Corrosion Study of Selective Laser Melted IN718 Alloy upon Post Heat Treatment and Shot Peening
Abstract
1. Introduction
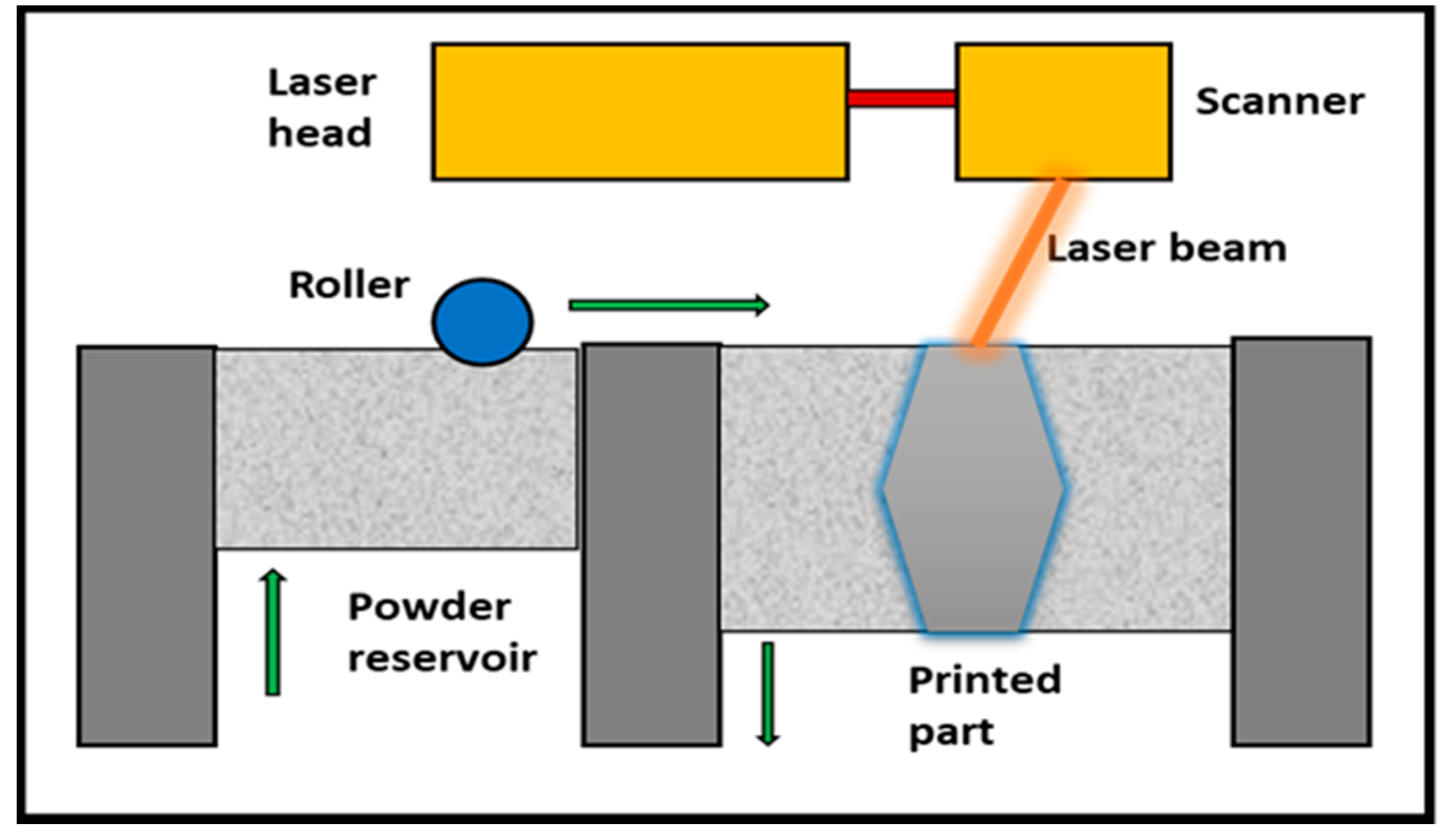
2. Materials and Methods
3. Results and Discussion
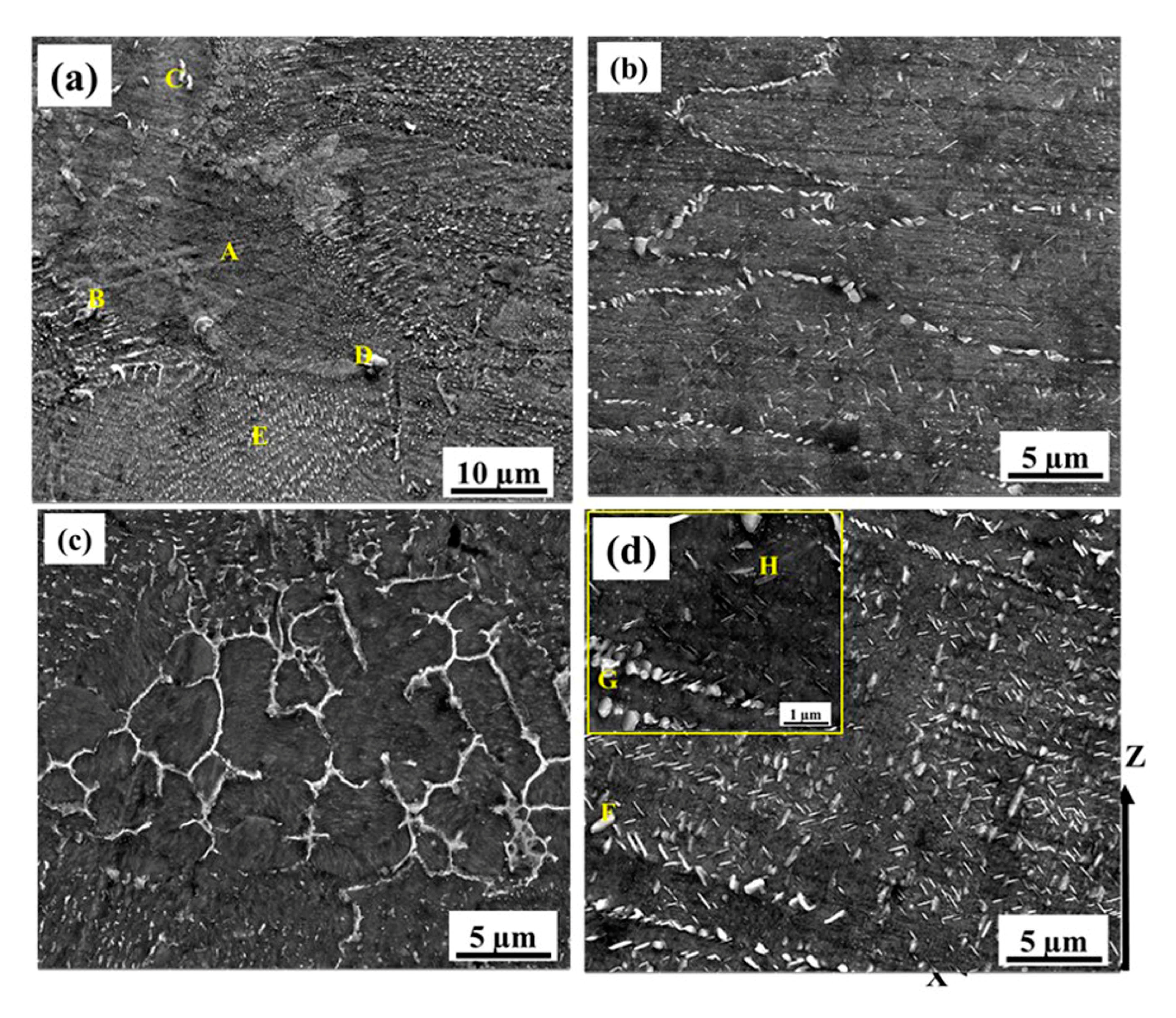
