Effect of CO2/H2S and Applied Stress on Corrosion Behavior of 15Cr Tubing in Oil Field Environment
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Immersion Tests
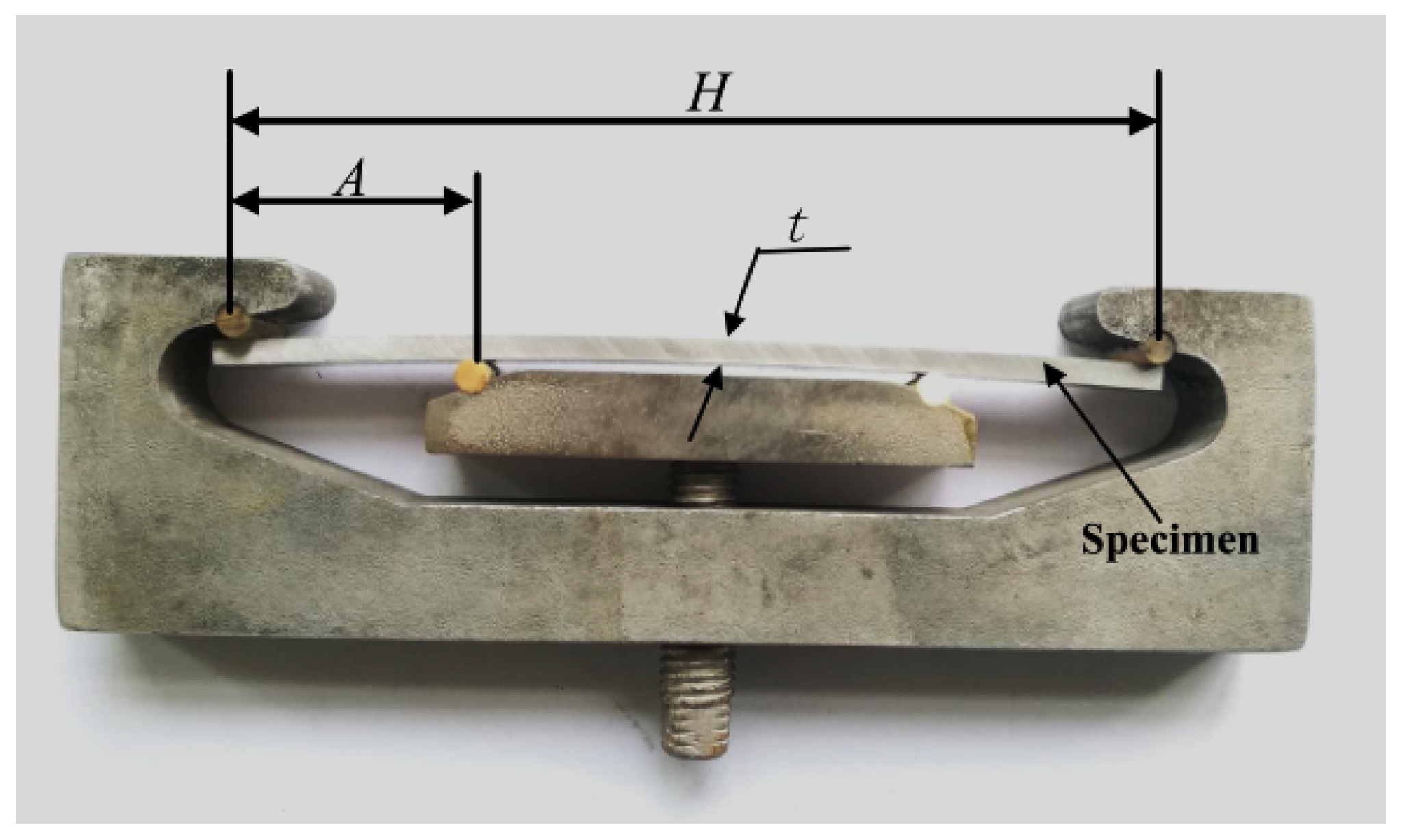
2.3. SCC Testing
2.4. Characterization
3. Results and Discussion
3.1. Average Corrosion Rate
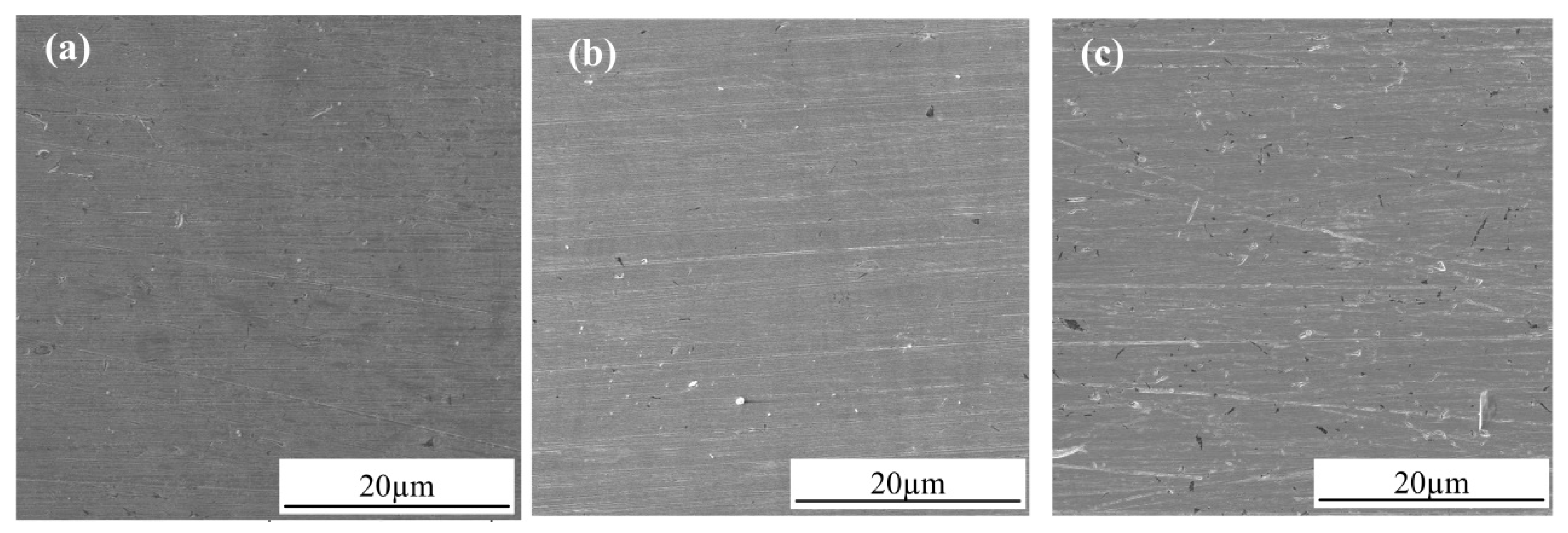
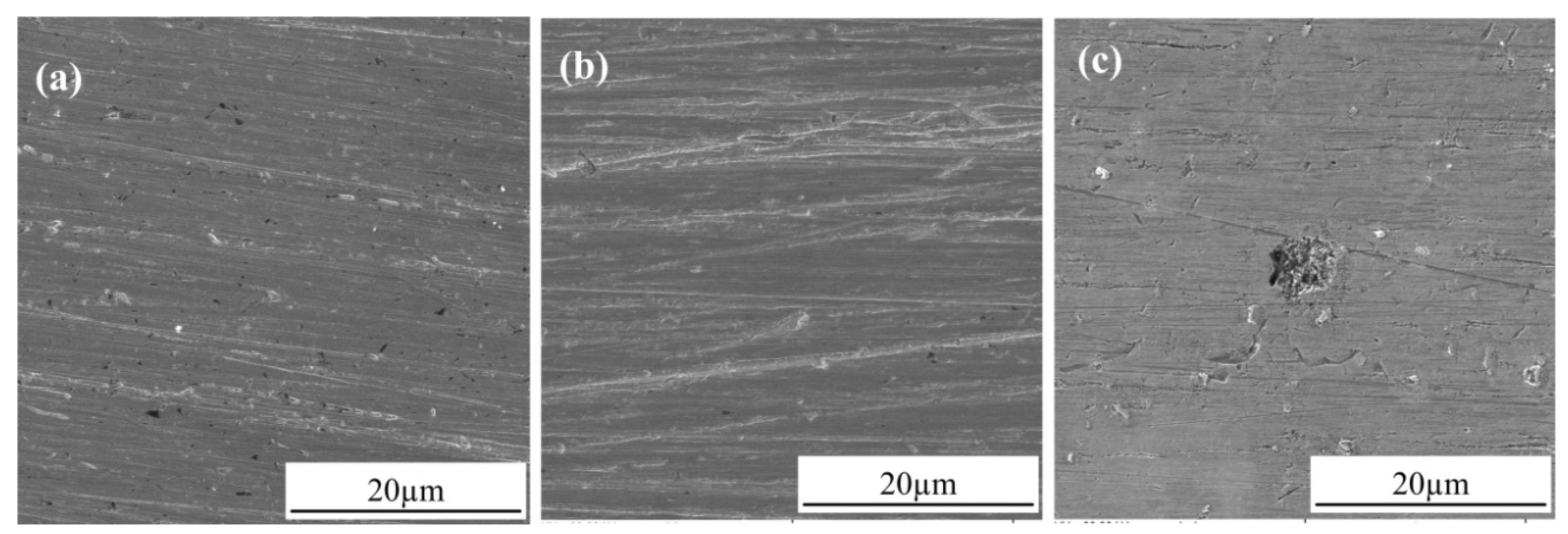
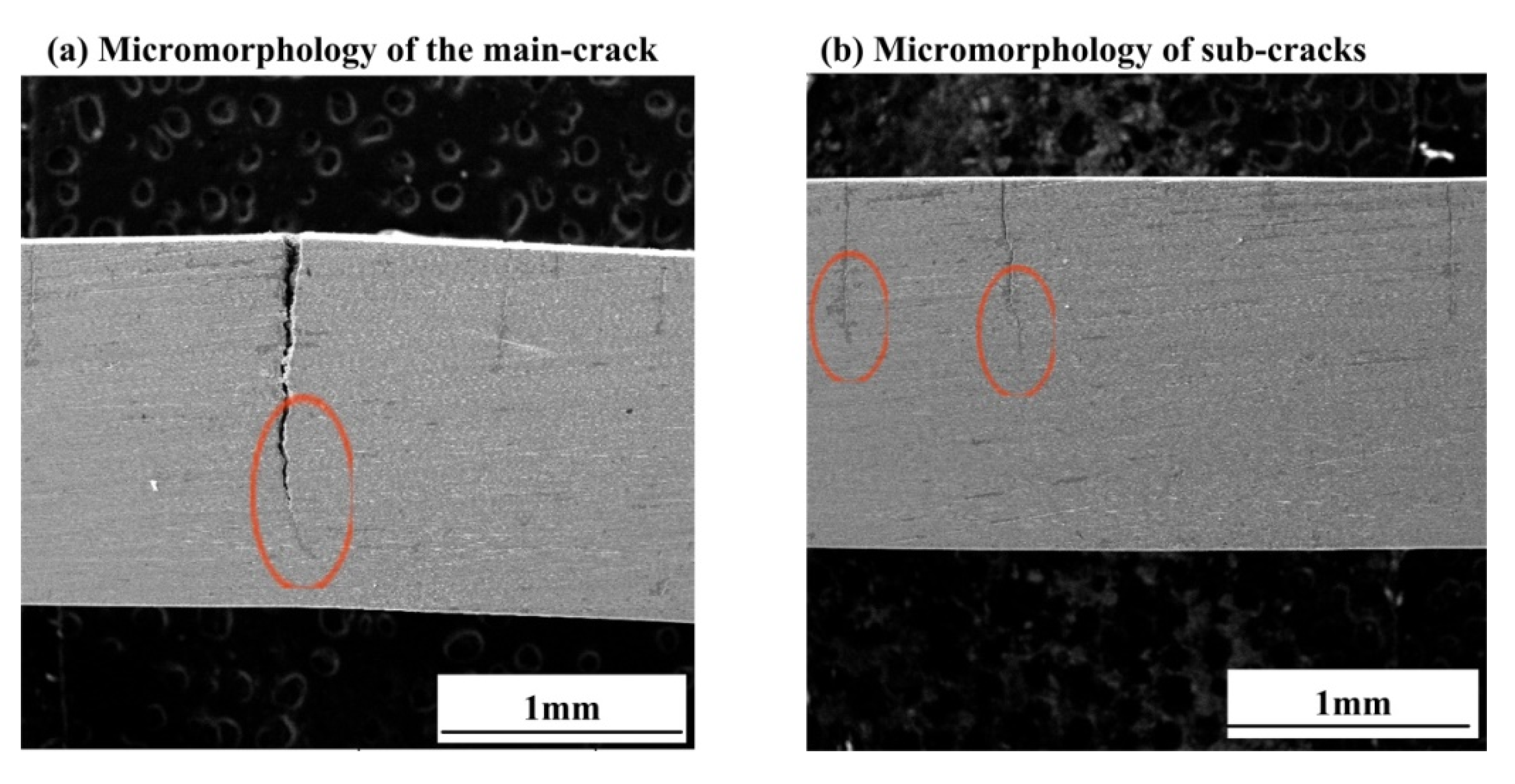
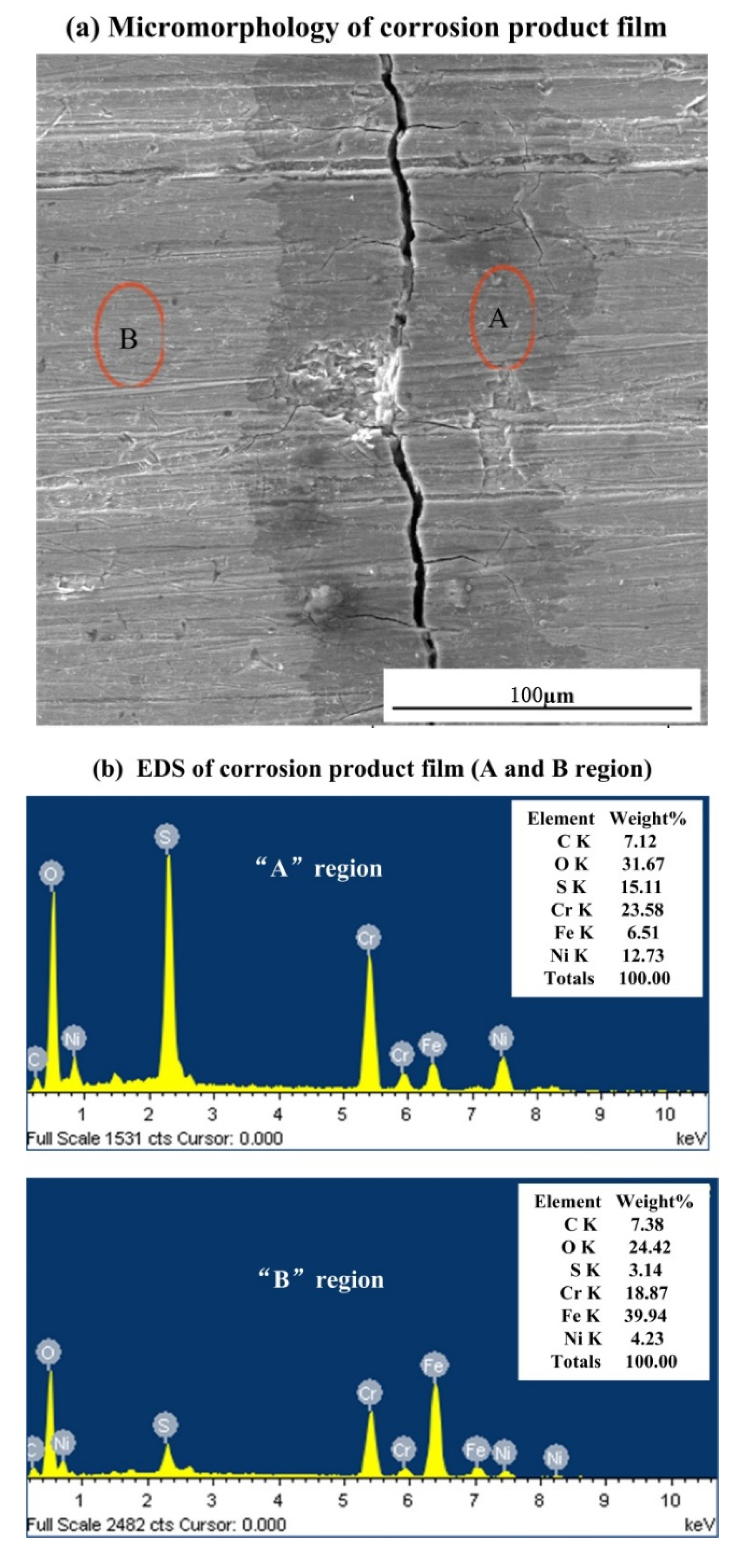
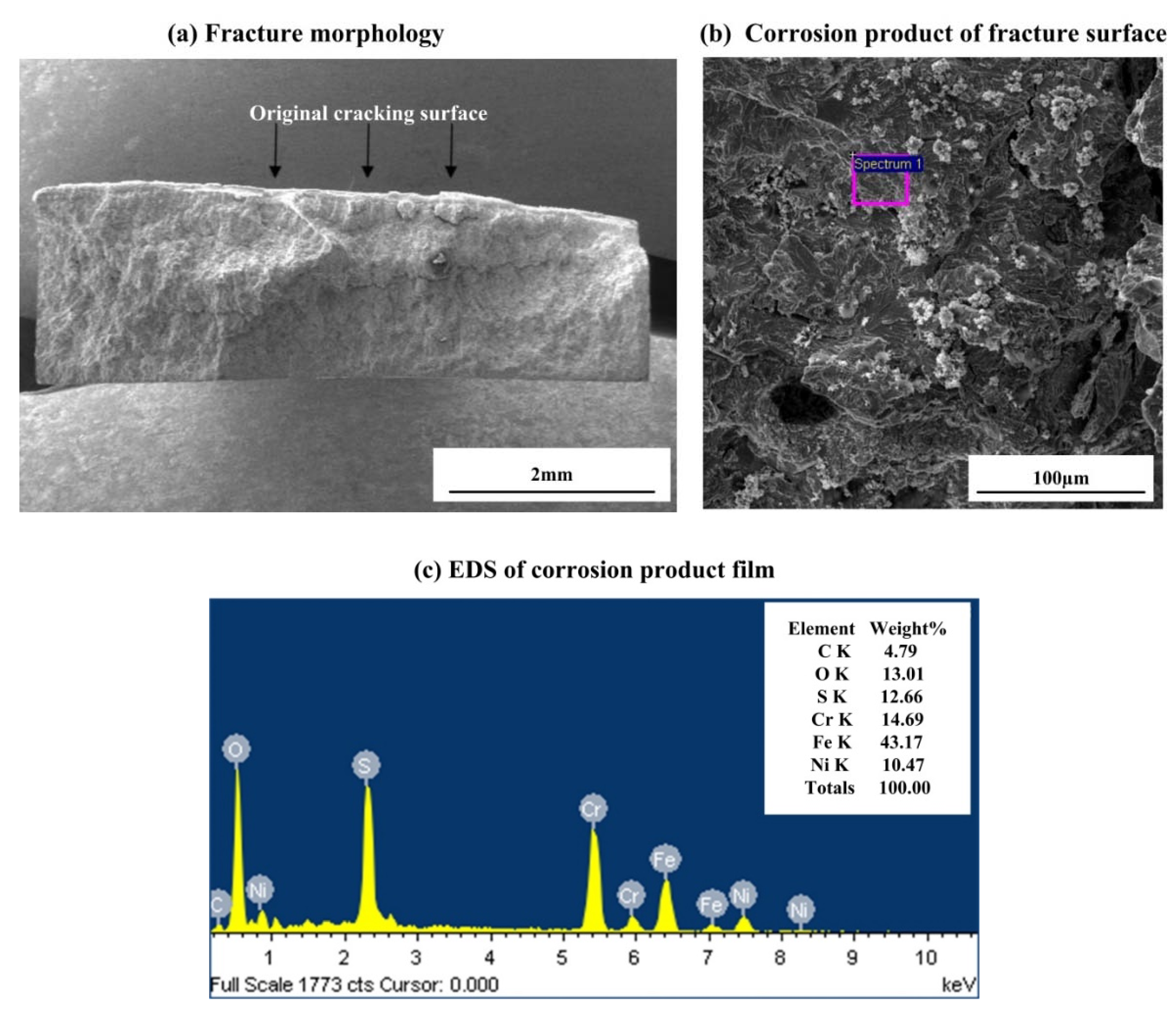
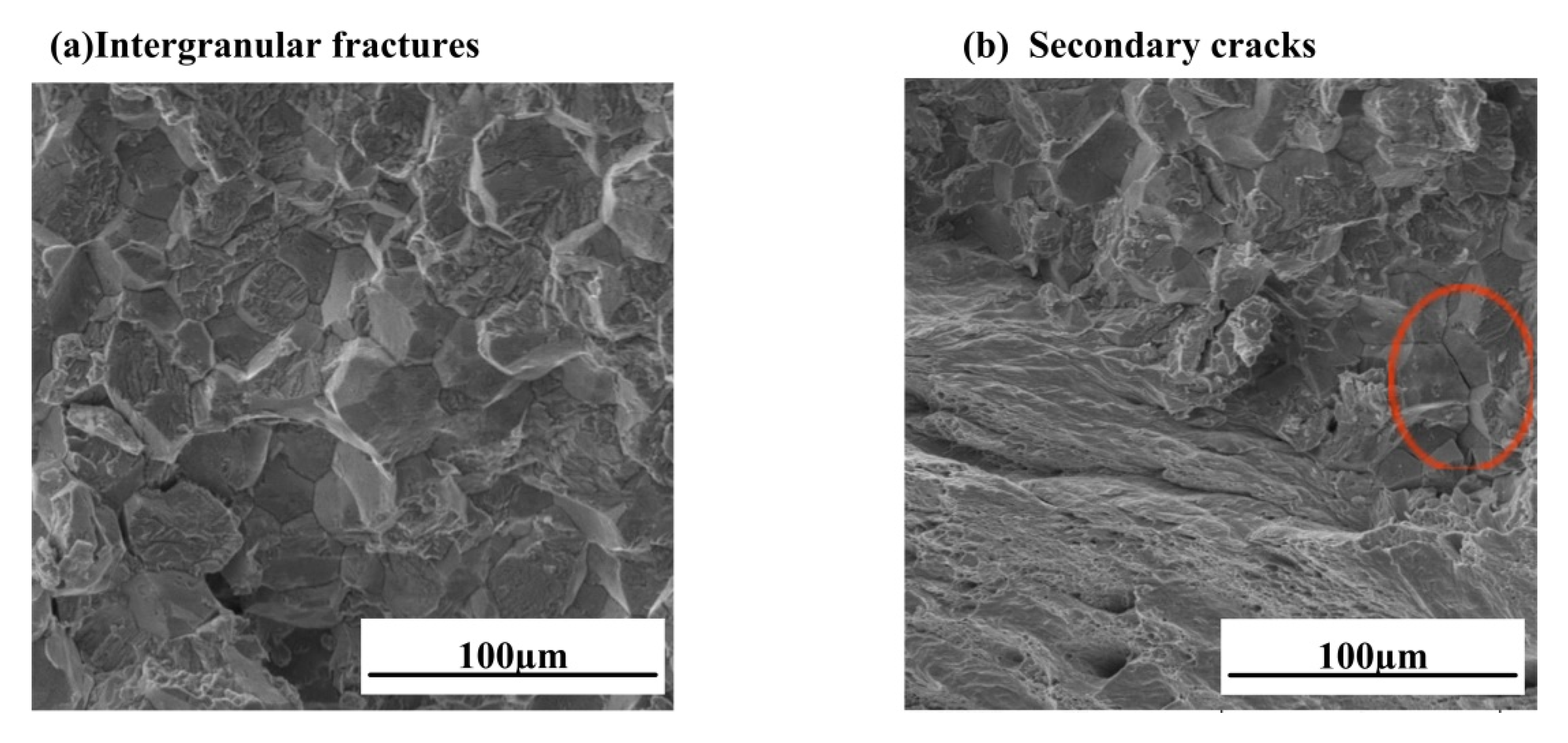
3.2. Observation of Corrosion Morphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.H.; Han, Y.; Bai, Z.Q.; Wei, B. The experiment research of corrosion behavior about Ni-based alloys in simulant solution containing H2S/CO2. Electrochim. Acta 2011, 56, 7725. [Google Scholar] [CrossRef]
