Quantification of the Dislocation Density, Size, and Volume Fraction of Precipitates in Deep Cryogenically Treated Martensitic Steels
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Design
- Binary Fe C alloy: Fe—0.6C
- Ternary Fe-C-Ni alloy: 2, 4, 12 and 20 wt % Ni
- Ternary Fe-C-Cr alloy: 4, 7 and 11 wt % Cr
2.2. Heat Treatments
2.3. Cryogenic Treatments
2.4. Optical and Electron Microscopy
2.5. Small Angle Neutron Scattering
2.5.1. Instrumentation
2.5.2. Small Angle Neutron Scattering from Cementite
2.5.3. Small Angle Neutron Scattering from Clusters—First Principles Calculations
2.5.4. Quantitative Fitting Procedure
2.6. X-ray Diffraction
2.7. Atom Probe Tomography
3. Results Part 1: Effect of Deep Cryogenic Treatment
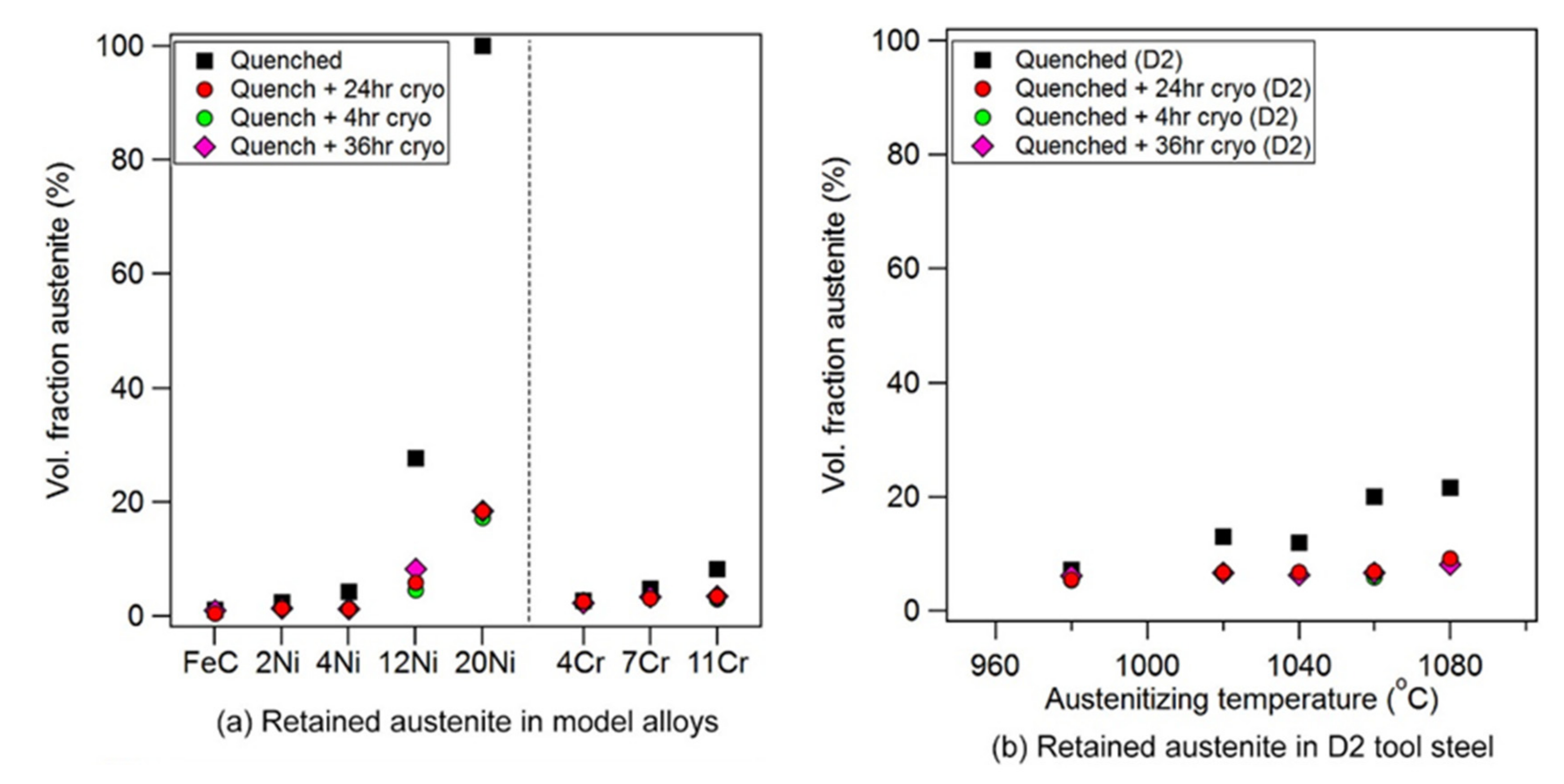
3.1. Microstructure
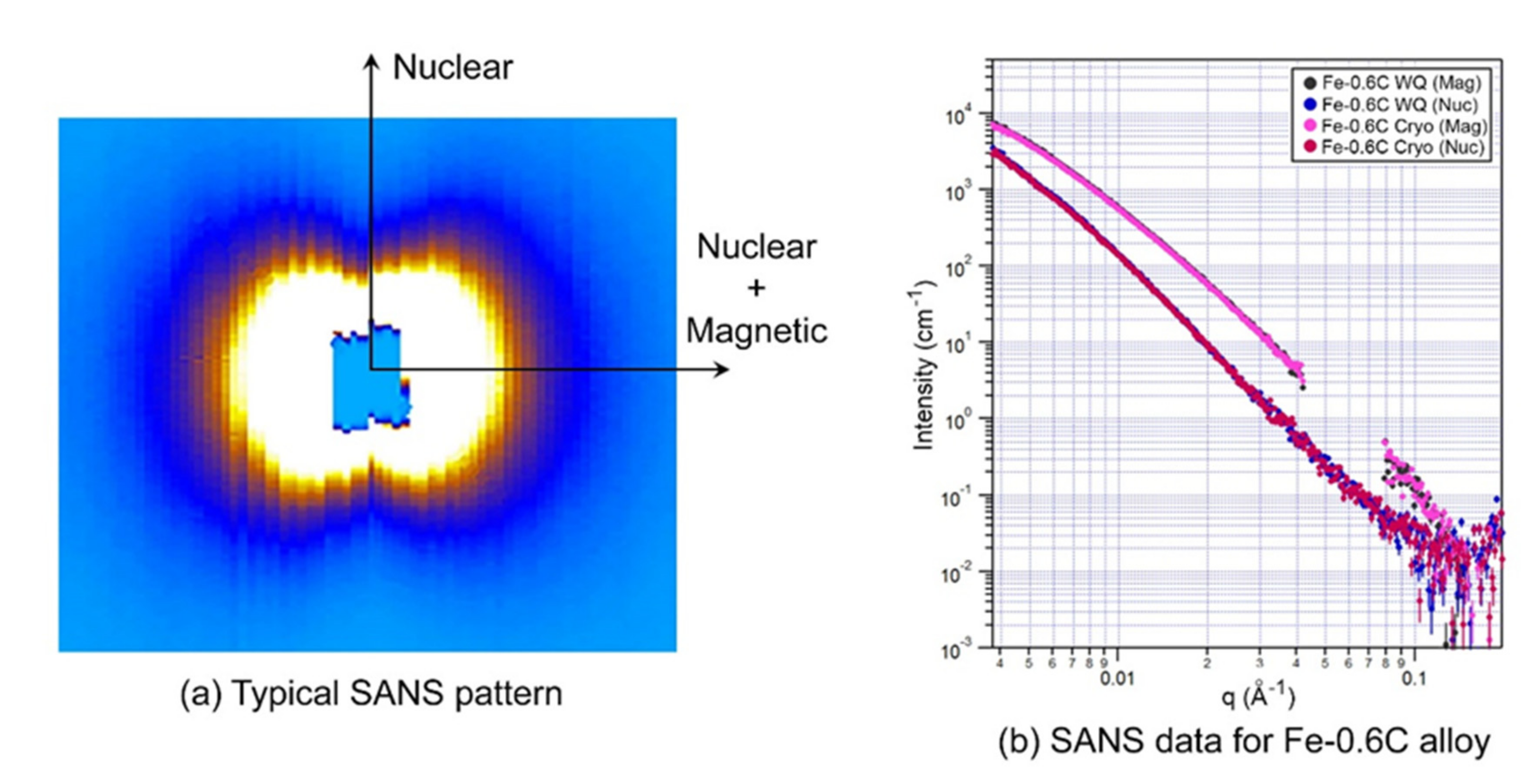
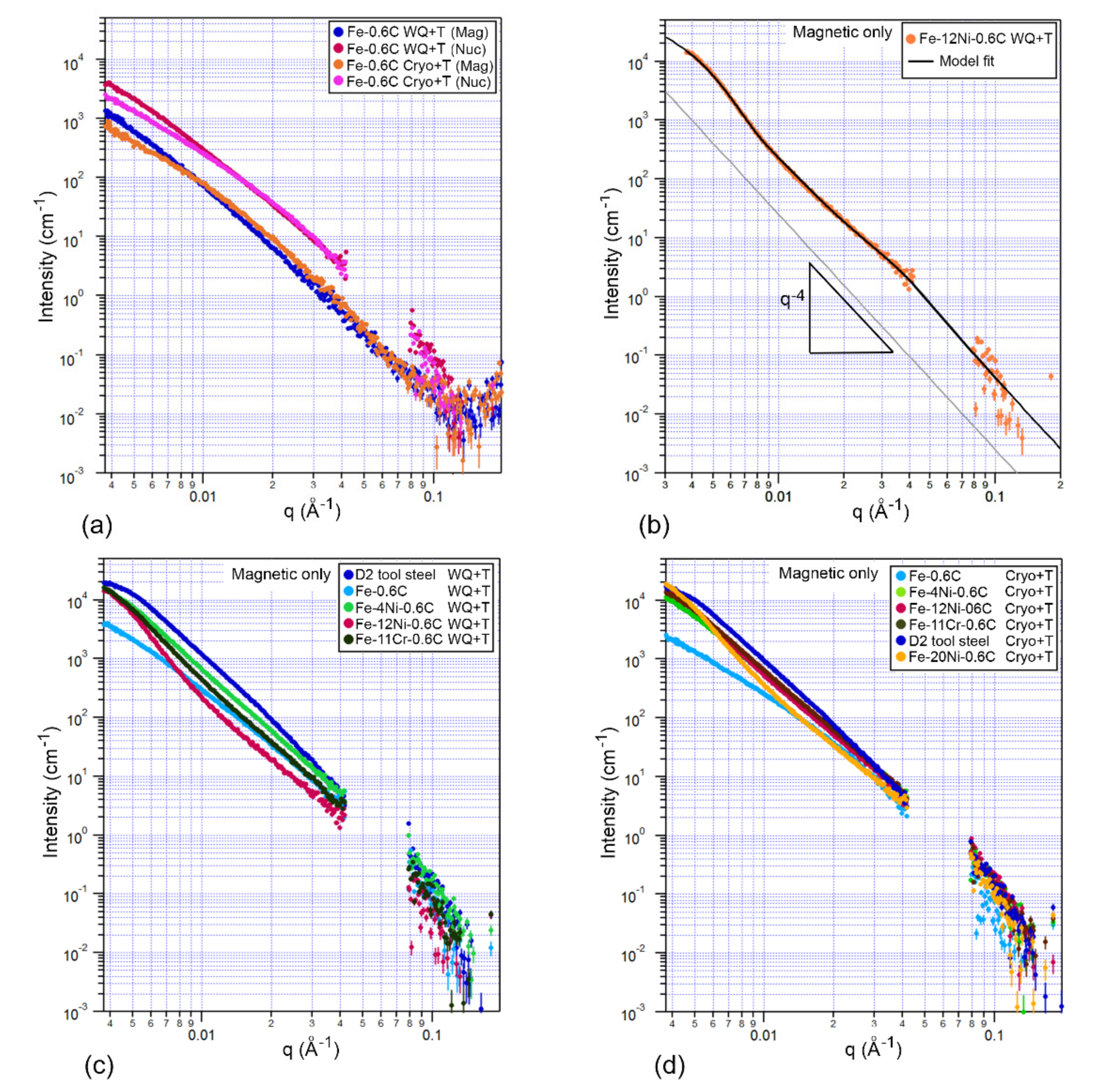
3.2. Small Angle Neutron Scattering
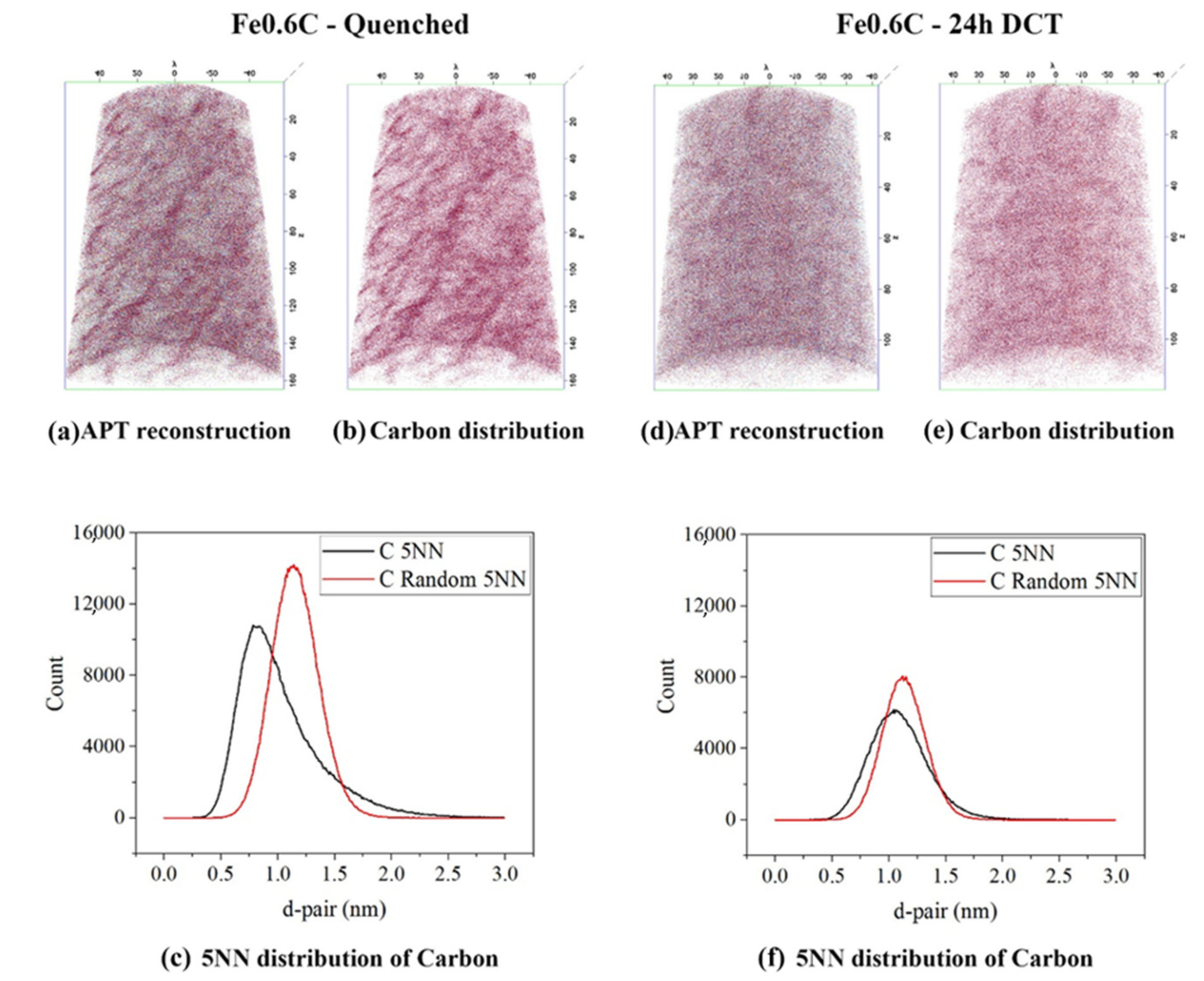
3.3. Atom Probe Tomography
4. Results Part 2: Effect of Tempering
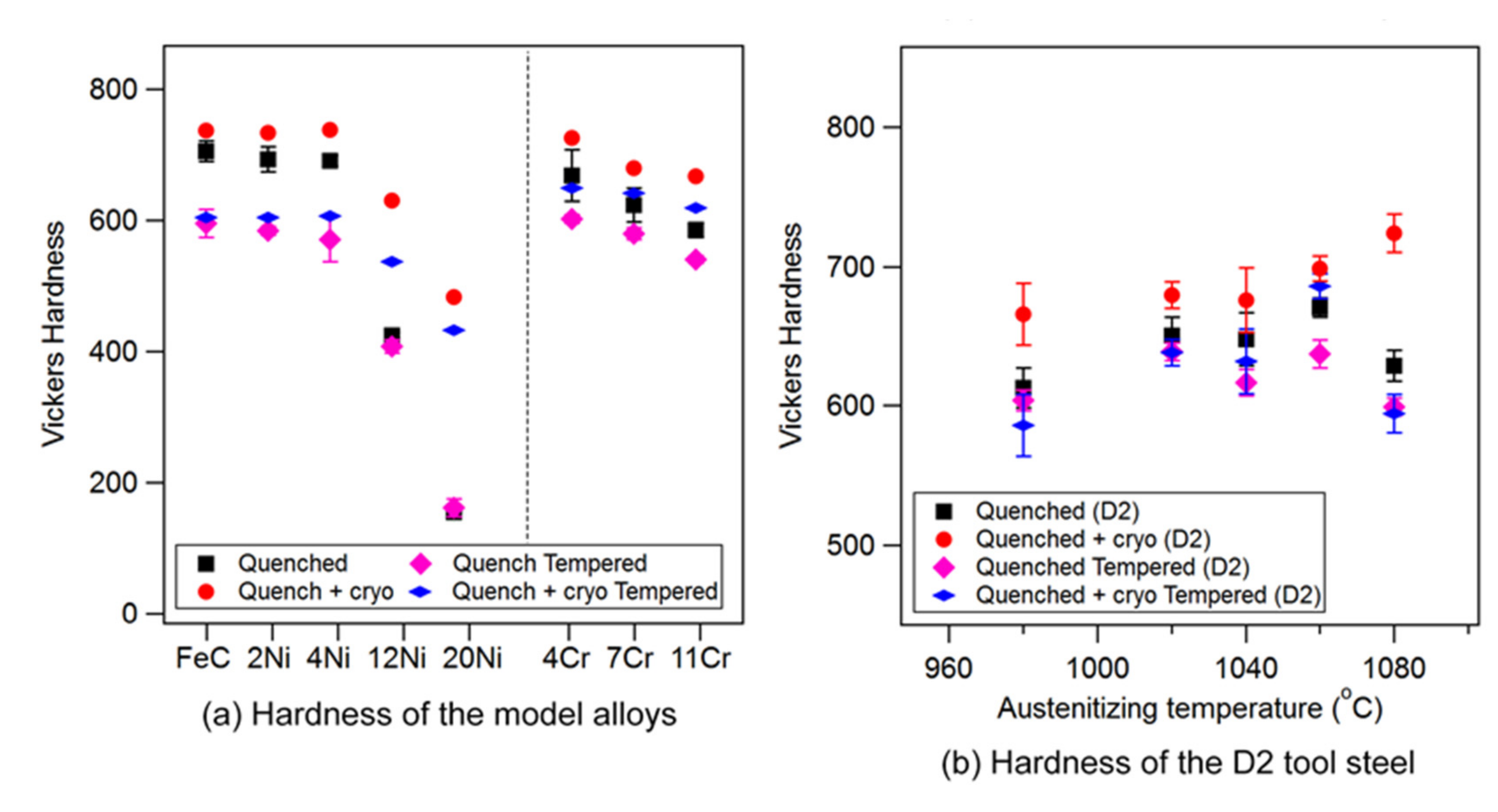