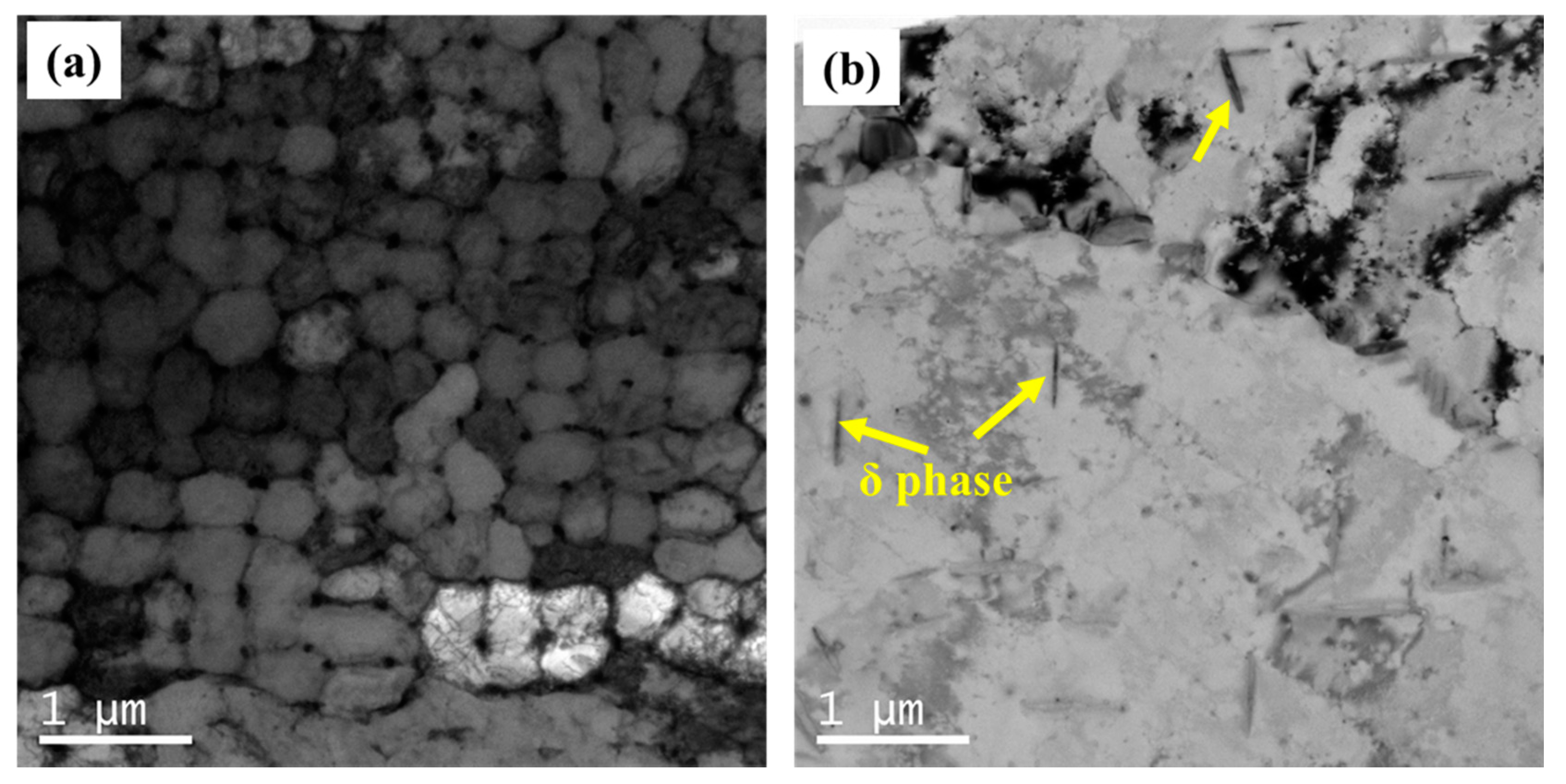
3.1. Microstructural Analysis
3.2. Surface Roughness
3.3. Electrochemical Measurements
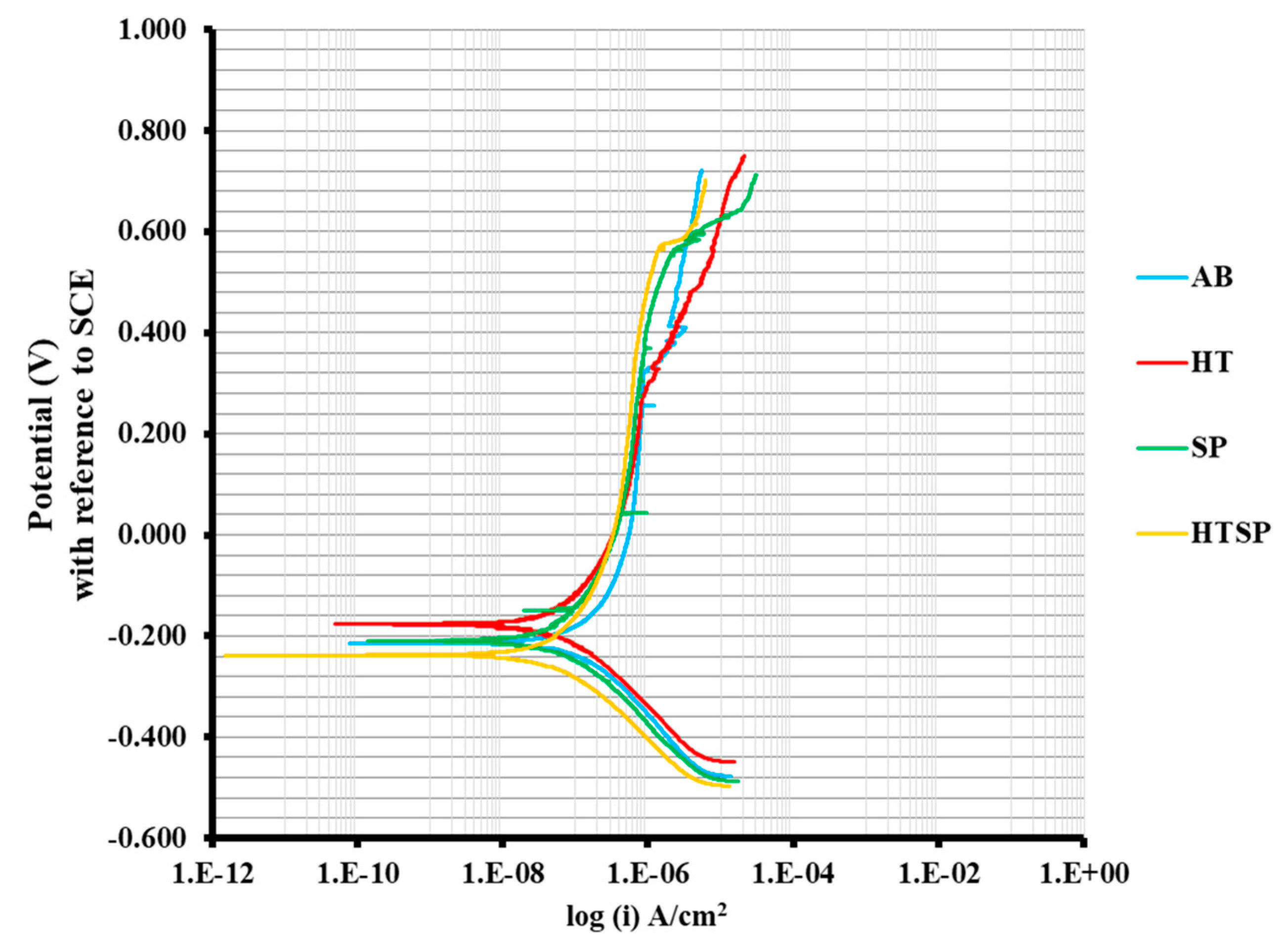
3.3.1. Potentiodynamic Polarization
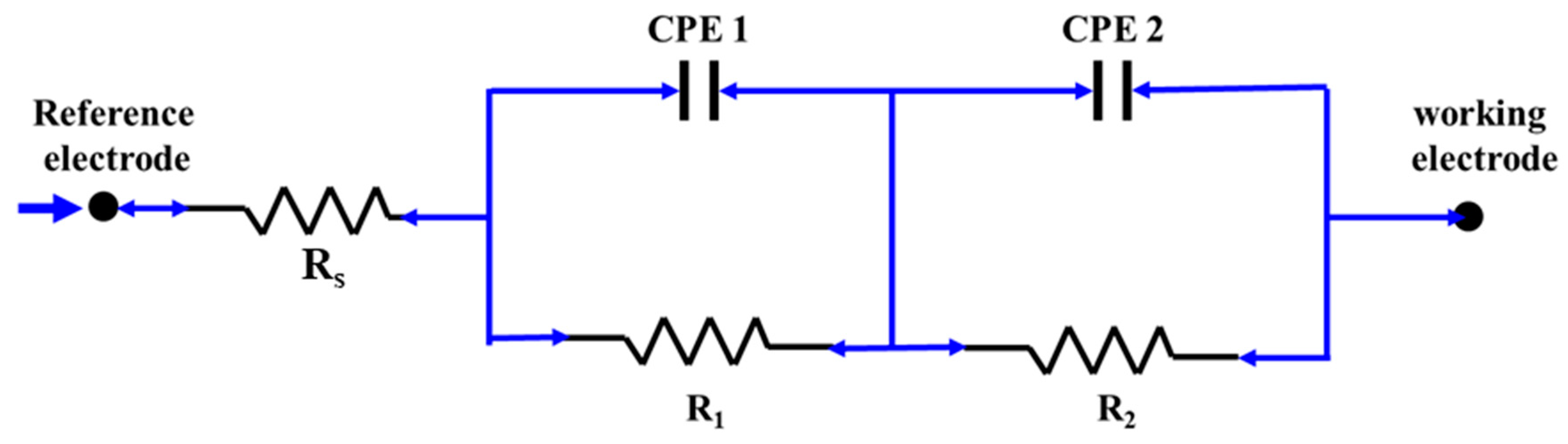