- Wang, H.T.; Han, E.H. Simulation of metastable corrosion pit development under mechanical stress. Electrochim. Acta 2013, 90, 128. [Google Scholar] [CrossRef]
- Zanotto, F.; Grassi, V.; Balbo, A. Stress corrosion cracking of LDX2101 duplex stainless steel in chloride solutions in the presence of thiosulphate. Corros. Sci. 2014, 80, 205. [Google Scholar] [CrossRef]
- Bueno, A.H.S.; Moreir, E.D.; Gomes, J.A.C.P. Evaluation of stress corrosion cracking and hydrogen embrittlement in an API grade steel. Eng. Fail. Anal. 2014, 36, 423. [Google Scholar] [CrossRef]
- Lu, L.S.; Song, W.W.; Yang, X.T. Corrosion Causes of Premium Connection S13Cr Tubing in a well. Corros. Prot. 2015, 36, 76. [Google Scholar]
- Fajardo, S.; Bastidas, D.M.; Criado, M. Electrochemical study on the corrosion behavior of a new low-nickel stainless steel in carbonated alkaline solution in the presence of chlorides. Electrochim. Acta 2014, 129, 60. [Google Scholar] [CrossRef]
- Machuca, L.L.; Bailey, S.I.; Gubner, R. Systematic study of the corrosion properties of selected high-resistance alloys in natural seawater. Corros. Sci. 2012, 64, 8. [Google Scholar] [CrossRef]
- Zhao, X.H.; Bai, Z.Q.; Feng, Y.R.; Wei, B.; Yin, C.X.; Wang, J.Z. Effects of heat treatment and precipitated phase on corrosion resistance of Ni-based alloy. Trans. Mater. Heat Treat. 2011, 33, 39. [Google Scholar]
- Sun, Y.L.; Bai, Z.Q.; Zhang, G.C.; Wei, B.; Zhu, S.D. Research Statue on Anticorrosion Properties of Bimetallic Composite Tubes in Oil and Gas Field. Total Corros. Control 2011, 25, 10. [Google Scholar]
- Zhao, S.; Lan, W. Present status and research progress of anticorrosion technology in pipeline. Surf. Technol. 2015, 44, 112. [Google Scholar]
