2O on the alloy surface 6061-T6, as a transformation product of basic aluminum (AlCl
3·H
2O) in the presence of NaCl [
39]. This layer is part of the entire surface (
Figure 3a), since the three zones (A–C) have a high oxygen content (
Table 3) [
4].
Figure 3c corresponds to the SEM image of the aluminum alloy 6061-T6 exposed in seawater at 30 days with flow after removal of the layer of corrosion products. An area on the surface damaged by cracking and fissures can be observed, while the surface exposed to seawater without flow (
Figure 3d) shows less damage, with pitting holes of several diameters. In contrast, on the surface exposed to laminar flow for this same time period, the pits are not clearly observable, but localised corrosion damage appeared in the form of fissures. This effect is due to the seawater flow, which facilitates the oxygen diffusion and its renewal at the metal interface, accelerating the corrosion process. Moreover, the seawater flow attributes to the detachment of the destroyed passive layer and the appearance of new “fresh” areas, where the oxygen diffuses at the cathodic sites, as an oxidising agent needed for the cathodic corrosion reaction. It is also observed that, on both surfaces (
Figure 3c,d), some particles remained (named as D and F), and according to EDS (
Table 3), they correspond to cathodic rich in Fe [
7], reported in the reference sample as α-Al(Fe,Mn,Cu)Si [
36]; these intermetallic particles promoted the preferential dissolution of the aluminum matrix [
41,
42].
3.4. Electrochemical Current Noise Measurement (ECN)
Figure 6 shows the current oscillations, and it can be seen that, at the beginning of the experiment (0 days), the current density of AA6061-T6 surface immersed in laminar flow of seawater is higher in approximately 50 µA cm
−2 than that current in stationary flow. This suggested that the corrosion of the aluminum alloy 6061-T6 surface in laminar flow initiates faster, when the oxide layer on the alloy begins to break down. However, at the end of the experiment (30 days), the current value diminished suddenly compared with the initial values. However, in stationary flow, the current shifted to one order higher values than those in laminar flow, suggesting an acceleration of the corrosion process at that time [
45]. Conversely, the current oscillations in stationary conditions (
Figure 6b) presented slow variations, while for flow conditions (
Figure 6a), intense current fluctuations were acquired with greater amplitude, which suggest greater corrosion [
46]. The observed current oscillations registered in (μA cm
−2) (
Figure 6) correspond to the variation of the free corrosion potential (φ
corr) values in several mV (
Figure 4).
The corrosion current density was calculated from the value of polarisation resistance
Rp (on the assumption that
Rp is equivalent to polarisation resistance noise
Rn) obtained by ECN tests, according to the Stern–Geary equation (Equation (5)).
Rn is calculated by dividing the standard deviation of potential by standard deviation of current. (The potential noise can be modeled as the action of the current noise on the metal-solution impedance):
where
Rp is the polarisation resistance, and
ba and
bc are the Tafel coefficients. In this research, the
B value employed was 0.26 V, taking common values for
ba and
bc of aluminum alloys [
47,
48]. The calculated values (
Table 5) show that the corrosion current of AA6061-T6 increases with the time of exposure, being more than one order higher when the alloy is exposed under laminar flow of seawater, while in stationary conditions, it maintains almost similar values up to 30 days.
Subsequently, Faraday’s law was applied to calculate the corrosion rate (CR,
Table 6) in the following form:
where
Ew stands for the equivalent mass of AA6061-T6,
icorr is the corrosion density (A cm
−2),
ρ is the metal density,
K is a constant (3272 mm/A cm year), and
A is the exposed specimen area (1 cm
2) [
49].
The corrosion rate values presented in
Table 6 indicate that, under laminar flow, the values varied 0.08 and 0.20 mm per year while, in stationary conditions, they were between 0.012 and 0.162 mm per year.
With the statistical data obtained from the corrosion current, the pitting index (PI) [
20] was calculated in order to reveal AA606-T6 susceptibility to localised corrosion for the laminar and stationary:
where
σi is the standard deviation and
irms the main square root of current noise.
Values of PI above 0.1 may indicate localised corrosion [
21,
50]. The pitting indexes are shown in
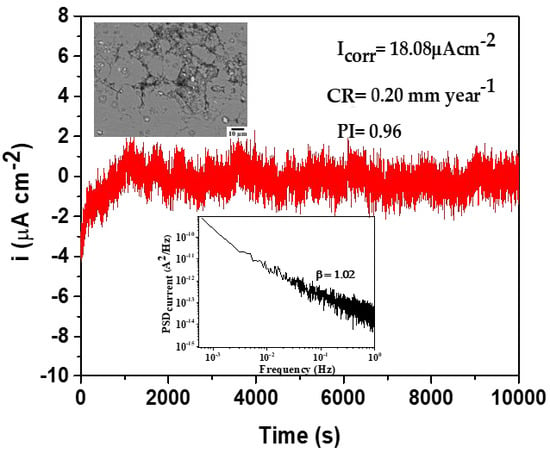
Table 7. Thus, the calculated PI value suggests that, at the end of the experiment (30 days), for both flow cases, AA606-T6 showed pitting corrosion approximately four times higher in flow conditions, reaching PI = 0.96. These facts agree with the SEM images (
Figure 3) comparing the corrosion attacks on AA6061-T6 exposed to both flow conditions.
The current fluctuations, considered as ECN, were transformed into the frequency domain to estimate PSD slopes (
β exponent).
Figure 7 compares the PSD plots in bi-logarithmic scale, corresponding to AA6061-T6 surfaces after 30 days of exposure in seawater under laminar and stationary flows. In each case, the
β exponent decreases as the frequency increases, and this fact could be associated with the advance of the localised corrosion attacks on the alloy surface [
35]. At 30 days,
β values are similar in laminar and stationary flows (1.0 and 0.94, respectively) and may be attributed to the fractional Gaussian noise (
fGn), associated with a persistent process [
27]. This type of noise (
fGn) is considered also as a stationary process [
25].
Figure 8 shows the spontaneous energy E during the corrosion process. At the beginning, after 5 days, the energy was of an order of magnitude higher in laminar flow (1.1 × 10
−4). This fact is consistent with the SEM image presented in
Figure 2, which showed the accelerated corrosion process causing severe damage to the alloy surface exposed to laminar flow.
However, at the end of the experiment (30 days), the energy diminished in magnitude, being very similar in the order of 10−7, for both flows probably on account of the formation of layers of corrosion products with different characteristics that act as a physical barrier on the alloy surfaces, slowing down the corrosion attack.