TiPd- and TiPt-Based High-Temperature Shape Memory Alloys: A Review on Recent Advances
Abstract
1. Introduction
2. Martensitic Transformation Temperature
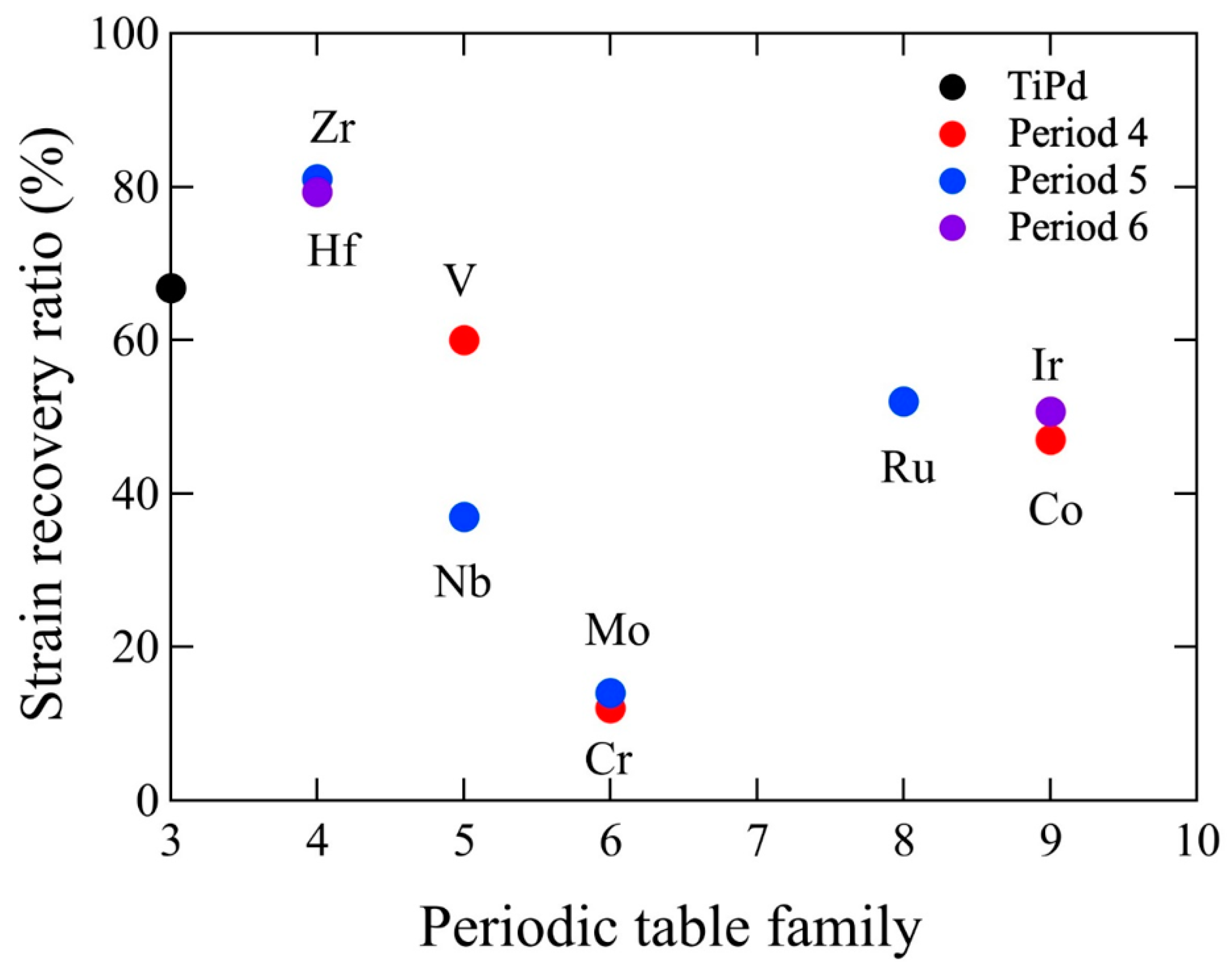
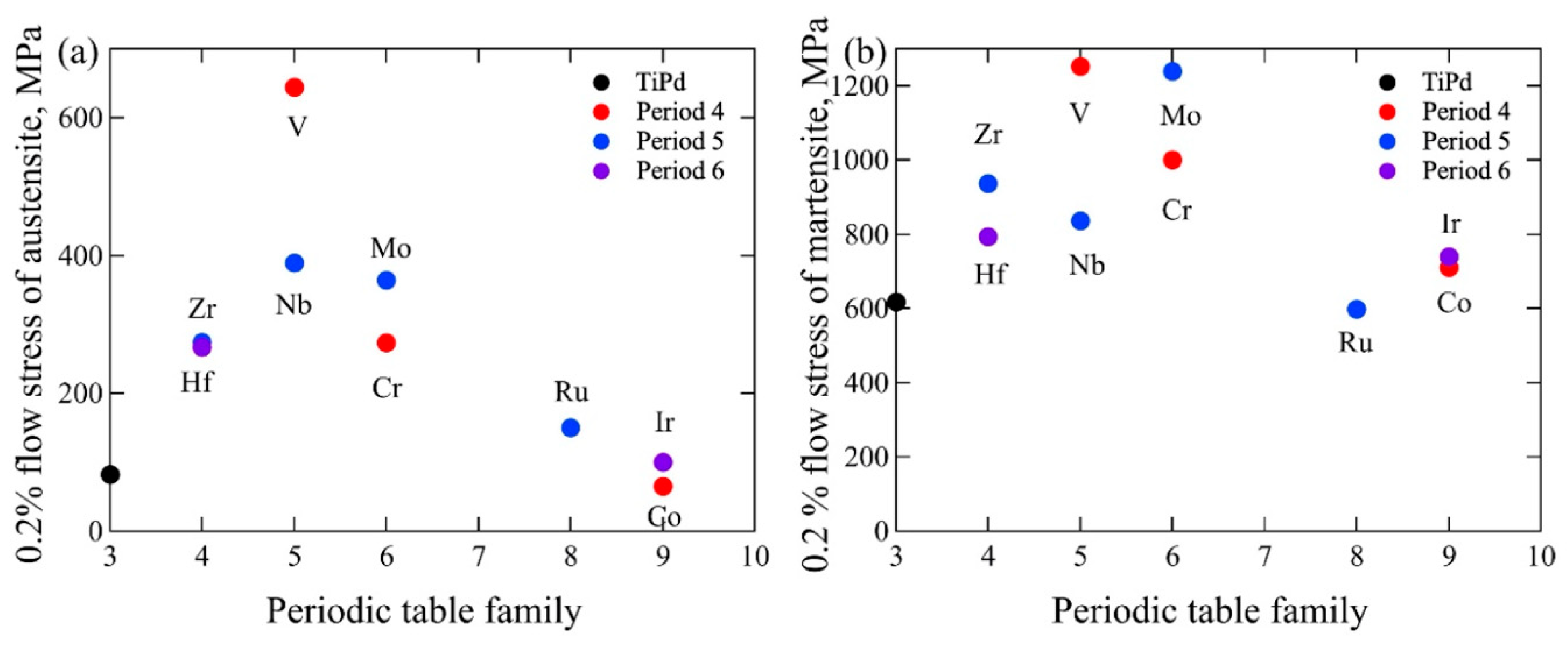