- Palimi, M.J.; Peymannia, M.; Ramezanzadeh, B. An evaluation of the anticorrosion properties of the spinel nanopigment filled epoxy composite coatings applied on the steel surface. Prog. Org. Coat. 2015, 80, 164–175. [Google Scholar] [CrossRef]
- Prabhu, R.A.; Venkatesha, T.V.; Shanbhag, A.V. Inhibition effects of some Schiff’s bases on the corrosion of mild steel in hydrochloric acid solution. Corros. Sci. 2008, 50, 3356. [Google Scholar] [CrossRef]
- Sumithra, K.; Kavita, Y.; Manivannan, R.; Noyel, V.S. Electrochemical investigation of the corrosion inhibition mechanism of Tectona grandis leaf extract for SS304 stainless steel in hydrochloric acid. Corros. Rev. 2017, 35, 111–121. [Google Scholar]
- Xiang, R.; Luo, D.D.; Wei, D. Economic evaluation of corrosion protection measures based on corrosion status of typical gathering pipelines. Saf. Environ. 2013, 13, 207. [Google Scholar]
- Ningshen, S.; Sakairi, M.; Suzuki, K.; Ukai, S. The corrosion resistance and passive film compositions of 12% Cr and 15% Cr oxide dispersion strengthened steels in nitric acid media. Corros. Sci. 2014, 78, 322. [Google Scholar] [CrossRef]
- Jiménez, H.; Olaya, J.J.; Alfonso, J.E.; Pineda-Vargas, C.A. Corrosion resistance of Ni-based coatings deposited by spray and fuse technique varying oxygen flow. Surf. Coat. Technol. 2017, 321, 341. [Google Scholar] [CrossRef]
- Mesquita, T.J.; Chauveau, E.; Mantel, M. Corrosion and metallurgical investigation of two supermartensitic stainless steels for oil and gas environments. Corros. Sci. 2014, 81, 152. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.S.; Li, J.; Liu, W.Y.; Liu, M.Q.; Gao, W.X.; Shi, T.H. Stress corrosion crack evaluation of super 13Cr tubing in high-temperature and high-pressure gas wells. Eng. Fail. Anal. 2019, 95, 263. [Google Scholar] [CrossRef]