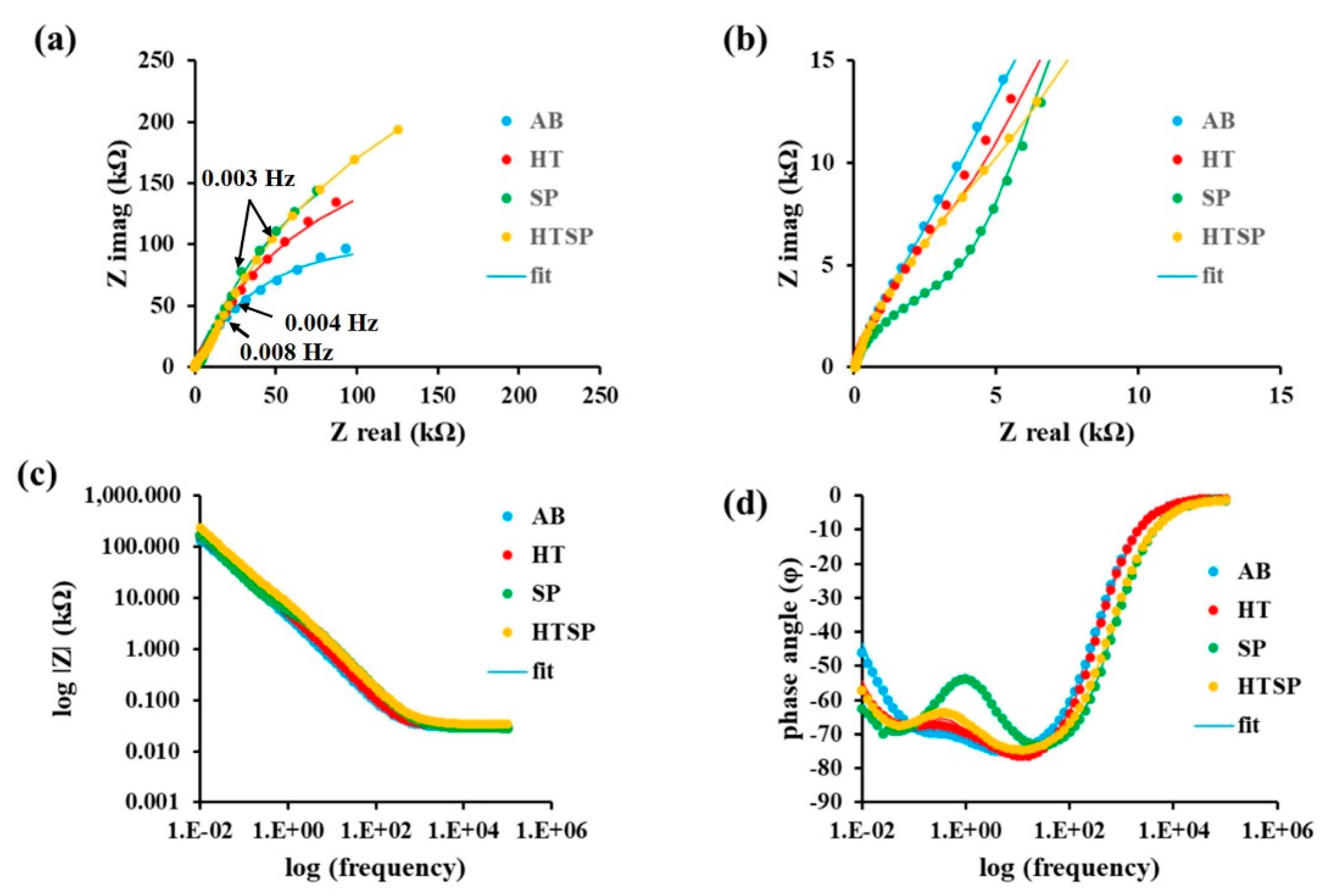
3.3.2. Electrochemical Impedance Spectroscopy Analysis
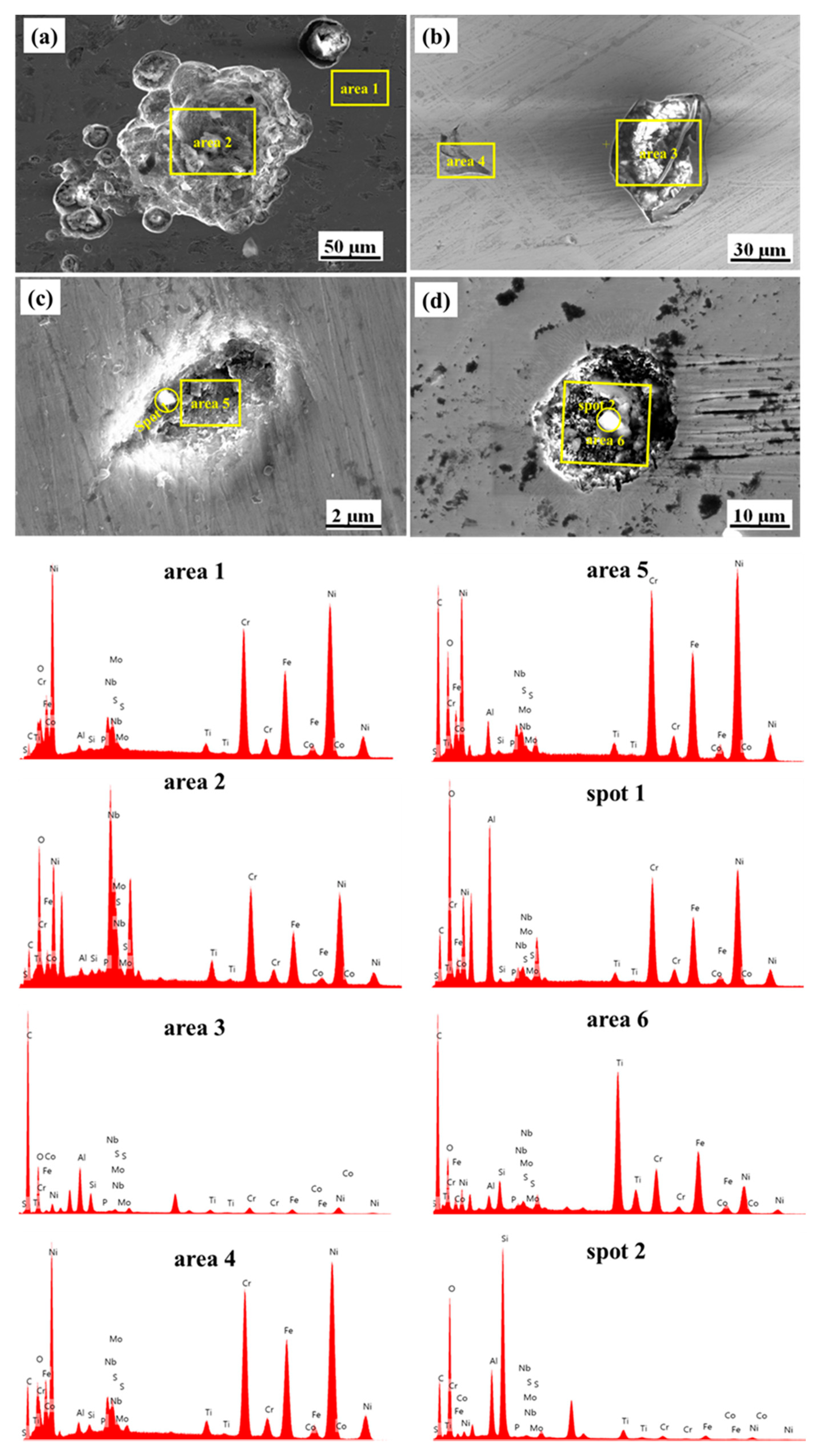
3.4. Post Corrosion Elemental Analysis of IN718
4. Conclusions
- As-built (AB) and shot-peened (SP) material were characterized by the presence of a Laves phase network surrounding the 500 nm-sized γ-matrix. The Laves network in HT and HTSP materials becomes dissolved, but needle-like δ particles were distributed along the γ matrix.