3. Strain Recovery Determined by Compression Test
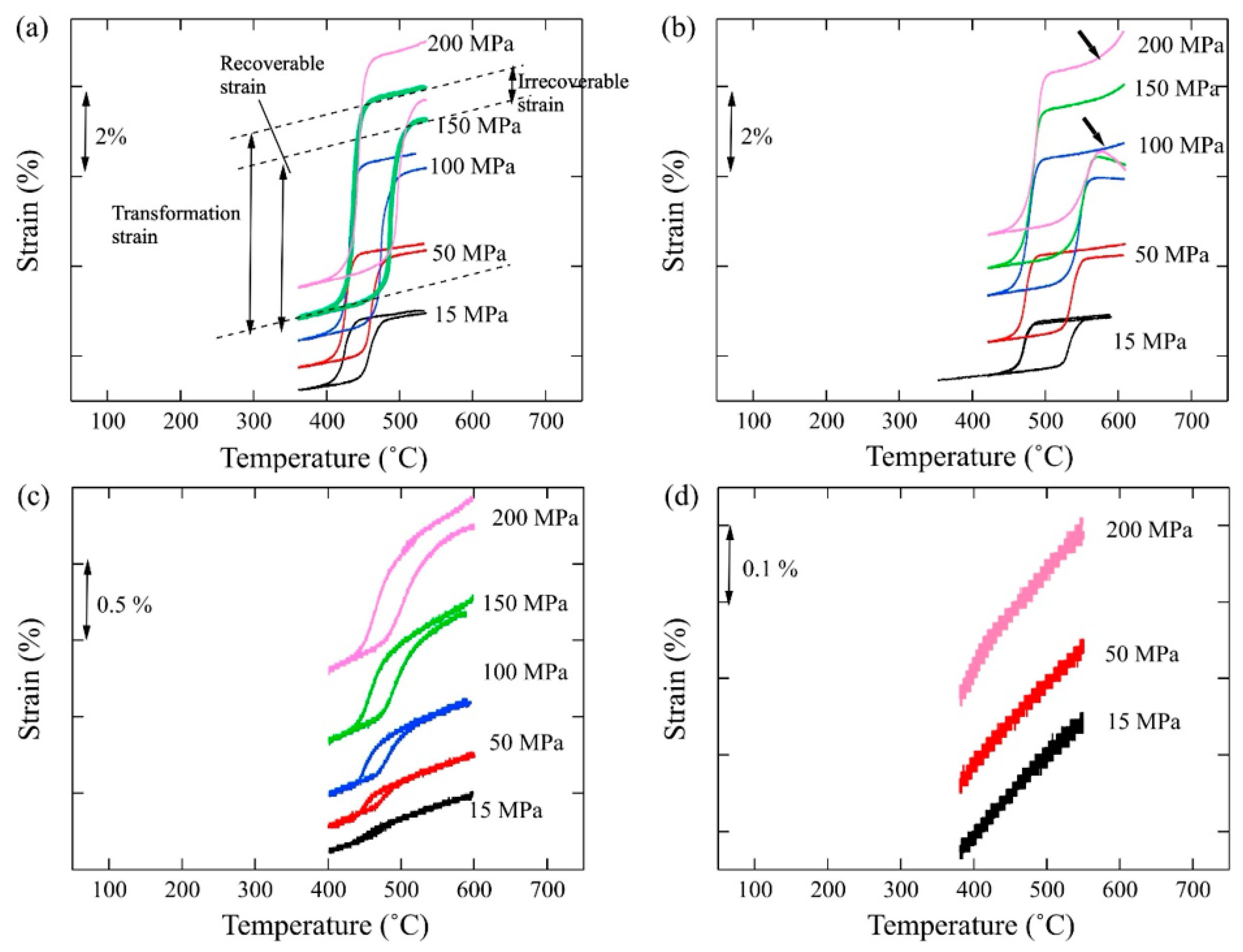
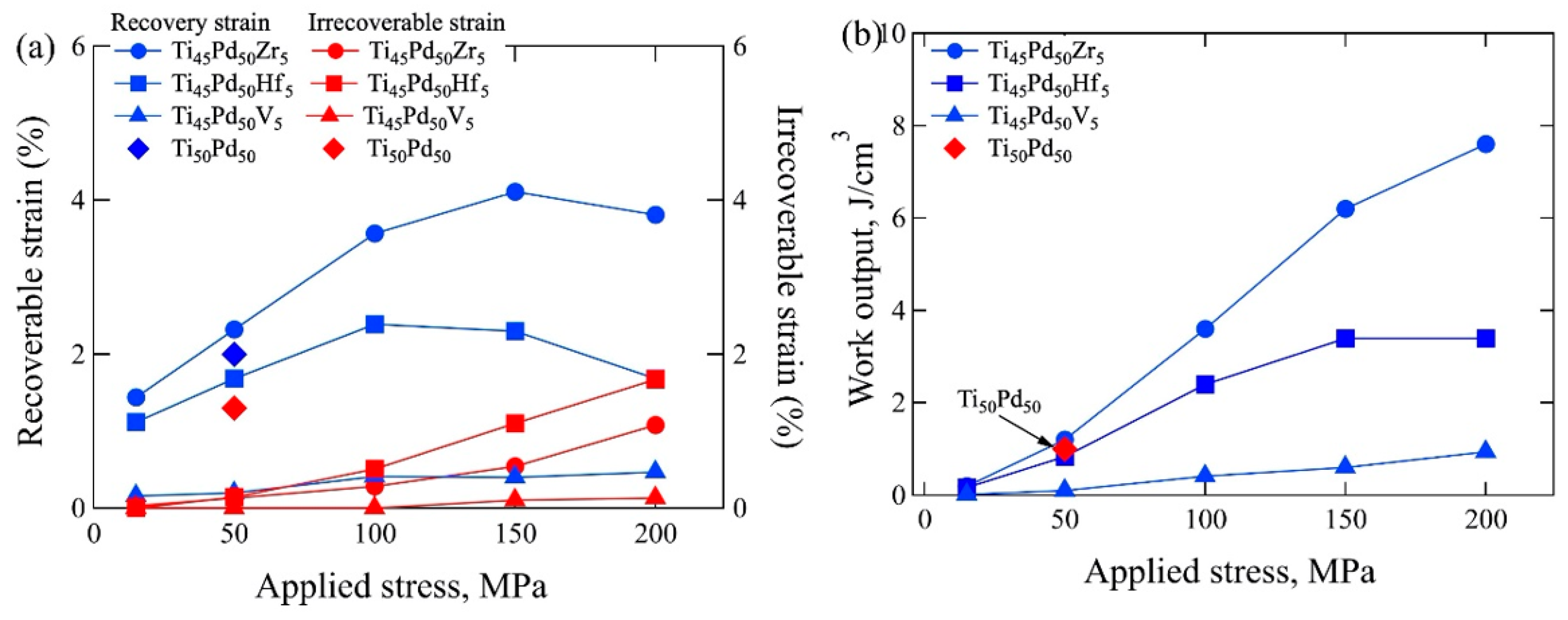
4. Strain Recovery Determined by Thermal Cyclic Test under Constant Stress
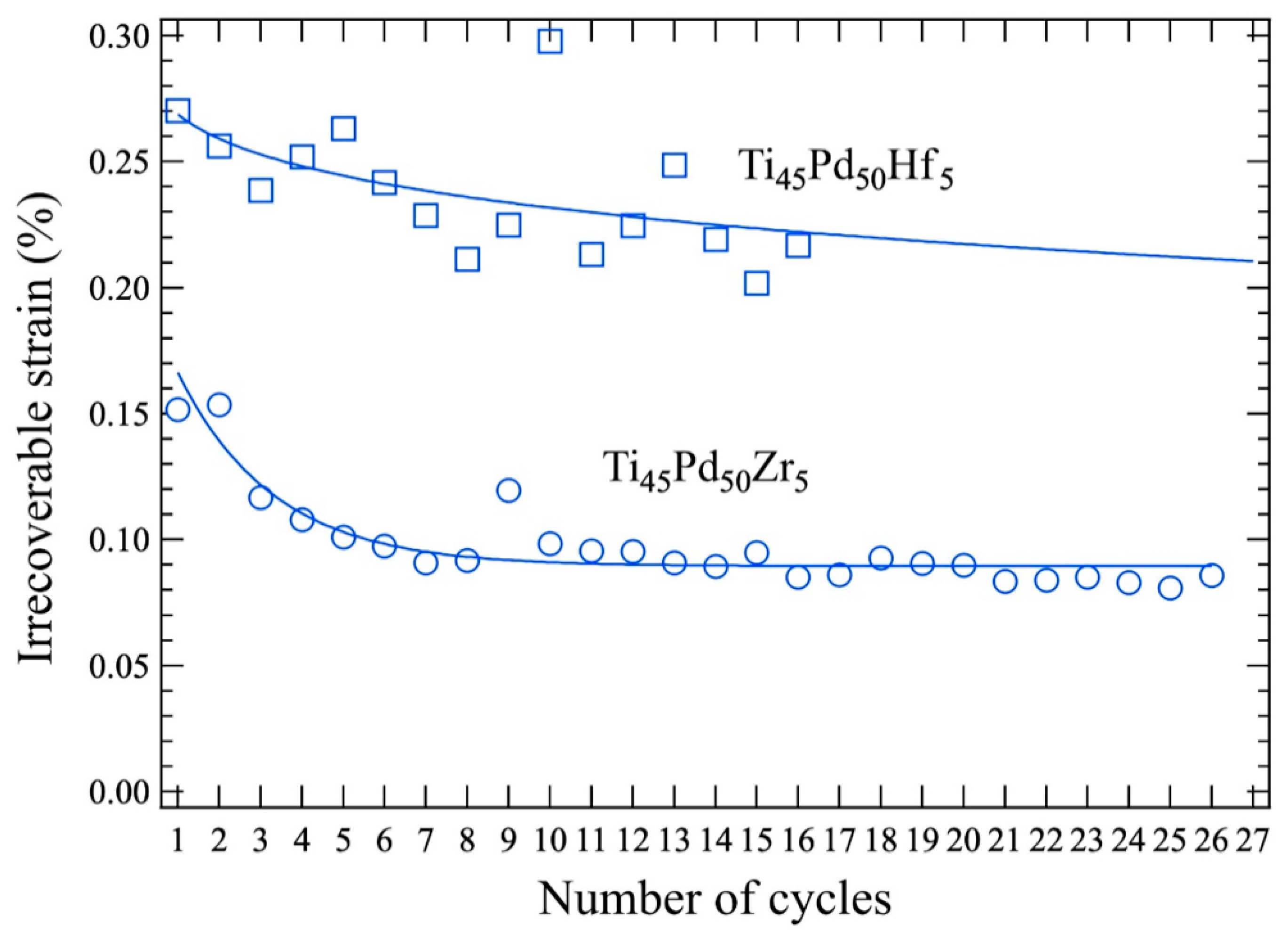