- Nacéra, S.M.; Brian, C.; Russell, K.; Tanmay, A.; James, S. Sour Service Limits of Martensitic Stainless Steels: A Review of Current Knowledge, Test Methods and Development Work. In CORROSION 2013; NACE International: Houston, TX, USA, 2013; p. 2639. [Google Scholar]
- Qiu, Z.C.; Liu, X.; Zhang, N. Corrosion Behavior of 13Cr Steel in Different Temperature. Adv. Mater. Res. 2014, 37, 168. [Google Scholar] [CrossRef]
- Lv, X.H.; Zhang, F.X.; Yang, X.T. Corrosion Performance of High Strength 15Cr Martensitic Stainless Steel in Severe Environments. J. Iron Steel Res. Int. 2014, 21, 774. [Google Scholar]
- Wan, J.Q.; Ran, Q.X.; Li, J.; Xu, Y.L. A new resource-saving, low chromium and low nickel duplex stainless steel 15Cr-xAl-2Ni-yMn. Mater. Des. 2014, 53, 43. [Google Scholar] [CrossRef]
- Meng, J.; Chambers, B.; Kane, R.; Skogsberg, J.; Kimura, M.; Shimamoto, K. Environmentally Assisted Cracking Testing of High Strength 15 Cr Steel in Sour Well Environments. In CORROSION 2011; NACE International: Houston, TX, USA, 2011; p. 100. [Google Scholar]
- Brian, C.; James, S.; John, M.; Mitsuo, K. Evaluation of Environmentally Assisted Cracking Resistance of High Strength 15Cr Steel in Sour Well Environments. In CORROSION 2012; No.C0001353; NACE International: Houston, TX, USA, 2012. [Google Scholar]
- ISO 15156-3-2015. Petroleum and Natural Gas Industries-Materials for Use in H2S-Containing Environments in Oil and Gas Production–Part 3: Cracking-Resistant CRAS (Corrosion-Resisitant Alloys) and Other Alloys; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- NACE International. Available online: http://eurocorr.efcweb.org/2014/abstracts/10/7368.pdf (accessed on 10 March 2020).