4.1. Tempered Microstructure
4.2. Small Angle Neutron Scattering of Tempered Samples
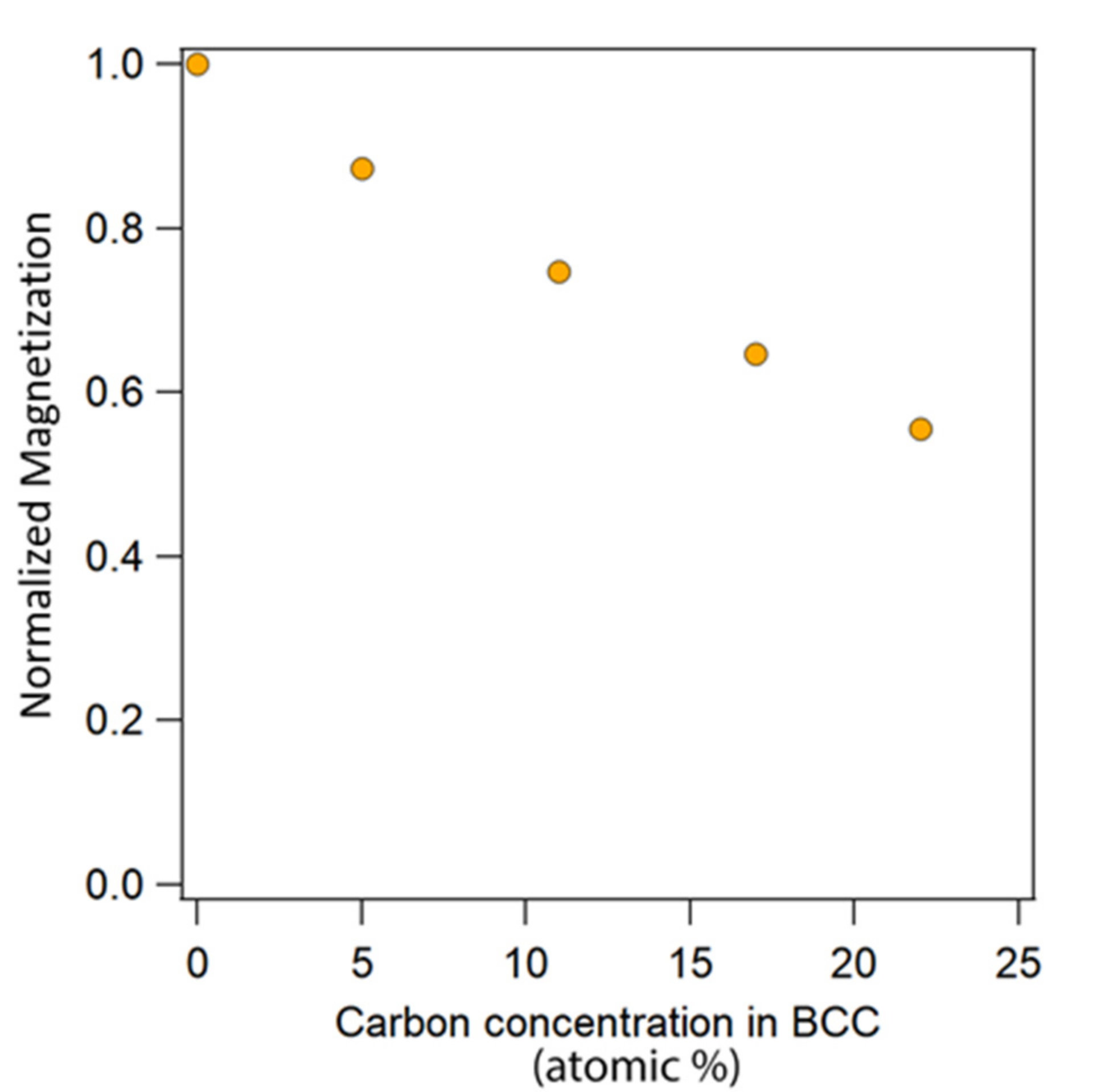
4.2.1. First Principles Calculations of Magnetic Properties
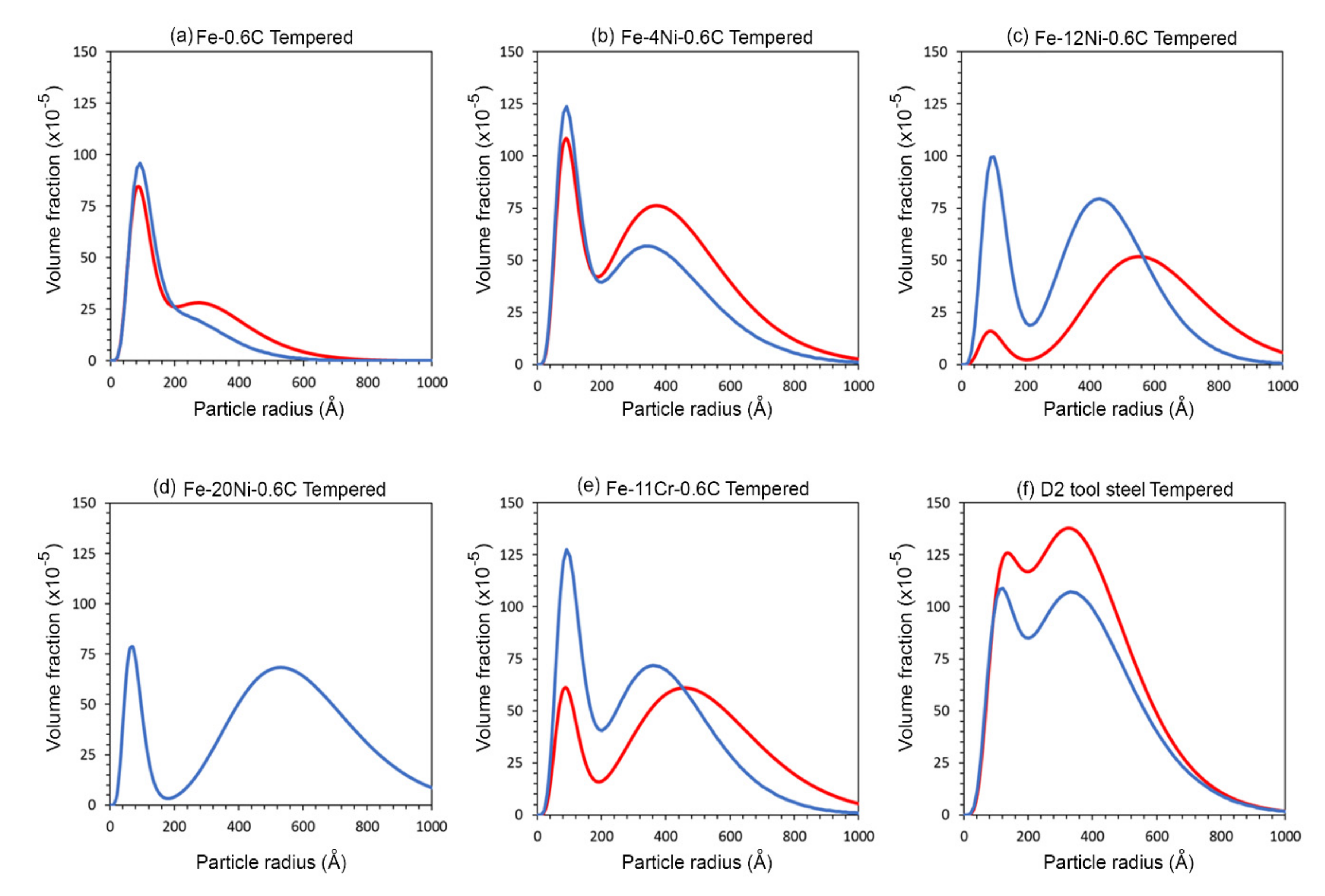
4.2.2. Quantitative Fitting of Small Angle Neutron Scattering Data for Tempered Samples
5. Discussion
5.1. Effect of DCT on Microstructure
5.2. Effect of DCT on Tempering Behavior
6. Conclusions
- In those alloys containing retained austenite, DCT transformed a substantial quantity of austenite to martensite.