5. Effects of Training
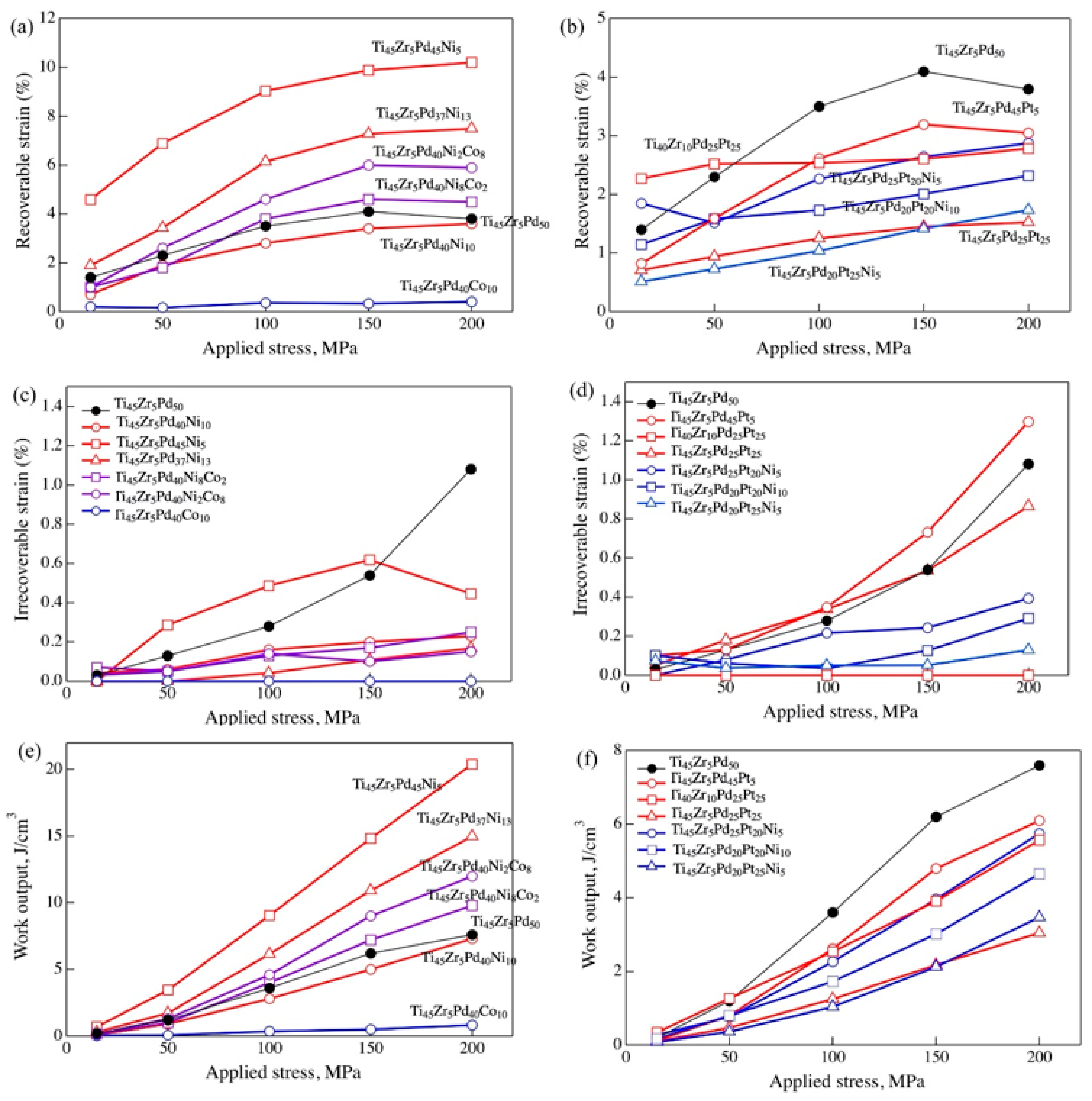
6. Strain Recovery of Multi-Component Alloys
7. Change in Microstructure before and after Training
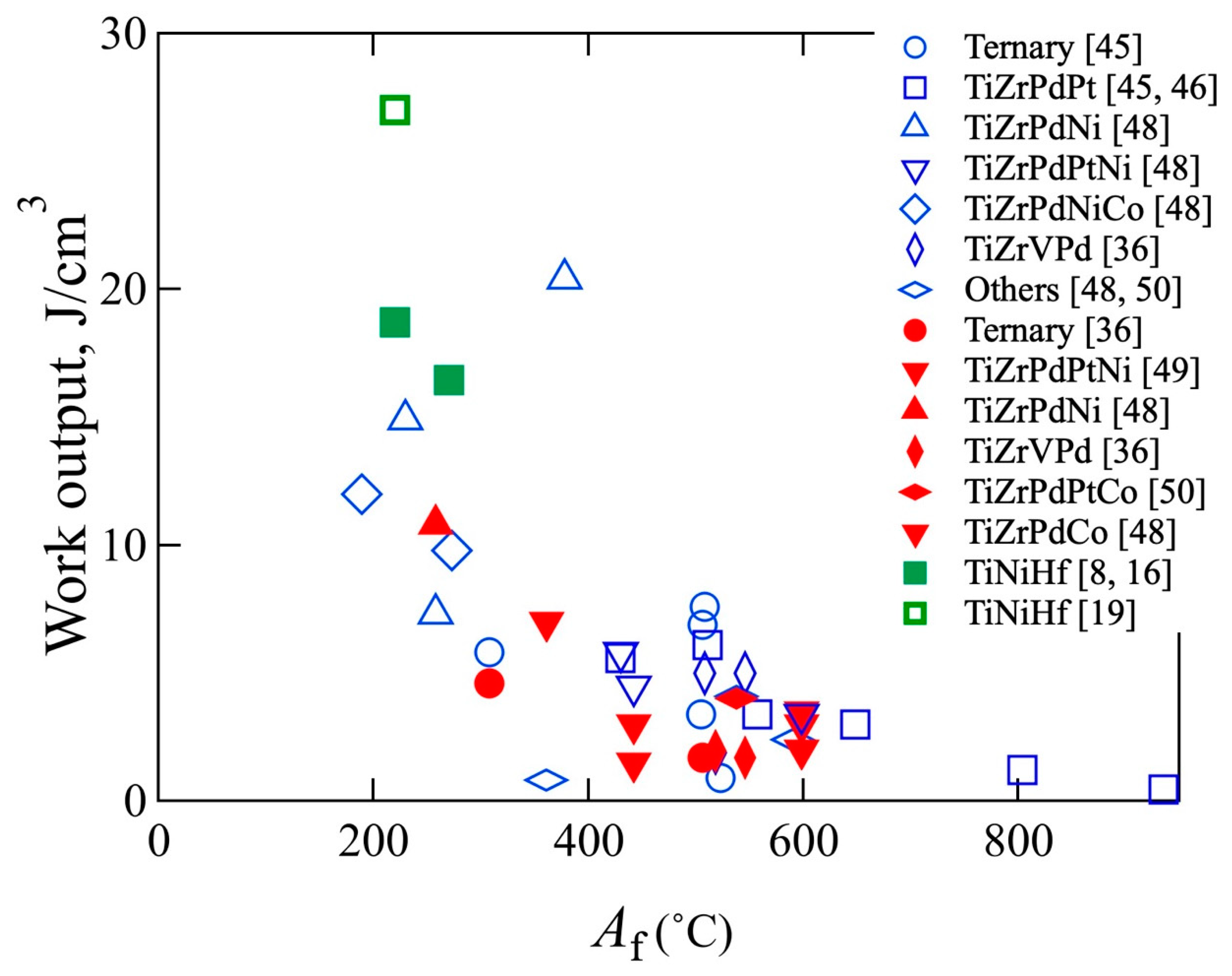
8. Potential of High-Temperature Shape Memory Alloys (HT-SMAs)
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- DeCastro, J.A.; Melcher, K.J.; Noebe, R.D. NASA/TM-2005-213834, AIAA-2005-3988. Available online: https://ntrs.nasa.gov/citations/20050203852 (accessed on 30 September 2020).