- The surface roughness values of the AB and HT samples reduced from approximately 12 to 2.5 µm after shot peening.
- Potentiodynamic polarization experiments confirmed that anodic dissolution in the AB material leads to lesser corrosion resistance and exhibited the least pitting potential of 220 mV. However, after post-processing, the specimens have shown passive behavior, with the HTSP material possessing the highest pitting potential of 570 mV.
- Nyquist plot confirmed the increasing corrosion resistance in accordance with the decreasing order of measured corrosion resistance, and the Bode plot has shown the presence of two RC time constants in all the tested materials. A high-frequency (100 Hz) time constant corresponds to the film/electrolyte interface and the low-frequency (100 mHz) interface corresponds to the metal/film interface.
- The improvement in surface morphology and alteration of phase constituents in the alloy achieved through shot peening and heat treatment, respectively, have improved the corrosion resistance of the SLMed IN718 alloy.
- The dissolution of the Laves phase network after heat treatment improved the corrosion resistance, while the shot-peening has a considerable effect on the corrosion behavior of both as-built and heat-treated material, as it reduced the surface roughness to almost 80% and produced a uniformly smoothened surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Why Choosing Inconel 718 for Aerospace Additive Manufacturing? | Farinia Group, (n.d.). Available online: https://www.farinia.com/additive-manufacturing/3d-materials/inconel-718-aerospace-additive-manufacturing (accessed on 8 April 2020).
- Lv, J.H.; Wang, W.Z.; Liu, S.W. Statistical Analysis of Failure Cases in Aerospace. Int. Sch. Sci. Res. Innov. 2018, 12, 497–501. [Google Scholar]
- El-Meligi, A.; Palatino, L.I. Effect of nanostructure on corrosion and corrosion resistance of materials. Curr. Chem. Res. 2012, 2, 47–56. [Google Scholar]
- Simon, H.; Thoma, I.M. Attack on Superalloys by Chemical and Electrolytic Processes. Aircr. Eng. Aerosp. Technol. 1981, 53, 22–25. [Google Scholar] [CrossRef]
- Groh, J.R.; Duvelius, R.W. Influence of Corrosion Pitting On Alloy 718 Fatigue Capability. In Superalloy 718, 625, 706, and Derivatives; Loria, E.A., Ed.; The Mining, Metallurgy, and Materials Society: Warrendale, PA, USA, 2001; pp. 583–592. [Google Scholar]
- Boodhun, K.; Surnam, B.Y.R. Corrosion of Aircrafts—A Case Study in Mauritius. Int. Conf. Comput. Sci. Data Min. Mech. Eng. 2015, 77–82. [Google Scholar] [CrossRef]
- Czaban, M. Aircraft corrosion—Review of corrosion processes and its effects in selected cases. Fatigue Aircr. Struct. 2018, 2018, 5–20. [Google Scholar] [CrossRef]
- Akca, E.; Gursel, A. A Review on Superalloys and IN718 Nickel-Based Inconel Superalloy. Period. Eng. Nat. Sci. 2015, 3, 15–27. [Google Scholar] [CrossRef]
- Thompson, M.K.; Moroni, G.; Vaneker, T.; Fadel, G.; Campbell, R.I.; Gibson, I.; Bernard, A.; Schulz, J.; Graf, P.; Ahuja, B.; et al. Design for Additive Manufacturing: Trends, opportunities, considerations, and constraints. CIRP Ann. 2016, 65, 737–760. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Directed Energy Deposition Processes BT—Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing. Johns. Matthey Technol. Rev. 2015, 59, 245–268. [Google Scholar] [CrossRef]
- Andreotta, R.; Ladani, L.; Brindley, W. Finite element simulation of laser additive melting and solidification of Inconel 718 with experimentally tested thermal properties. Finite Elem. Anal. Des. 2017, 135, 36–43. [Google Scholar] [CrossRef]
- Promoppatum, P.; Yao, S.C.; Pistorius, P.C.; Rollett, A.D. A Comprehensive Comparison of the Analytical and Numerical Prediction of the Thermal History and Solidification Microstructure of Inconel 718 Products Made by Laser Powder-Bed Fusion. Engineering 2017, 3, 685–694. [Google Scholar] [CrossRef]
- Kantzos, C.; Pauza, J.; Cunningham, R.; Narra, S.P.; Beuth, J.; Rollett, A. An Investigation of Process Parameter Modifications on Additively Manufactured Inconel 718 Parts. J. Mater. Eng. Perform. 2019, 28, 620–626. [Google Scholar] [CrossRef]
- Trosch, T.; Strößner, J.; Völkl, R.; Glatzel, U. Microstructure and mechanical properties of selective laser melted Inconel 718 compared to forging and casting. Mater. Lett. 2016, 164, 428–431. [Google Scholar] [CrossRef]
- Kaynak, Y.; Tascioglu, E. Post-processing effects on the surface characteristics of Inconel 718 alloy fabricated by selective laser melting additive manufacturing. Prog. Addit. Manuf. 2019, 5, 221–234. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Li, X. Corrosion of metallic materials fabricated by selective laser melting. Mater. Degrad. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Turker, M.; Godlinski, D.; Petzoldt, F. Effect of production parameters on the properties of IN 718 superalloy by three-dimensional printing. Mater. Charact. 2008, 59, 1728–1735. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, K.; Gao, M.; Li, X.; Chen, X.; Zeng, X. The microstructure and mechanical properties of deposited-IN718 by selective laser melting. J. Alloys Compd. 2012, 513, 518–523. [Google Scholar] [CrossRef]