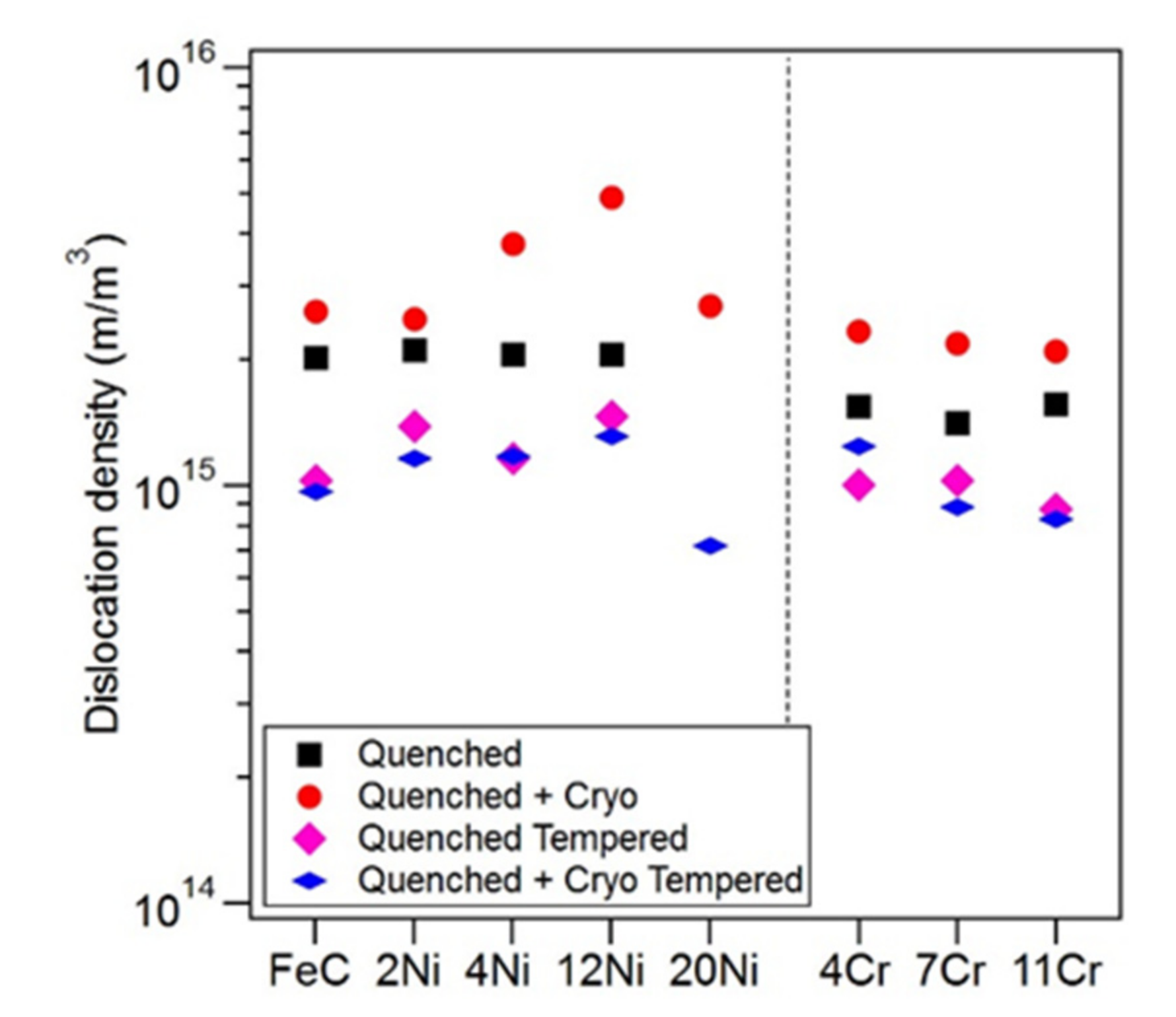
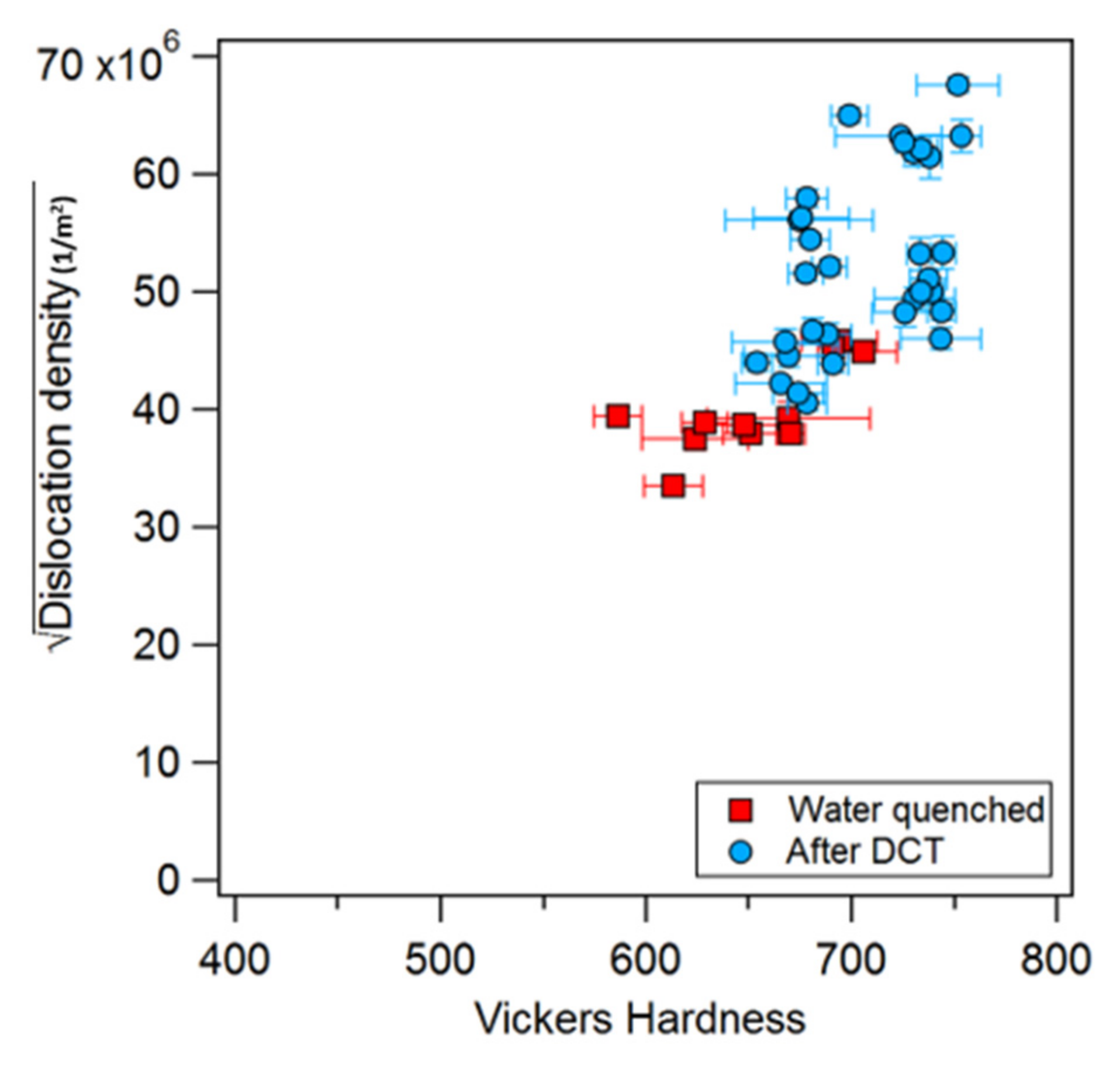
- DCT resulted in an increase in alloy hardness due to two factors: an increase in martensite volume fraction and an increase in martensite dislocation density.
- It was found that the dislocation density of the martensite increased in all specimens, even those that did not exhibit transformation during DCT. This is believed to be the first such observation.
- The increase in dislocation density of the martensite that results from DCT is proposed to be due to local differences in thermal expansion within the complex martensite structure.
- Tempering was shown to change the SANS scattering signal in all alloys. Quantification of the SANS signal confirmed that the size and volume fraction of cementite in all alloys was measurably changed as a result of DCT prior to tempering.