- Stebner, A.; Padula, S., II; Noebe, R.; Lerch, B.; Quinn, D. Development, Characterization, and Design Considerations of Ni19.5Ti50.5Pd25Pt5 High-temperature Shape Memory Alloy Helical Actuators. J. Intell. Mater. Syst. Struct. 2009, 20, 2107–2126. [Google Scholar] [CrossRef]
- Benefan, O.; Brown, J.; Calkins, F.T.; Kumar, P.; Stebner, A.P.; Turner, T.L.; Vaidyanathan, R.; Webster, J.; Young, M.L. Shape memory alloy actuator design: CASMART collaborative best practices and case studies. Int. J. Mech. Mater. Des. 2014, 10, 1–42. [Google Scholar] [CrossRef]
- Ma, J.; Karaman, I.; Noebe, R.D. High temperature shape memory alloys. Int. Mater. Rev. 2010, 55, 257–315. [Google Scholar] [CrossRef]
- Bigelow, G.S.; Padula, S.A., II; Garg, A.; Gaydosh, D.; Noebe, R.D. Characterization of ternary NiTiPd high-temperature shape-memory alloys under load-biased thermal cycling. Metall. Mater. Trans. A 2010, 41, 3065–3079. [Google Scholar] [CrossRef]
- “Development of a Numerical Model for High-Temperature Shape Memory Alloys” NASA Technical Reports Server, 2013. Available online: https://ntrs.nasa.gov/citations/20060028493 (accessed on 30 September 2020).
- Atli, K.C.; Karaman, I.; Noebe, R.D.; Garg, A.; Chumlyakov, Y.I.; Kireeva, I.V. Improvement in the Shape Memory Response of Ti50.5Ni24.5Pd25 High-Temperature Shape Memory Alloy with Scandium Microalloying. Metall. Mater. Trans. A 2010, 41, 2485–2497. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D.; Maier, H.J. Comparative analysis of the effects of severe plastic deformation and thermomechanical training on the functional stability of Ti50.5Ni24.5Pd25 high-temperature shape memory alloy. Scr. Mater. 2011, 64, 315–318. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D. Work output of the two-way shape memory effect in Ti50.5Ni24.5Pd25 high-temperature shape memory alloy. Scr. Mater. 2011, 65, 903–906. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D.; Garg, A.; Chumlyakov, Y.I.; Kireeva, I.V. Shape memory characteristics of Ti50.5Ni24.5Pd25Sc0.5 high-temperature shape memory alloy after severe plastic deformation. Acta Mater. 2011, 59, 4747–4760. [Google Scholar] [CrossRef]
- Yang, F.; Coughlin, D.R.; Phillips, P.J.; Yang, L.; Devaraj, A.; Kovarik, L. Structure analysis of a precipitate phase in an Ni-rich high-temperature NiTiHf shape memory alloy. Acta Mater. 2013, 61, 3335–3346. [Google Scholar] [CrossRef]
- Santamarta, R.; Arroyave, R.R.; Pons, J.; Evrigen, A.; Karaman, I.; Karacka, H.E.; Noebe, R.D. TEM study of structural and microstructural characteristics of a precipitate phase in NI-rich Ni-Ti-Hf and Ni-Ti-Zr shape memory alloys. Acta Mater. 2013, 61, 6191–6206. [Google Scholar] [CrossRef]
- Bigelow, G.S.; Garg, A.; Padula, S.A., II; Gaydosh, D.J.; Noebe, R.D. Load-biased shape-memory and superelastic properties of a precipitation strengthened high-temperature Ni50.3Ti29.7Hf20 alloy. Scr. Mater. 2011, 64, 725–728. [Google Scholar] [CrossRef]
- Coughlin, D.R.; Phillips, P.J.; Bigelow, G.S.; Garg, A.; Noebe, R.D.; Mills, M.J. Characterization of the microstructure and mechanical properties of a 50.3Ni-29.7Ti-20Hf shape memory alloy. Scr. Mater. 2012, 67, 112–115. [Google Scholar] [CrossRef]
- Evirgen, A.; Basner, F.; Karaman, I.; Noeve, R.D.; Pons, J.; Santamarta, R. Effect of Aging on the martensitic transformation characteristics of a Ni-rich NiTiHf high temperature shape meory alloys. Funct. Mater. Lett. 2012, 5, 1250038. [Google Scholar] [CrossRef]
- Karaca, H.E.; Saghaian, S.M.; Ded, G.; Tobe, H.; Basaran, B.; Maier, H.J.; Noebe, R.D.; Chumlyakov, Y.I. Effects of nanoprecipitation on the shape memory and material properties of an Ni-rich NiTiHf high temperature shape memory alloy. Acta Mater. 2013, 61, 7422–7431. [Google Scholar] [CrossRef]
- Benafan, O.; Garg, A.; Noebe, R.D.; Bigelow, G.S.; Padula, S.A.; Gaydosh, D.J.; Schell, N.; Mabe, J.H.; Vaidyanathan, R. Mechanical and functional behavior of Ni-rich Ni50.3Ti29.7Hf20 high temperature shape memory alloy. Intermet 2014, 50, 94–107. [Google Scholar] [CrossRef]
- Evirgen, A.; Karaman, I.; Santamarta, R.; Pons, J.; Hayrettin, C.; Noebe, R.D. Relationship between crystallographic compatibility and thermal hysteresis in Ni-rich NiTiHf and NiTiZr high temperature shape memory alloys. Acta Mater. 2016, 121, 374–383. [Google Scholar] [CrossRef]