- Witzel, J.; Schrage, J.; Gasser, A.; Kelbassa, I. Additive manufacturing of a blade-integrated disk by laser metal deposition. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, Laser Institute of America, Orlando, FL, USA, 23–27 October 2011; pp. 250–256. [Google Scholar] [CrossRef]
- Tillmann, W.; Schaak, C.; Nellesen, J.; Schaper, M.; Aydinöz, M.E.; Hoyer, K.P. Hot isostatic pressing of IN718 components manufactured by selective laser melting. Addit. Manuf. 2017, 13, 93–102. [Google Scholar] [CrossRef]
- Jiang, R.; Mostafaei, A.; Pauza, J.; Kantzos, C.; Rollett, A.D. Varied heat treatments and properties of laser powder bed printed Inconel 718. Mater. Sci. Eng. A 2019, 755, 170–180. [Google Scholar] [CrossRef]
- Jain, S.; Corliss, M.; Tai, B.; Hung, W.N. Electrochemical polishing of selective laser melted Inconel 718. Procedia Manuf. 2019, 34, 239–246. [Google Scholar] [CrossRef]
- Caliari, F.R.; Candioto, K.C.G.; Couto, A.A.; Nunes, C.Â.; Reis, D.A.P. Effect of Double Aging Heat Treatment on the Short-Term Creep Behavior of the Inconel 718. J. Mater. Eng. Perform. 2016, 25, 2307–2317. [Google Scholar] [CrossRef]
- Bagehorn, S.; Wehr, J.; Maier, H.J. Application of mechanical surface finishing processes for roughness reduction and fatigue improvement of additively manufactured Ti-6Al-4V parts. Int. J. Fatigue 2017, 102, 135–142. [Google Scholar] [CrossRef]
- Mostafa, A.; Rubio, I.P.; Brailovski, V.; Jahazi, M.; Medraj, M. Structure, texture and phases in 3D printed IN718 alloy subjected to homogenization and HIP treatments. Metals 2017, 7, 196. [Google Scholar] [CrossRef]
- Sugavaneswaran, M.; Jebaraj, A.V.; Kumar, M.D.B.; Lokesh, K.; Rajan, A.J. Enhancement of surface characteristics of direct metal laser sintered stainless steel 316L by shot peening. Surf. Interfaces 2018, 12, 31–40. [Google Scholar] [CrossRef]
- Zhuo, L.; Wang, Z.; Zhang, H.; Yin, E.; Wang, Y.; Xu, T.; Li, C. Effect of post-process heat treatment on microstructure and properties of selective laser melted AlSi10Mg alloy. Mater. Lett. 2019, 234, 196–200. [Google Scholar] [CrossRef]
- Damon, J.; Dietrich, S.; Vollert, F.; Gibmeier, J.; Schulze, V. Process dependent porosity and the influence of shot peening on porosity morphology regarding selective laser melted AlSi10Mg parts. Addit. Manuf. 2018, 20, 77–89. [Google Scholar] [CrossRef]
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.R.; Singer, L.; Thomas, S.; Kelly, R.G.; Scully, J.R.; Birbilis, N. Corrosion of additively manufactured alloys: A review. Corrosion 2018, 74, 1318–1350. [Google Scholar] [CrossRef]
- Zhang, B.; Xiu, M.; Tan, Y.T.; Wei, J.; Wang, P. Pitting corrosion of SLM Inconel 718 sample under surface and heat treatments. Appl. Surf. Sci. 2019, 490, 556–567. [Google Scholar] [CrossRef]
- Amin, M.A.; El-Bagoury, N.; Saracoglu, M.; Ramadan, M. Electrochemical and corrosion behavior of cast re-containing inconel 718 alloys in sulphuric acid solutions and the effect of Cl−. Int. J. Electrochem. Sci. 2014, 9, 5352–5374. [Google Scholar]
- Chen, T.; Nutter, J.; Hawk, J.; Liu, X. Corrosion fatigue crack growth behavior of oil-grade nickel-base alloy 718. Part 1: Effect of corrosive environment. Corros. Sci. 2014, 89, 146–153. [Google Scholar] [CrossRef]
- Nutter, J.S.; Chen, T.; Hawk, J.; Liu, X. Effect of aging treatment on pitting corrosion and corrosion fatigue crack propagation behavior of oil-grade alloy 718. In Proceedings of the 8th International Symposium on Superalloy 718 and Derivatives, Hoboken, NJ, USA, 11 November 2014; pp. 643–658. [Google Scholar]
- Osoba, L.O.; Oladoye, A.M.; Ogbonna, V.E. Corrosion evaluation of superalloys Haynes 282 and Inconel 718 in Hydrochloric acid. J. Alloys Compd. 2019, 804, 376–384. [Google Scholar] [CrossRef]
- Caliari, F.R.; Rosa, E.F.D.; Silva, M.A.D.; Reis, D.A.P. Analysis of pitting corrosion on an Inconel 718 alloy submitted to aging heat treatment. Tecnologia em Metalurgia, Materiais e Mineração 2014, 11, 189–194. [Google Scholar] [CrossRef]
- Luo, S.; Huang, W.; Yang, H.; Yang, J.; Wang, Z.; Zeng, X. Microstructural evolution and corrosion behaviors of Inconel 718 alloy produced by selective laser melting following different heat treatments. Addit. Manuf. 2019, 30, 100875. [Google Scholar] [CrossRef]
- Klapper, H.S.; Molodtsov, N.; Burns, M.; Wangenheim, C. Critical Factors Affecting the Pitting Corrosion Resistance of Additively Manufactured Nickel Alloy in Chloride Containing Environments. Corrosion 2017, 2017, 1–8. [Google Scholar]
- Landeta, D. Performance Analysis of 3d Printed 718 Alloy for Corrosive Environments. Master’s Thesis, University of Manchester, Manchester, UK, 2015. [Google Scholar]
- Li, H.; Feng, S.; Li, J.; Gong, J. Effect of heat treatment on the δ phase distribution and corrosion resistance of selective laser melting manufactured Inconel 718 superalloy. Mater. Corros. 2018, 69, 1350–1354. [Google Scholar] [CrossRef]
- Jiao, L.; Chua, Z.; Moon, S.; Song, J.; Bi, G.; Zheng, H. Femtosecond Laser Produced Hydrophobic Hierarchical Structures on Additive Manufacturing Parts. Nanomaterials 2018, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Basic Overview of the Working Principle of a Potentiostat/Galvanostat (PGSTAT)-Electrochemical Cell Setup. Metrohm Autolab. BV. 2011. Available online: https://www.metrohm.com/en-gb/applications/AN-EC-008# (accessed on 19 November 2020).