- JB/T (1999). GB/T Standard 7901. Metal Materials-Uniform Corrosion-Methods of Laboratory Immersion Testing; Institute of Integrated Technology and Economics of Mechanical Industrial Instruments and Meters: Beijing, China, 2001. [Google Scholar]
- GB/T (2000). GB/T Standard 15970.2. Corrosion of Metals and Alloys-Stress Corrosion Testing-Part 2: Preparation and Use of Bent-Beam Specimens; China Standards Press: Beijing, China, 2001. [Google Scholar]
- Hinds, G.; Wickstrom, L.; Mingard, K.; Turnbull, A. Impact of surface condition on sulphide stress corrosion cracking of 316L stainless steel. Corros. Sci. 2013, 71, 43–52. [Google Scholar] [CrossRef]
- Jiang, W.J. Piping Material Selection for Wet H2S Environment of Hydrotreating Unit. Shandong Chem. Ind. 2019, 48, 118. [Google Scholar]
- Kittel, J.; Ropital, F.; Grosjean, F.; Sutter, E.M.M.; Tribollet, B. Corrosion mechanisms in aqueous solutions containing dissolved H2S. Part 1: Characterisation of H2S reduction on a 316L rotating disc electrode. Corros. Sci. 2013, 66, 324. [Google Scholar] [CrossRef]
- Smith, S.N. Current Understanding of Corrosion Mechanisms Due to H2S in Oil and Gas Production Environments. In CORROSION 2015; NACE: Dallas, TX, USA, 2015; p. 5485. [Google Scholar]
- Bao, M.Y.; Ren, C.Q.; Hu, J.S.; Liu, B.; Li, J.; Wang, F.; Guo, X. Stress Induced Corrosion Electrochemical Behavior of Steels for Oil and Gas Pipes. Chin. Soc. Corros. Prot. 2017, 37, 504. [Google Scholar]
- Plennevaux, C.; Kittel, J.; Frégonèse, M.; Normand, B.; Ropital, F.; Grosjean, F.; Cassagne, T. Contribution of CO2 on hydrogen evolution and hydrogen permeation in low alloy steels exposed to H2S environment. Electrochem. Commun. 2013, 26, 17. [Google Scholar] [CrossRef]
- Sun, W.; Nesic, S.; Papavinasam, S.K. Kinetics of Iron Sulfide and Mixed Iron Sulfide/Carbonate Scale Precipitation in CO2/H2S Corrosion. In Corrosion 2006; No. 06644; NACE International: San Diego, CA, USA, 2006. [Google Scholar]
- Choi, Y.S.; Nesic, S.; Ling, S. Effect of H2S on the CO2 corrosion of carbon steel in acidic solutions. Electrochim. Acta 2011, 56, 1752. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Zhang, Z.; Zhao, W.T.; Liu, L.; Shi, T.H. Corrosion Evaluation of Tubing Steels and Material Selection in the CO2/H2S Coexistent Environment. In CORROSION 2018; NACE International: Phoenix, AZ, USA, 2018. [Google Scholar]
- Lu, Y.; Zhang, Y.; Liu, Z.G.; Zhang, Y.; Wang, C.M.; Guo, H.J. Corrosion control in CO2/H2S-produced water of offshore oil fields. Anti Corros. Methods Mater. 2014, 61, 166. [Google Scholar] [CrossRef]
- Jingen, D.; Wei, Y.; Xiaorong, L.; Xiaoqin, D. Influence of H2S Content on CO2 Corrosion Behaviors of N80 Tubing Steel. Pet. Sci. Technol. 2011, 29, 1387. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, Y.; Guo, X.P.; Jiang, F.; Shi, D.Y.; Chen, Z.Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012, 65, 37. [Google Scholar] [CrossRef]

| Condition Number | Test Conditions | Vcorr (µm/y), w—Range | |||
|---|---|---|---|---|---|
| 90 °C | 150 °C | ||||
| 0% | 80%σs | 0% | 80%σs | ||
| 1 | PH2S = 0.1 MPa | 0.73 (w = 0.20) | 1.30 (w = 0.35) | 1.24 (w = 0.36) | 1.91 (w = 0.06) |
| 2 | PH2S = 0.5 MPa | 1.0 (w = 0.36) | 1.60 (w = 0.40) | 1.7 (w = 0.20) | 3.2 (w = 0.42) |
| 3 | PH2S = 1 MPa | 11.03 (w = 0.20) | 15.20 (w = 0.43) | 29.03 (w = 0.4) | Fracture |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Huang, W.; Li, G.; Feng, Y.; Zhang, J. Effect of CO2/H2S and Applied Stress on Corrosion Behavior of 15Cr Tubing in Oil Field Environment. Metals 2020, 10, 409. https://doi.org/10.3390/met10030409
Zhao X, Huang W, Li G, Feng Y, Zhang J. Effect of CO2/H2S and Applied Stress on Corrosion Behavior of 15Cr Tubing in Oil Field Environment. Metals. 2020; 10(3):409. https://doi.org/10.3390/met10030409
Chicago/Turabian StyleZhao, Xuehui, Wei Huang, Guoping Li, Yaorong Feng, and Jianxun Zhang. 2020. "Effect of CO2/H2S and Applied Stress on Corrosion Behavior of 15Cr Tubing in Oil Field Environment" Metals 10, no. 3: 409. https://doi.org/10.3390/met10030409
APA StyleZhao, X., Huang, W., Li, G., Feng, Y., & Zhang, J. (2020). Effect of CO2/H2S and Applied Stress on Corrosion Behavior of 15Cr Tubing in Oil Field Environment. Metals, 10(3), 409. https://doi.org/10.3390/met10030409