- Saghaian, S.M.; Karaca, H.E.; Tobe, H.; Turabi, A.S.; Saedi, S.; Saghaian, S.E.; Chumlyakov, Y.I.; Noebe, R.D. High strength NiTiHf shape memory alloys with tailorable properties. Acta Mater. 2017, 134, 211–220. [Google Scholar] [CrossRef]
- Amin-Ahmadi, B.; Gallmeyer, T.; Pauza, J.G.; Duerig, T.W.; Noebe, R.D.; Stebner, A.P. Effect of a pre-aging treatment on the mechanical behaviors of Ni50.3Ti49.7−xHfx(x < 9 at.%) Shape memory alloys. Scr. Mater. 2018, 147, 11–15. [Google Scholar]
- Amin-Ahmadi, B.; Pauza, J.G.; Shamimi, A.; Duerig, T.W.; Noebe, R.D.; Stebner, A.P. Coherency strains of H-phase precipitates and their influence on functional properties of nickel-titanium-hafnium shape memory alloys. Scr. Mater. 2018, 147, 83–87. [Google Scholar] [CrossRef]
- Evirgen, A.; Pons, J.; Karaman, I.; Santamarta, R.; Noebe, R.D. H-Phase Precipitation and Martensitic Transformation in Ni-rich Ni-Ti-Hf and Ni-Ti-Zr High-Temperature Shape Memory Alloys. Shap. Mem. Superelasticity 2018, 4, 85–92. [Google Scholar] [CrossRef]
- Amin-Ahmadi, B.; Noebe, R.D.; Stebner, A.P. Crack propagation mechanisms of an aged nickel-titanium-hafnium shape memory alloy. Scr. Mater. 2019, 159, 85–88. [Google Scholar] [CrossRef]
- Karakoc, O.; Atli, K.C.; Evirgen, A.; Pons, J.; Santamarta, R.; Benafan, O.; Noebe, R.D.; Karaman, I. Effects of training on the thermomechanical behavior of NiTiHf and NiTiZr high temperature shape memory alloys. Mater. Sci. Eng. A 2020, 794, 139857. [Google Scholar] [CrossRef]
- ASM Alloy Phase Diagrams Center; Villars, P.; Okamoto, H.; Cenzual, K. (Eds.) ASM International, Materials Park, OH, 2006. Available online: http://www1.asminternational.org/AsmEnterprise/APD (accessed on 4 September 2020).
- Donkersloot, H.C.; van Vocht, J.H.N. Martensitic transformations in gold-titanium, palladium-titanium and platinum-titanium alloys near the equiatomic composition. J. Less Common Met. 1970, 20, 83–91. [Google Scholar] [CrossRef]
- Biggs, T.; Witcomb, M.J.; Cornish, L.A. Martensite-type transformations in platinum alloys. Mater. Sci. Eng. A 1999, 273–278, 204–207. [Google Scholar] [CrossRef]
- Biggs, T.; Corite, M.B.; Witcomb, M.J.; Cornish, L.A. Martensitic Transformations, Microstructure, and Mechanical Workability of TiPt. Metall. Trans. A 2003, 32A, 2267–2272. [Google Scholar] [CrossRef]
- Otsuka, K.; Oda, K.; Ueno, Y.; Piao, M.; Ueki, T.; Horikawa, H. The shape memory effect in a Ti50Pd50 alloy. Scr. Met. Mater. 1993, 29, 1355–1358. [Google Scholar] [CrossRef]
- Declairieux, C.; Denquin, A.; Ochin, P.; Poriter, R.; Vermaut, P. On the potential of Ti50Au50compound as a high temperature shape memory alloy. Intermetallics 2011, 19, 1461–1465. [Google Scholar] [CrossRef]
- Wadood, A.; Hosoda, H.; Yamabe-Mitarai, Y. Phase transformation, osidation and shape memory properties of Ti-50Au-10Zr alloy for high temperature applications. J. Alloys Compd. 2014, 595, 200–205. [Google Scholar] [CrossRef]
- Wadood, A.; Yamabe-Mitarai, Y. Silver- and Zirconium-added ternary and quaternary TiAu based high temperature shape memory alloys. J. Alloys Compd. 2015, 646, 1172–1177. [Google Scholar] [CrossRef]
- Kawakita, M.; Takahashi, M.; Takahashi, S.; Yamabe-Mitarai, Y. Effect of Zr on phase transformation and high-temperature shape memory effect in TiPd alloys. Mater. Lett. 2012, 89, 336–338. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y. Development of High-Temperature Shape Memory Alloys above 673 K. Mater. Sci. Forum 2017, 879, 107–112. [Google Scholar] [CrossRef]
- Arockiakumar, R.; Takahashi, M.; Takahashi, S.; Yamabe-Mitarai, Y. Microstructure, mechanical and shape memory properties of Ti-55Pd-5x (x = Zr, Hf, V, Nb) alloys. Mater. Sci. Eng. A 2013, 585, 86–93. [Google Scholar] [CrossRef]