- It is suggested that the change in dislocation density that results from DCT causes the change in cementite size distribution. Calculations showed that in some cases, the diffusivity of carbon was doubled by the increase in dislocation density, and this change in the diffusivity of carbon may be responsible for the different nucleation and growth behavior of cementite.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
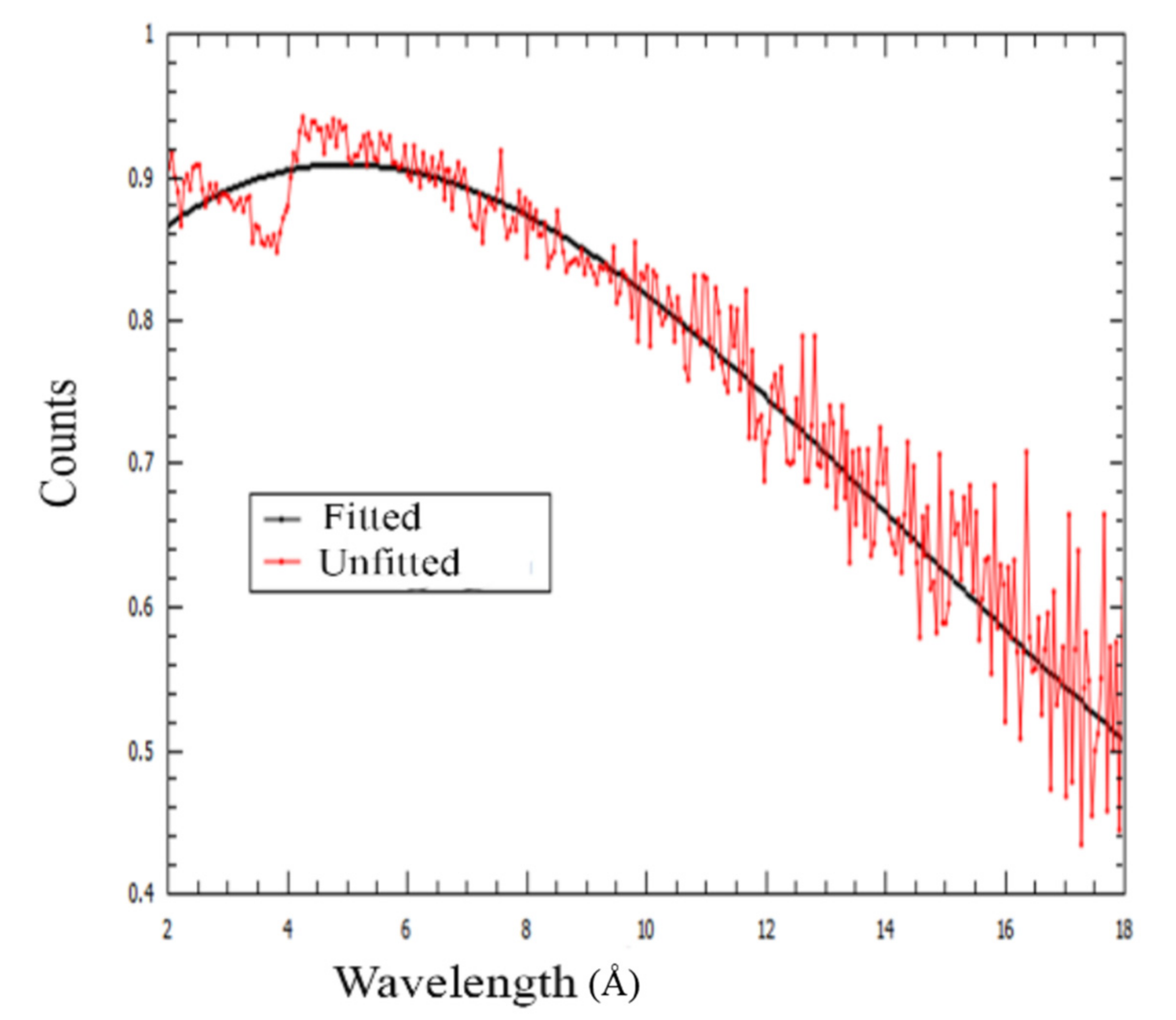
Appendix B
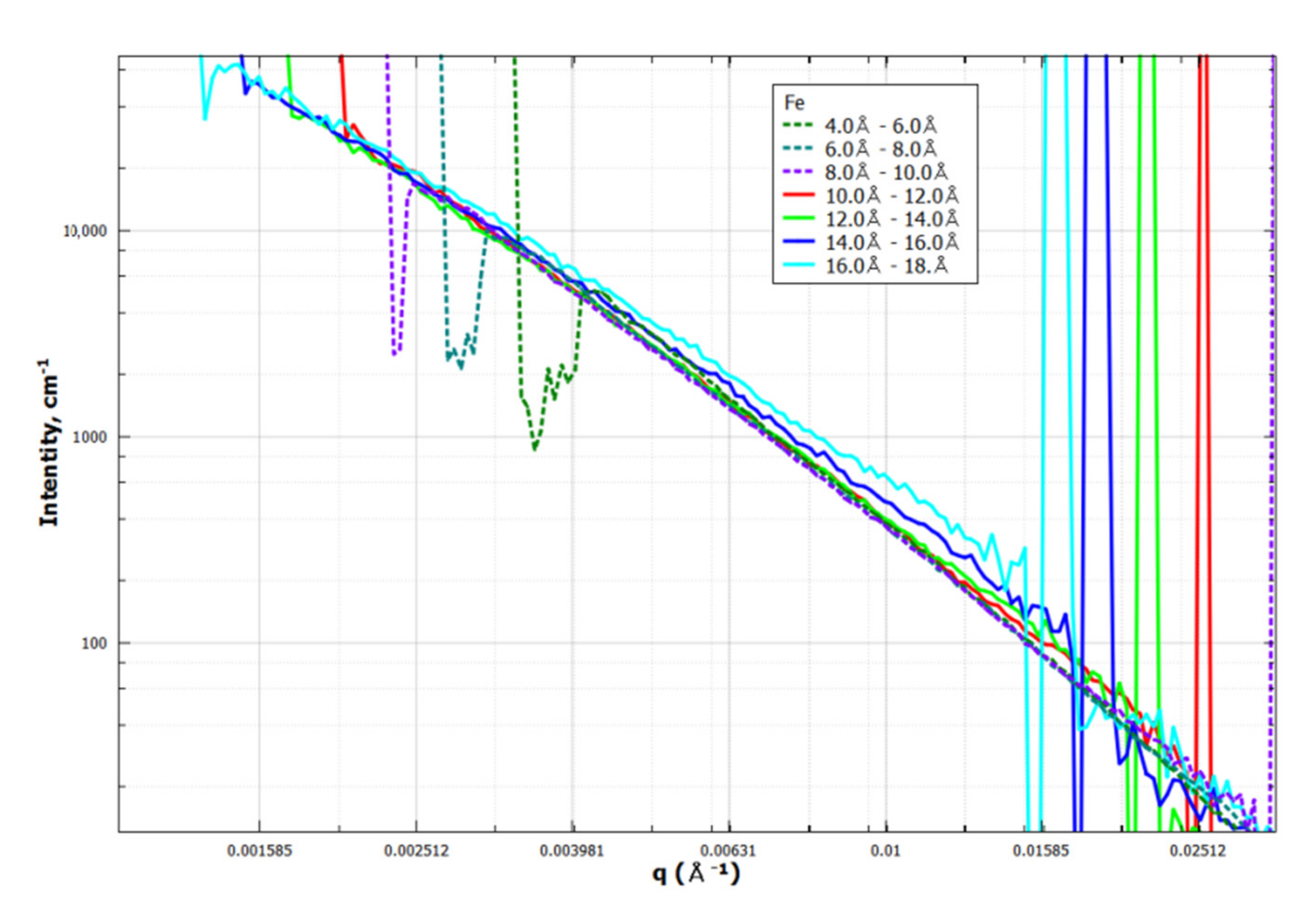
Appendix C
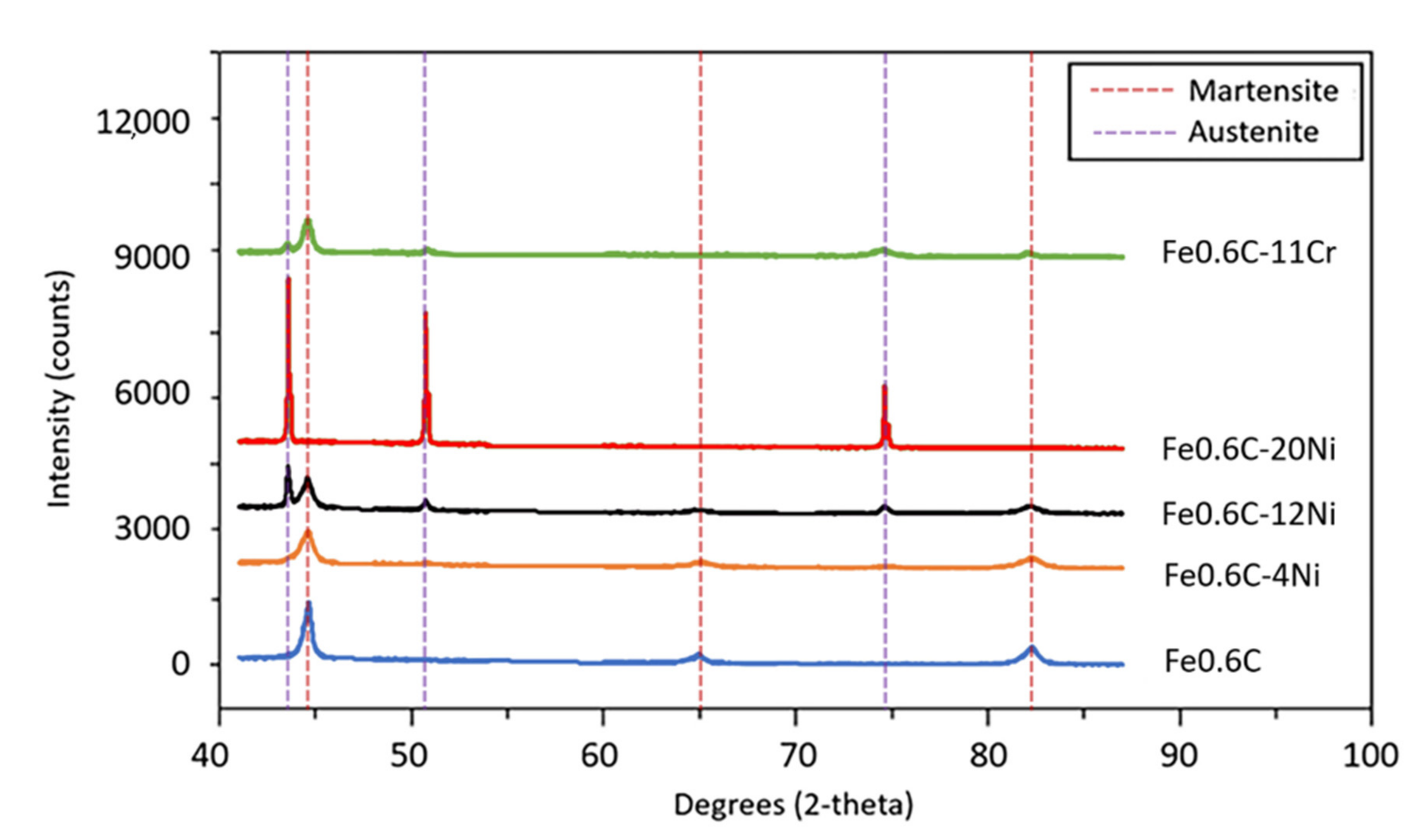
Appendix D

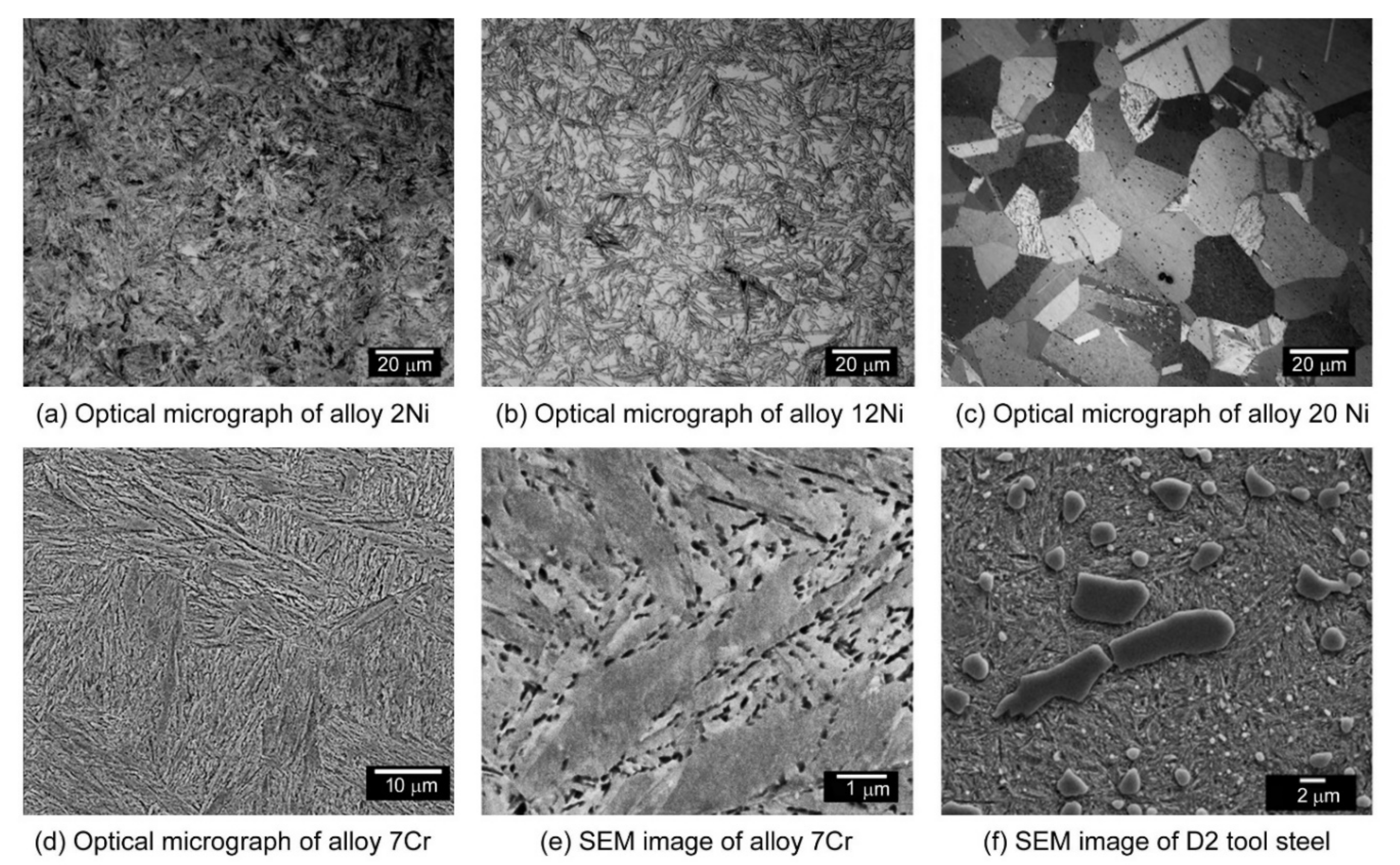
Appendix E

References
- Diekman, F. Cold Treating and Cryogenic Treatment of Steel; ASM Handbook; ASM International: Materials Park, OH, USA, 1991; Volume 4, pp. 203–206. [Google Scholar]
- Ray, K.K.; Das, D. Improved wear resistance of steels by cryotreatment: The current state of understanding. Mater. Sci. Technol. 2016, 33, 340–354. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Wang, Y.; Jin, X. Effect of Cryogenic Treatment on Microstructure and Wear Resistance of Carburized 20CrNi2MoV Steel. Metals 2018, 8, 808. [Google Scholar] [CrossRef]
- Wang, K.; Gu, K.; Misra, R.D.K.; Chen, L.; Liu, X.; Weng, Z.; Wang, J. On the Optimization of Microstructure and Mechanical Properties of CrWMn Tool Steel by Deep Cryogenic Treatment. Steel Res. Int. 2019, 90, 1800523. [Google Scholar] [CrossRef]
- Prieto, G.; Tuckart, W.R. Wear behaviour of cryogenically treated AISI 420 martensitic stainless steel. In Proceedings of the VIII Iberian Conference on Tribology, Cartagena, Spain, 18–19 June 2015. [Google Scholar]
- Das, D.; Dutta, A.; Ray, K. On the enhancement of wear resistance of tool steels by cryogenic treatment. Philos. Mag. Lett. 2008, 88, 801–811. [Google Scholar] [CrossRef]
- Gunes, I.; Cicek, A.; Aslantas, K.; Kara, F.; Çiçek, A. Effect of Deep Cryogenic Treatment on Wear Resistance of AISI 52100 Bearing Steel. Trans. Indian Inst. Met. 2014, 67, 909–917. [Google Scholar] [CrossRef]
- Molinari, A.; Pellizzari, M.; Gialanella, S.; Straffelini, G.; Stiasny, K. Effect of deep cryogenic treatment on the mechanical properties of tool steels. J. Mater. Process. Technol. 2001, 118, 350–355. [Google Scholar] [CrossRef]
- Akhbarizadeh, A.; Shafyei, A.; Golozar, M.A. Effects of cryogenic treatment on wear behaviour of D6 tool steel. Mater. Des. 2009, 30, 3259–3264. [Google Scholar] [CrossRef]
- Amini, K.; Nategh, S.; Shafyei, A.; Rezaeian, A. Influence of different cryo treatments on tribological behaviour of 80CrMo12 5 cold work tool steel. Mater. Des. 2010, 31, 4666–4675. [Google Scholar] [CrossRef]
- Akhbarizadeh, A.; Amini, K.; Javadpour, S. Effects of applying an external magnetic field during the deep cryogenic heat treatment on the corrosion resistance and wear behaviour of 1.2080 tool steel. Mater. Des. 2012, 41, 114–123. [Google Scholar] [CrossRef]
- Mahmudi, R.; Ghasemi, H.M.; Faradji, H.R. Effects of cryogenic treatments on the mechanical properties and wear behaviour of high speed steel M2. Heat Treat. Met. 2000, 27, 69–72. [Google Scholar]
- Bensely, A.; Prabhakaran, A.; Lal, D.M.; Nagarajan, G. Enhancing the wear resistance of case carburized steel (En 353) by cryogenic treatment. Cryogenics 2005, 45, 747–754. [Google Scholar] [CrossRef]
- Das, D.; Dutta, A.; Ray, K. Correlation of microstructure with wear behaviour of deep cryogenically treated AISI D2 steel. Wear 2009, 267, 1371–1380. [Google Scholar] [CrossRef]
- Das, D.; Dutta, A.K.; Ray, K.K. Inconsistent wear behaviour of cryotreated tool steels: Role of mode and mechanism. Mater. Sci. Technol. 2009, 25, 1249–1257. [Google Scholar] [CrossRef]
- Gill, S.S.; Singh, J.; Singh, R.; Singh, H. Effect of Cryogenic Treatment on AISI M2 High Speed Steel: Metallurgical and Mechanical Characterization. J. Mater. Eng. Perform. 2012, 21, 1320–1326. [Google Scholar] [CrossRef]
- Vimal, A.J.; Bensely, A.; Lal, D.M.; Srinivasan, K. Deep Cryogenic Treatment Improves Wear Resistance of En 31 Steel. Mater. Manuf. Process. 2008, 23, 369–376. [Google Scholar] [CrossRef]
- Katoch, S.; Sehgal, R.; Singh, V. Evolution of mechanical properties and microstructure of differently cryogenically treated hot die steel AISI–H13. Int. J. Mater. Res. 2017, 108, 173–184. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Y.; Zhao, Y.; An, Z.; Xue, F.; Yao, J.; Sun, Z.; Chang, J. Enhanced Surface Properties and Microstructure Evolution of Cr12MoV Using Ultrasonic Surface Rolling Process Combined with Deep Cryogenic Treatment. J. Mater. Eng. Perform. 2019, 28, 1132–1140. [Google Scholar] [CrossRef]
- Das, D.; Ray, K.K. Structure–property correlation of sub-zero treated AISI D2 steel. Mater. Sci. Eng. A 2012, 541, 45–60. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; Wang, Y.; Jiang, Y.; Song, Y.; Ge, N. Effect of deep cryogenic pretreatment on microstructure and mechanical properties of warm-deformed 7 Mn steel after intercritical annealing. Mater. Sci. Eng. A 2019, 764, 138202. [Google Scholar] [CrossRef]