- Ni, M.; Chen, C.; Wang, X.; Wang, P.; Li, R.; Zhang, X.; Zhou, K. Anisotropic tensile behavior of in situ precipitation strengthened Inconel 718 fabricated by additive manufacturing. Mater. Sci. Eng. A 2017, 701, 344–351. [Google Scholar] [CrossRef]
- Amato, K.N.; Gaytan, S.M.; Murr, L.E.; Martinez, E.; Shindo, P.W. Microstructures and mechanical behavior of Inconel 718 fabricated by selective laser melting. Acta Mater. 2012, 60, 2229–2239. [Google Scholar] [CrossRef]
- Xiao, H.; Xie, P.; Cheng, M.; Song, L. Enhancing mechanical properties of quasi-continuous-wave laser additive manufactured Inconel 718 through controlling the niobium-rich precipitates. Addit. Manuf. 2020, 34, 101278. [Google Scholar] [CrossRef]
- Tian, Y.; McAllister, D.; Colijn, H.; Mills, M.; Farson, D.; Nordin, M.; Babu, S. Rationalization of microstructure heterogeneity in INCONEL 718 builds made by the direct laser additive manufacturing process. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 4470–4483. [Google Scholar] [CrossRef]
- Parimi, L.L.; Ravi, G.; Clark, D.; Attallah, M.M. Microstructural and texture development in direct laser fabricated IN718. Mater. Charact. 2014, 89, 102–111. [Google Scholar] [CrossRef]
- Stevens, E.L.; Toman, J.; To, A.C.; Chmielus, M. Variation of hardness, microstructure, and Laves phase distribution in direct laser deposited alloy 718 cuboids. Mater. Des. 2017, 119, 188–198. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lin, X.; Yu, X.B.; Xu, J.J.; Lei, M.; Huang, W.D. Effect of Ti addition on cracking and microhardness of Inconel 625 during the laser solid forming processing. J. Alloys Compd. 2017, 711, 267–277. [Google Scholar] [CrossRef]
- Zhang, D.; Niu, W.; Cao, X.; Liu, Z. Effect of standard heat treatment on the microstructure and mechanical properties of selective laser melting manufactured Inconel 718 superalloy. Mater. Sci. Eng. A 2015, 644, 32–40. [Google Scholar] [CrossRef]
- Keller, T.; Lindwall, G.; Ghosh, S.; Ma, L.; Lane, B.M.; Zhang, F.; Kattner, U.R.; Lass, E.A.; Heigel, J.C.; Idell, Y.; et al. Application of finite element, phase-field, and CALPHAD-based methods to additive manufacturing of Ni-based superalloys. Acta Mater. 2017, 139, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.L.; Horikawa, S.; Kakehi, K. The effect of interdendritic δ phase on the mechanical properties of Alloy 718 built up by additive manufacturing. Mater. Des. 2017, 116, 411–418. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Dong, J.; Zhang, M. An investigation of microsegregation and liquid density redistribution during solidification of inconel 718. Chem. Eng. Commun. 2009, 196, 754–765. [Google Scholar] [CrossRef]
- Lindwall, G.; Campbell, C.E.; Lass, E.A.; Zhang, F.; Stoudt, M.R.; Allen, A.J.; Levine, L.E. Simulation of TTT Curves for Additively Manufactured Inconel 625. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2019, 50, 457–467. [Google Scholar] [CrossRef]
- Gribbin, S.; Ghorbanpour, S.; Ferreri, N.C.; Bicknell, J.; Tsukrov, I.; Knezevic, M. Role of grain structure, grain boundaries, crystallographic texture, precipitates, and porosity on fatigue behavior of Inconel 718 at room and elevated temperatures. Mater. Charact. 2019, 149, 184–197. [Google Scholar] [CrossRef]
- Lawitzki, R.; Hassan, S.; Karge, L.; Wagner, J.; Wang, D.; von Kobylinski, J.; Krempaszky, C.; Hofmann, M.; Gilles, R.; Schmitz, G. Differentiation of γ′- and γ″- precipitates in Inconel 718 by a complementary study with small-angle neutron scattering and analytical microscopy. Acta Mater. 2019, 163, 28–39. [Google Scholar] [CrossRef]
- Nunes, R.M.; Pereira, D.; Clarke, T.; Hirsch, T.K. Delta phase characterization in inconel 718 alloys through X-ray diffraction. ISIJ Int. 2015, 55, 2450–2454. [Google Scholar] [CrossRef]
- Rapp, R.A.; Zhang, Y.S. Hot corrosion of materials: Fundamental studies. JOM 1994, 46, 47–55. [Google Scholar] [CrossRef]
- Fontana, M.G. Nomograph of corrosion rates. Ind. Eng. Chem. 1955, 47, 81A–82A. [Google Scholar] [CrossRef]
- Abdallah, M.; Jahdaly, A.L.; Salem, M.M.; Fawzy, A.; Abdel Fattah, A.A. Pitting corrosion of nickel alloys and stainless steel in chloride solutions and its inhibition using some inorganic compounds. J. Mater. Environ. Sci. 2017, 8, 2599–2607. [Google Scholar]