- Sato, H.; Kim, H.Y.; Shimojo, M.; Yamabe-Mitarai, Y. Training effect on microstructure and shape recovery in Ti-Pd-Zr alloys. Mater. Trans. 2017, 10, 1479–1486. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Wadood, A.; Arockiakumar, R.; Hara, T.; Takahashi, M.; Takahashi, S.; Hosoda, H. High-Temperature Shape Memory Alloys Based on Ti-Platinum Group Metals compounds. Mater. Sci. Forum 2014, 783–786, 2541–2545. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Miura, S.; Hosoda, H. Mechanical properties of Ti-50(Pt, Ir) high-temperature shape memory alloys. Mater. Trans. 2006, 47, 650–657. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Miura, S.; Hosoda, H. Phase transformation and shape memory effect of Ti(Pt, Ir). Metall. Trans. A 2012, 43A, 2901–2911. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Arockiakumar, R.; Wadood, A.; Suresh, K.S.; Kitashima, T.; Hara, T.; Shimojo, M.; Tasaki, W.; Takahashi, M.; Takahashi, S.; et al. Ti(Pt, Pd, Au) based high temperature shape memory alloys. Mater. Today 2015, 2S, S517–S522. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Kitashima, T.; Miura, S.; Hosoda, H. Composition dependence of phase transformation behavior and shape memory effect of Ti(Pt, Ir). J. Alloys Compd. 2013, 577S, S399–S403. [Google Scholar] [CrossRef]
- Wadood, A.; Takahashi, M.; Takahashi, S.; Hosoda, H.; Yamabe-Mitarai, Y. High-temperature mechanical and shape memory properties of TiPt-Zr and TiPt-Ru alloys. Mater. Sci. Eng. A 2013, 564, 34–41. [Google Scholar] [CrossRef]
- Wadood, A.; Yamabe-Mitarai, Y. TiPt-Co and TiPt-Ru high temperature shape memory alloys. Mater. Sci. Eng. A 2014, 610, 106–110. [Google Scholar] [CrossRef]
- Wadood, A.; Yamabe-Mitarai, Y. Recent research and developments related to near-equiatomic titanium-platinum alloys for high-temperature applications. Platinum Metals Rev. 2014, 2, 61–67. [Google Scholar] [CrossRef]
- Tasaki, W.; Shimojo, M.; Yamabe-Mitarai, Y. Thermal cyclic properties of Ti-Pd-Pt-Zr high-temperature shape memory alloys. Crystals 2019, 9, 595. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Takebe, W.; Shimojo, M. Phase transformation and shape memory effect of Ti-Pd-Pt-Zr high-temperature shape memory alloys. Shap. Mem. Superelasticity 2017, 3, 381–391. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Tasaki, W.; Ohl, B.; Sato, H. Effect of Ni, Co, and Pt on Phase transformation and shape recovery of TiPd high temperature shape memory alloys. In Proceedings of the International Conference on Processing & Manufacturing of Advanced Materials, Paris, France, 9–13 July 2018. [Google Scholar]
- Yamabe-Mitarai, Y.; Ohl, B.; Bogdanowicz, K.; Muszalska, E. Effect of Ni and Co on Phase Transformation and Shape Memory Effect of Ti-Pd-Zr alloys. Shap. Mem. Superelasticity 2020, 6, 170–180. [Google Scholar] [CrossRef]
- Matsuda, H.; Sato, H.; Shimojo, M.; Yamabe-Mitarai, Y. Improvement of high-temperature shap-memory effect by multi-component alloyin for TiPd alloys. Mater. Trans. 2019, 11, 2282–2291. [Google Scholar] [CrossRef]
- Matsuda, H.; Shimojo, M.; Murakami, H.; Yamabe-Mitarai, Y. Martensitic Transformation of High-Entropy and Medium-Entropy Shape Memory Alloys. Mater. Sci. Forum 2021, 1016, 1802–1810. [Google Scholar]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Canter, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Firstov, G.S.; Kosorukova, T.A.; Koval, Y.N.; Odnosum, V.V. High entropy shape memory alloys. Mater. Today 2015, 2S, S499–S504. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, Y.-J. Shape memory characteristics of (TiZrHf)50Ni25Co10Cu15 high entropy shape memory alloy. Scr. Mater. 2019, 162, 185–189. [Google Scholar] [CrossRef]
- Canadinc, D.; Trehern, W.; Ma, J.; Karaman, I.; Sun, F.; Chaudhry, Z. Ultra-high temperature multi-component shape memory alloys. Scr. Mater. 2019, 158, 83–87. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Phasha, M.; Ngoepe, P.; Chikwanda, H. Phase transformation and crystal structure of IrTi. Intermetallics 2012, 31, 26–33. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Atomic_radii_of_the_elements_(data_page) (accessed on 1 September 2020).
- Hisada, S.; Matsuda, M.; Yamabe-Mitarai, Y. Shape Change and Crystal Orientation of B19 Martensite in Equiatomic TiPd Alloy by Isobaric Test. Metals 2020, 10, 375. [Google Scholar] [CrossRef]
- Atli, K.C.; Franco, E.B.; Karaman, I.; Gaydosh, D.; Noebe, R.D. Influence of crystallographic compatibility on residual strain of TiNi based shape memory alloys during thermo-mechanical cycling. Mater. Sci. Eng. A 2013, 574, 9–16. [Google Scholar] [CrossRef]