- Zare, A.; Mansouri, H.; Hosseini, S.R. Influence of the holding time of the deep cryogenic treatment on the strain hardening behavior of HY-TUF steel. Int. J. Mech. Mater. Eng. 2015, 10, 306. [Google Scholar] [CrossRef]
- Yuan, X.H.; Yang, M.S.; Zhao, K.Y. Effects of Microstructure Transformation on Strengthening and Toughening for Heat-Treated Low Carbon Martensite Stainless Bearing Steel. Mater. Sci. Forum 2015, 817, 667–674. [Google Scholar] [CrossRef]
- Das, D.; Dutta, A.K.; Toppo, V.; Ray, K.K. Effect of deep cryogenic treatment on the carbide precipitation and tribological behaviour of D2 steel. Mater. Manuf. Process. 2007, 22, 474–480. [Google Scholar] [CrossRef]
- Villa, M.; Pantelon, K.; Somers, M.A.J. Evolution of compressive strains in retained austenite during sub-zero Celcius martensite formation and tempering. Acta Mater. 2014, 65, 383–392. [Google Scholar] [CrossRef]
- Xie, C.; Zhou, L.; Min, N.; Wu, X. Effect of deep cryogenic treatment on carbon segregation in Cr8Mo2SiV tool steel during tempering. Philos. Mag. Lett. 2017, 97, 372–377. [Google Scholar] [CrossRef]
- Katoch, S.; Singh, V.; Sehgal, R. Mechanical Properties and Microstructure Evaluation of Differently Cryogenically Treated AISI-H11 Steel. Int. J. Steel Struct. 2019, 19, 1381–1392. [Google Scholar] [CrossRef]
- Li, S.; Xiao, M.; Ye, G.; Zhao, K.; Yang, M. Effects of deep cryogenic treatment on microstructural evolution and alloy phases precipitation of a new low carbon martensitic stainless bearing steel during aging. Mater. Sci. Eng. A 2018, 732, 167–177. [Google Scholar] [CrossRef]
- Akhbarizadeh, A.; Javadpour, S. Investigating the effect of as-quenched vacancies in the final microstructure of 1.2080 tool steel during the deep cryogenic heat treatment. Mater. Lett. 2013, 93, 247–250. [Google Scholar] [CrossRef]
- Li, S.; Wu, X. Microstructural evolution and corresponding property changes after deep cryotreatment of tool steel. Mater. Sci. Technol. 2015, 31, 1867–1878. [Google Scholar] [CrossRef]
- Abudaia, F.B.; Fessatwi, E.A. Effectiveness of Cryotreatment on Steel with Low Potential for Carbide Precipitation and Austenite Retention. Al-Ostath. August 2015, pp. 41–51. Available online: https://cryogenictreatmentdatabase.org/article/effectiveness_of_cryotreatment_on_steel_with_low_potential_for_carbide_precipitation_and_austenite_retention/ (accessed on 22 November 2020).
- Sonar, T.; Lomte, S.; Gogte, C. Cryogenic Treatment of Metal—A Review. Mater. Today Proc. 2018, 5, 25219–25228. [Google Scholar] [CrossRef]
- Baldissera, P.; Delprete, C. Deep Cryogenic Treatment: A Bibliographic Review. Open Mech. Eng. J. 2008, 2, 1–11. [Google Scholar] [CrossRef]
- Calzada, M.P.; Pellizzari, M. Influence of deep cryogenic treatment on the thermal decomposition of Fe–C martensite. J. Mater. Sci. 2014, 49, 8183–8191. [Google Scholar] [CrossRef]
- Meng, F.; Tagashira, K.; Azuma, R.; Sohma, H. Role of Eta-carbide Precipitations in the Wear Resistance Improvements of Fe-12Cr-Mo-V-1.4C Tool Steel by Cryogenic Treatment. ISIJ Int. 1994, 34, 205–210. [Google Scholar] [CrossRef]
- Gill, S.S.; Singh, J.; Singh, R.; Singh, H. Metallurgical principles of cryogenically treated tool steels—a review on the current state of science. Int. J. Adv. Manuf. Technol. 2010, 54, 59–82. [Google Scholar] [CrossRef]
- Li, S.; Min, N.; Li, J.; Wu, X.; Li, C.; Tang, L. Experimental verification of segregation of carbon and precipitation of carbides due to deep cryogenic treatment for tool steel by internal friction method. Mater. Sci. Eng. A 2013, 575, 51–60. [Google Scholar] [CrossRef]
- Tyshchenko, A.; Theisen, W.; Oppenkowski, A.; Siebert, S.; Razumov, O.; Skoblik, A.; Sirosh, V.; Petrov, Y.; Gavriljuk, V. Low-temperature martensitic transformation and deep cryogenic treatment of a tool steel. Mater. Sci. Eng. A 2010, 527, 7027–7039. [Google Scholar] [CrossRef]
- Kumar, T.V.; Thirumurugan, R.; Viswanath, B. Influence of cryogenic treatment on the metallurgy of ferrous alloys: A review. Mater. Manuf. Process. 2017, 32, 1789–1805. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Firstov, S.A.; Sirosh, V.A.; Tyshchenko, A.I.; Mogilny, G.S. Carbon distribution in low temperature isothermal iron based martensite and its tegragonality. Metallofiz. Noveishie Teknol. 2016, 38, 455–475. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Sirosh, V.A.; Petrov, Y.N.; Tyshchenko, A.I.; Theisen, W.; Kortmann, A. Carbide Precipitation During Tempering of a Tool Steel Subjected to Deep Cryogenic Treatment. Met. Mater. Trans. A 2014, 45, 2453–2465. [Google Scholar] [CrossRef]
- Oppenkowski, A.; Weber, S.; Theisen, W. Evaluation of factors influencing deep cryogenic treatment that affect the properties of tool steels. J. Mater. Process. Technol. 2010, 210, 1949–1955. [Google Scholar] [CrossRef]