- Orazem, M.E.; Tribollet, B. An integrated approach to electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 7360–7366. [Google Scholar] [CrossRef]
- Gramy Instruments Inc. Basics of Electrochemical Impedance Spectroscopy Impedance Values; Gramy Instruments Inc.: Warminster, PA, USA, 2014. [Google Scholar]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Orazem, M.E.; Pebere, N.; Tribollet, B. Enhanced Graphical Representation of Electrochemical Impedance Data. J. Electrochem. Soc. 2006, 153, B129–B1336. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pébère, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric Properties of Materials Showing Constant-Phase-Element (CPE) Impedance Response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, Z. The Cause Analysis of the Incomplete Semi-Circle Observed in High Frequency Region of EIS Obtained from TEL-Covered Pure Copper. Int. J. Electrochem. Sci. 2013, 8, 8282–8290. [Google Scholar]
- Chen, T.; John, H.; Xu, J.; Lu, Q.; Hawk, J.; Liu, X. Influence of surface modifications on pitting corrosion behavior of nickel-base alloy 718. Part 1: Effect of machine hammer peening. Corros. Sci. 2013, 77, 230–245. [Google Scholar] [CrossRef]
- Du, D.; Dong, A.; Shu, D.; Zhu, G.; Sun, B.; Li, X.; Lavernia, E. Influence of build orientation on microstructure, mechanical and corrosion behavior of Inconel 718 processed by selective laser melting. Mater. Sci. Eng. A 2019, 760, 469–480. [Google Scholar] [CrossRef]




| Element | Ni | Fe | Cr | Nb | Mo | Ti | Al | C | S | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| Wt % | 53.1 | 18.63 | 17.65 | 4.79 | 3.07 | 0.86 | 0.60 | 0.04 | 0.03 | 0.02 |
| Weight Percentage of Elements | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Ni | Cr | Fe | Al | Ti | Nb | Mo | C | O | Si |
| A | 52.5 | 21.4 | 19.5 | 0.1 | 1.3 | 2.5 | 1.6 | – | – | – |
| B | 51.7 | 21.5 | 19.2 | – | 1.5 | 3.9 | 2.2 | – | – | – |
| C | 51.2 | 21.4 | 19.4 | – | 1.5 | 4.1 | 2.6 | – | – | – |
| D | 40.0 | 17.3 | 15.6 | 4.0 | 1.3 | 3.2 | – | – | 9.8 | 5.5 |
| E | 50.8 | 21.5 | 19.5 | – | 1.7 | 3.8 | 2.7 | – | – | – |
| F | 52.6 | 20.6 | 18.6 | – | 1.8 | 3.7 | 1.2 | – | – | – |
| G | 47.8 | 20.2 | 18.5 | 2.2 | 1.7 | 3.8 | 2.3 | 0.8 | 2.9 | – |
| H | 50.8 | 21.5 | 19.3 | – | 1.5 | 3.5 | 2.2 | 0.6 | 0.7 | – |
| Specimen Condition | Ecorr (mV) (vs. SCE) | Icorr (µA/cm2) | Corrosion Rate (mpy) | Epit (mV) (vs. SCE) |
|---|---|---|---|---|
| AB | –200 ± 3 | 0.21 ± 0.002 | 0.135 ± 0.06 | 220 ± 18 |
| HT | –190 ± 4 | 0.08 ± 0.003 | 0.045 ± 0.05 | 330 ± 21 |
| SP | –210 ± 3 | 0.06 ± 0.002 | 0.031 ± 0.04 | 560 ± 7 |
| HTSP | –238 ± 5 | 0.04 ± 0.002 | 0.025 ± 0.05 | 570 ± 6 |
| Sample Conditions | Characteristic Frequency (fc) Hz | Rs (Ω cm2) | R1 (kΩ cm2) | Yo of CPE1 (Ω−1 cm−2 sα) | α of CPE1 | R2 (kΩ cm2) | Yo of CPE2 (Ω−1 cm−2 sα) | α of CPE2 | Impedance of the Film (R1+R2) kΩ cm2 |
|---|---|---|---|---|---|---|---|---|---|
| AB | 0.008 | 29.5 ± 0.2 | 1.6 ± 0.7 | 175 × 10−6 | 0.92 | 234 ± 11 | 57 × 10−6 | 0.86 | 235.6 |
| HT | 0.004 | 31.3 ± 0.3 | 3.7 ± 0.9 | 78 × 10−6 | 0.92 | 407 ± 33 | 57 × 10−6 | 0.87 | 410.7 |
| SP | 0.003 | 28.9 ± 0.3 | 3.9 ± 0.3 | 29 × 10−6 | 0.89 | 558 ± 60 | 63 × 10−6 | 0.88 | 561.9 |
| HTSP | 0.003 | 33.5 ± 0.2 | 6.2 ± 1.0 | 51 × 10−6 | 0.87 | 676 ± 81 | 41 × 10−6 | 0.86 | 682.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mythreyi, O.V.; Raja, A.; Nagesha, B.K.; Jayaganthan, R. Corrosion Study of Selective Laser Melted IN718 Alloy upon Post Heat Treatment and Shot Peening. Metals 2020, 10, 1562. https://doi.org/10.3390/met10121562
Mythreyi OV, Raja A, Nagesha BK, Jayaganthan R. Corrosion Study of Selective Laser Melted IN718 Alloy upon Post Heat Treatment and Shot Peening. Metals. 2020; 10(12):1562. https://doi.org/10.3390/met10121562
Chicago/Turabian StyleMythreyi, Opadhrishta Venkataramana, Allavikutty Raja, Bommanahalli Kenchappa Nagesha, and Rengaswamy Jayaganthan. 2020. "Corrosion Study of Selective Laser Melted IN718 Alloy upon Post Heat Treatment and Shot Peening" Metals 10, no. 12: 1562. https://doi.org/10.3390/met10121562
APA StyleMythreyi, O. V., Raja, A., Nagesha, B. K., & Jayaganthan, R. (2020). Corrosion Study of Selective Laser Melted IN718 Alloy upon Post Heat Treatment and Shot Peening. Metals, 10(12), 1562. https://doi.org/10.3390/met10121562