- Podgornik, B.; Paulin, I.; Zajec, B.; Jacobson, S.; Leskovšek, V. Deep cryogenic treatment of tool steels. J. Mater. Process. Technol. 2016, 229, 398–406. [Google Scholar] [CrossRef]
- Pillai, N.; Karthikeyan, R.; Paulo Davim, J. A review on effects of cryogenic treatment of ASIS D series cold working tool steels. Rev. Adv. Mater. Sci. 2017, 51, 149–159. [Google Scholar]
- Hoyos, J.J.; Velez, J.M.; Ghilarducci, A.A. Comment on Experimental verification of segregation of carbon and precipitation of carbides due to deep cryogenic treatment for tool steel by internal friction method. Mater. Sci. Eng. A 2014, 613, 1–2. [Google Scholar] [CrossRef]
- Xie, C.; Zhou, L.; Min, N.; Wu, X. Effect of deep cryogenic treatment on carbon partition of tempered high carbon high alloy tool steel SDC99. Chin. J. Mater. Res. 2016, 30, 801–810. [Google Scholar]
- Li, S.; Deng, L.; Wu, X. The mechanism investigation of deep cryogenic treatment on high alloy martensitic steel by low frequency internal friction. Cryogenics 2010, 50, 433–438. [Google Scholar] [CrossRef]
- Li, S.; Zhao, K.; Wang, K.; Yang, M. Microstructural evolution and thermal stability after aging of a cobalt-containing martensitic bearing steel. Mater. Charact. 2017, 124, 154–164. [Google Scholar] [CrossRef]
- Sokolova, A.; Whitten, A.E.; De Campo, L.; Christoforidis, J.; Eltobaji, A.; Barnes, J.; Darmann, F.; Berry, A. Performance and characteristics of the BILBY time-of-flight small-angle neutron scattering instrument. J. Appl. Crystallogr. 2019, 52, 1–12. [Google Scholar] [CrossRef]
- Dorin, T.; Wood, K.; Taylor, A.; Hodgson, P.; Stanford, N. Quantitative examination of carbide and sulphide precipitates in chemically complex steels processed by direct strip casting. Mater. Charact. 2016, 112, 259–268. [Google Scholar] [CrossRef]
- Simm, T.H.; Sun, L.; Galvin, D.R.; Hill, P.; Rawson, M.; Birosca, S.; Gilbert, E.P.; Bhadeshia, H.; Perkins, K. The Effect of a Two-Stage Heat-Treatment on the Microstructural and Mechanical Properties of a Maraging Steel. Materials 2017, 10, 1346. [Google Scholar] [CrossRef]
- Leitner, H.; Staron, P.; Clemens, H.; Marsoner, S.; Warbichler, P. Analysis of the precipitation behaviour in a high-speed steel by means of small-angle neutron scattering. Mater. Sci. Eng. A 2005, 398, 323–331. [Google Scholar] [CrossRef]
- Osamura, K.; Okuda, H.; Asano, K.; Furusaka, M.; Kishida, K.; Kurosawa, F.; Uemori, R. SANS Study of Phase Decomposition in Fe-Cu Alloy with Ni and Mn Addition. ISIJ Int. 1994, 34, 346–354. [Google Scholar] [CrossRef]
- Häglund, J.; Grimvall, G.; Jarlborg, T. Electronic structure, X-ray photoemission spectra, and transport properties ofFe3C (cementite). Phys. Rev. B 1991, 44, 2914–2919. [Google Scholar] [CrossRef] [PubMed]
- Rossat-Mignot, J.; Zhu, Y. (Eds.) Chapter 2: Small-angle neutron scattering. In Modern Techniques for Characterizing Magnetic Materials; Springer: Boston, MA, USA, 2005; pp. 65–105. [Google Scholar]
- Squires, G.L. Introduction to the Theory of Thermal Neutron Scattering; Cambridge University Press (CUP): Cambridge, UK, 2009. [Google Scholar]
- Schenk, F. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatability of the first and second kinds. Medial Phys. 1996, 23, 815–850. [Google Scholar] [CrossRef] [PubMed]
- Lipert, K.; Kazmierczak, J.; Pelech, I.; Narkiewicz, U.; Slawska-Waniewska, A.; Lachowicz, H.K. Magnetic properties of cementite (Fe3C) nanoparticle agglomerates in a carbon matrix. Mater. Sci. Pol. 2007, 25, 399–404. [Google Scholar]
- Morsdorf, L.; Tasan, C.; Ponge, D.; Raabe, D. 3D structural and atomic-scale analysis of lath martensite: Effect of the transformation sequence. Acta Mater. 2015, 95, 366–377. [Google Scholar] [CrossRef]
- Thompson, S.; Tanner, B.K. The magnetic properties of specially prepared pearlitic steels of varying carbon content as a function of plastic deformation. J. Magn. Magn. Mater. 1994, 132, 71–88. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total energy calculations using a plane wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kline, S.R. Reduction and analysis of SANS and USANS data using IGOR Pro. J. Appl. Crystallogr. 2006, 39, 895–900. [Google Scholar] [CrossRef]
- De Geuser, F.; Deschamps, A. Precipitate characterisation in metallic systems by small-angle X-ray or neutron scattering. C. R. Phys. 2012, 13, 246–256. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E. III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Wilde, J.; Cerezo, A.; Smith, G. Three-dimensional atomic-scale mapping of a cottrell atmosphere around a dislocation in iron. Scr. Mater. 2000, 43, 39–48. [Google Scholar] [CrossRef]
- Shatov, A.V.; Ponomarev, S.S.; Firstov, S.A. Comprehensive Hard Materials: Hardness and Deformation of Hard Metals at Room Temperature; Elsevier: Boston, MA, USA, 2014. [Google Scholar]
- Lee, S.-J.; Lusk, M.; Lee, Y.-K. Conversional model of transformation strain to phase fraction in low alloy steels. Acta Mater. 2007, 55, 875–882. [Google Scholar] [CrossRef]
- Inoue, T.; Raniecki, B. Determination of thermal-hardening stress in steels by use of thermoplasticity theory. J. Mech. Phys. Solids 1978, 26, 187–212. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Liao, X.; Beyerlein, I.; Bourke, M.; Mitchell, T. Microstructure of cryogenic treated M2 tool steel. Mater. Sci. Eng. A 2003, 339, 241–244. [Google Scholar] [CrossRef]
- Jablonka, A.; Harste, K.; Schwerdtfeger, K. Thermomechanical properties of iron and iron-carbon alloys: Density and thermal contraction. Steel Res. 1991, 62, 24–33. [Google Scholar] [CrossRef]
- Danilchenko, V.; Filatov, A.; Mazanko, V.F.; Iakovlev, V.E. Cyclic Martensitic Transformations Influence on the Diffusion of Carbon Atoms in Fe-18 wt.%Mn-2 wt.%Si alloy. Nanoscale Res. Lett. 2017, 12, 194. [Google Scholar] [CrossRef][Green Version]
- Hart, E. On the role of dislocations in bulk diffusion. Acta Met. 1957, 5, 597. [Google Scholar] [CrossRef]
- Le Claire, A. LII. Grain boundary diffusion in metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1951, 42, 468–474. [Google Scholar] [CrossRef]
- Ruoff, A.L.; Balluffi, R.W. Strain-Enhanced Diffusion in Metals. II. Dislocation and Grain-Boundary Short-Circuiting Models. J. Appl. Phys. 1963, 34, 1848–1853. [Google Scholar] [CrossRef]
- Lübbehusen, M.; Mehrer, H. Self-diffusion in α-iron: The influence of dislocations and the effect of the magnetic phase transition. Acta Met. Mater. 1990, 38, 283–292. [Google Scholar] [CrossRef]
- Da Silva, J.; McLellan, R.B. Diffusion of carbon and nitrogen in B.C.C. iron. Mater. Sci. Eng. 1976, 26, 83–87. [Google Scholar] [CrossRef]
- Huang, S.; Worthington, D.L.; Asta, M.; Ozolins, V.; Ghosh, G.; Liaw, P.K. Calculation of impurity diffusivities in α-Fe using first-principles methods. Acta Mater. 2010, 58, 1982–1993. [Google Scholar] [CrossRef]
- Brown, L.M. Precipitation and dispersion hardening. In Proceedings of the 5th International Conference, Aachen, Germany, 27–31 August 1979; Volume 3, pp. 1551–1571. [Google Scholar]
- Davidson, P.A. An Introduction to Electrodynamics; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Heremans, J.; Olk, C.H.; Morelli, D.T. Magnetic susceptibility of carbon structures. Phys. Rev. B 1994, 49, 15122–15125. [Google Scholar] [CrossRef] [PubMed]



| Type | Alloy | Composition (wt %) | |||
|---|---|---|---|---|---|
| Fe | C | Ni | Cr | ||
| Base Alloy | Fe-0.6C | 99.2 | 0.57 | <0.0015 | 0.009 |
| Ni | Fe-0.6C-2Ni | 97.1 | 0.55 | 2.1 | 0.019 |
| Fe-0.6C-4Ni | 95.2 | 0.63 | 3.9 | 0.019 | |
| Fe-0.6C-12Ni | 87.2 | 0.66 | 11.8 | 0.021 | |
| Fe-0.6C-20Ni | 79.4 | 0.55 | 19.7 | 0.018 | |
| Cr | Fe-0.6C-4Cr | 95.2 | 0.54 | 0.014 | 4.1 |
| Fe-0.6C-7Cr | 91.7 | 0.57 | 0.017 | 7.5 | |
| Fe-0.6C-11Cr | 88.8 | 0.53 | 0.016 | 10.4 | |
| Element | Composition (wt %) | Element | Composition (wt %) |
|---|---|---|---|
| C | 1.568 | Cu | 0.085 |
| Si | 0.275 | Nb | 0.017 |
| Mn | 0.266 | Ti | 0.002 |
| P | 0.007 | V | 0.69 |
| S | <0.0005 | W | 0.114 |
| Cr | 12.26 | Zr | 0.01 |
| Mo | 0.694 | B | 0.0005 |
| Ni | 0.23 | Zn | 0.0006 |
| Al | 0.031 | N | 0.091 |
| Co | 0.054 | Fe | 83.58 |
| Alloy | Increase in Diffusion Coefficient (%) | ||
|---|---|---|---|
| 4 DCT | 24 DCT | 36 DCT | |
| Fe-0.6C | 16.0 | 29.3 | 40.6 |
| 2Ni | 19.4 | 19.2 | 35.8 |
| 4Ni | 86.4 | 84.5 | 88.5 |
| 4Cr | 58.8 | 51.3 | 37.9 |
| 7Cr | 53.0 | 55.0 | 37.1 |
| 11Cr | 27.2 | 34.3 | 24.0 |
| D2 tool steel (980 °C) | 46.4 | 58.8 | 52.7 |
| D2 tool steel (1020 °C) | 119.0 | 106.1 | 84.5 |
| D2 tool steel (1040 °C) | 124.4 | 111.6 | 81.5 |
| D2 tool steel (1060 °C) | 177.3 | 193.7 | 172.8 |
| D2 tool steel (1080 °C) | 164.4 | 226.6 | 202.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antony, A.; Schmerl, N.M.; Sokolova, A.; Mahjoub, R.; Fabijanic, D.; Stanford, N.E. Quantification of the Dislocation Density, Size, and Volume Fraction of Precipitates in Deep Cryogenically Treated Martensitic Steels. Metals 2020, 10, 1561. https://doi.org/10.3390/met10111561
Antony A, Schmerl NM, Sokolova A, Mahjoub R, Fabijanic D, Stanford NE. Quantification of the Dislocation Density, Size, and Volume Fraction of Precipitates in Deep Cryogenically Treated Martensitic Steels. Metals. 2020; 10(11):1561. https://doi.org/10.3390/met10111561
Chicago/Turabian StyleAntony, Ajesh, Natalya M. Schmerl, Anna Sokolova, Reza Mahjoub, Daniel Fabijanic, and Nikki E. Stanford. 2020. "Quantification of the Dislocation Density, Size, and Volume Fraction of Precipitates in Deep Cryogenically Treated Martensitic Steels" Metals 10, no. 11: 1561. https://doi.org/10.3390/met10111561
APA StyleAntony, A., Schmerl, N. M., Sokolova, A., Mahjoub, R., Fabijanic, D., & Stanford, N. E. (2020). Quantification of the Dislocation Density, Size, and Volume Fraction of Precipitates in Deep Cryogenically Treated Martensitic Steels. Metals, 10(11), 1561. https://doi.org/10.3390/met10111561