- Pearson’s Crystal Data—Crystal Structure Database for Inorganic Compounds (on CD-ROM); Villars, P., Cenzual, K., Eds.; ASM International: Materials Park, OH, USA, 2010. [Google Scholar]
- Karaca, H.E.; Acar, E.; Tobe, H.; Saghaian, S.M. NiTiHf-based shape memory alloys. Mater. Sci. Technol. 2014, 30, 1530–1544. [Google Scholar] [CrossRef]

| Alloy | Austenite Start Temperature As | Austenite Finish Temperature Af | Martensite Start Temperature Ms | Martensite Finish Temperature Mf | Temperature Hysteresis Af–Ms | Average Decrease in Ms per 1 at% ΔMs/at% | Ref. |
|---|---|---|---|---|---|---|---|
| Ti50Pd50 | 568 | 587 | 527 | 515 | 60 | - | [33] |
| Ti48Pd50Hf2 | 538 | 570 | 494 | 472 | 76 | - | |
| Ti45Pd50Hf5 | 481 | 505 | 460 | 429 | 45 | −15 | [34] |
| Ti40Pd50Hf10 | 227 | 306 | 276 | 188 | 30 | - | |
| Ti40Pd55Hf5 | 528 | 551 | 471 | 487 | 80 | - | [35] |
| Ti47Pd50Zr3 | 520 | 533 | 491 | 470 | 42 | - | |
| Ti45Pd50Zr5 | 492 | 508 | 467 | 445 | 41 | −15 | [33] |
| Ti43Pd50Zr7 | 491 | 506 | 464 | 443 | 42 | - | [36] |
| Ti40Pd50Zr10 | 256 | 308 | 221 | 204 | 87 | - | [33,36] |
| Ti50Pd45Zr5 | 324 | 374 | 344 | 312 | 30 | - | |
| Ti47Pd48Zr5 | 429 | 450 | 370 | 331 | 80 | - | |
| Ti43Pd52Zr5 | 482 | 529 | 393 | 366 | 136 | - | |
| Ti40Pd55Zr5 | 530 | 593 | 451 | 438 | 142 | - | [35] |
| Ti45Pd50V5 | 508 | 523 | 462 | 440 | 61 | −15 | [34] |
| Ti40Pd55V5 | 437 | 559 | 442 | 398 | 117 | - | [35] |
| Ti45Pd50Nb5 | 486 | 508 | 431 | 408 | 77 | −20 | [34] |
| Ti40Pd55Nb5 | 487 | 535 | 409 | 360 | 126 | - | [35] |
| Ti45Pd50Ta5 * | 561 | 579 | 512 | 489 | 67 | −4 | [34] |
| Ti45Pd50Cr5 | 268 | 356 | 347 | 293 | 9 | −37 | [34] |
| Ti45Pd50Mo5 | 267 | 383 | 401 | 268 | −18 | −27 | [34] |
| Ti45Pd50W5 * | 558 | 576 | 513 | 492 | 63 | −4 | [34] |
| Ti50Pd48Ru2 | 561 | 576 | 516 | 510 | 60 | - | [37] |
| Ti50Pd46Ru4 | 440 | 482 | 436 | 393 | 46 | −23 | [37] |
| Ti50Pd42Ru8 | 298 | 354 | 333 | 275 | 22 | - | [37] |
| Ti50Pd37.5Ru12.5 | - | - | - | - | - | - | [37] |
| Ti50Pd25Ru25 | - | - | - | - | - | - | [37] |
| Ti50Pd48Ir2 | 556 | 581 | 508 | 500 | 73 | - | [37] |
| Ti50Pd46Ir4 | 525 | 552 | 510 | 484 | 42 | −4 | [37] |
| Ti50Pd42Ir8 | 478 | 521 | 479 | 439 | 43 | - | [37] |
| Ti50Pd38Ir12 | 471 | 508 | 452 | 395 | 56 | - | |
| Ti50Pd37.5Ir12.5 | 478 | 492 | 452 | 433 | 39 | - | [37] |
| Ti50Pd25Ir25 | - | - | - | - | - | - | [37] |
| Ti50Pd12.5Ir37.5 | - | - | - | - | - | - | [37] |
| Ti50Pd48Co2 | 523 | 545 | 478 | 464 | 67 | - | [37] |
| Ti50Pd46Co4 | 473 | 493 | 455 | 427 | 38 | −18 | [37] |
| Ti50Pd42Co8 | 375 | 398 | 259 | 327 | 34 | - | [37] |
| Ti50Pt50 | 1000 | 1057 | 989 | 963 | 68 | - | [38] |
| Ti50Pt45Ir5 | 1050 | 1081 | 1030 | 1008 | 51 | - | [39,40] |
| Ti50Pt37.5Ir12.5 | 1036 | 1103 | 1000 | 978 | 103 | - | [38] |
| Ti50Pt25Ir25 | 1121 | 1190 | 1110 | 1068 | 80 | - | [38] |
| Ti50Pt20Ir30 | 1159 | 1189 | 1145 | 1127 | 44 | - | [39] |
| Ti50Pt12.5Ir37.5 | 1175 | 1218 | 1184 | 1169 | 34 | - | [38] |
| Ti55Pt35Ir10 | 931 | 959 | - | - | - | - | [41] |
| Ti50Pt45Ru5 | 925 | 975 | 913 | 856 | 62 | −20 | [40,42] |
| Ti45Pt50Zr5 | 939 | 985 | 897 | 840 | 88 | −23 | [40,42] |
| Ti50Pt45Co5 | 959 | 1003 | 913 | 855 | 90 | −15 | [40,43] |
| Ti45Pt50Hf5 | 952 | 996 | 905 | 855 | 91 | −17 | [40,44] |
| Alloy | As | Af | Ms | Mf | Af–Ms | ΔSmix | Alloy Classification | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ti45Zr5Pd45Ir5 | 457 | 475 | 422 | 398 | 53 | 1.0R | Low-entropy alloy LEA | - |
| Ti45Zr5Pd35Ir15 | 313 | 348 | 295 | 256 | 53 | 1.2R | Medium-entropy alloy MEA | - |
| Ti45Zr5Pd25Ir25 | - | - | - | - | - | 1.2R | MEA | - |
| Ti45Zr5Pd45Pt5 | 497 | 511 | 464 | 439 | 47 | 1.0R | LEA | [45] |
| Ti45Zr5Pd35Pt15 | 539 | 557 | 485 | 471 | 72 | 1.2R | MEA | [45] |
| Ti45Zr5Pd25Pt25 | 628 | 648 | 571 | 539 | 77 | 1.2R | MEA | [45] |
| Ti45Zr5Pd15Pt35 | 725 | 804 | 713 | 590 | 91 | 1.2R | MEA | [46] |
| Ti45Zr5Pd5Pt45 | 896 | 935 | 774 | 713 | 112 | 1.0R | LEA | [46] |
| Ti40Zr10Pd25Pt25 | 390 | 430 | 245 | 220 | 288 | 1.3R | MEA | [47] ST |
| Ti45Zr4V1Pd50 | 497 | 508 | 409 | 384 | 99 | 0.9R | LEA | [36] |
| Ti45Zr2.5V2.5Pd50 | 523 | 546 | 393 | 368 | 153 | 0.9R | LEA | [36] |
| Ti45Zr1V4Pd50 | 496 | 518 | 441 | 424 | 77 | 0.9R | LEA | [36] |
| Ti45Zr5Pd45Ni5 | 348 | 378 | 328 | 297 | 50 | 1.0R | LEA | [47] |
| Ti45Zr5Pd40Ni10 | 216 | 258 | 206 | 177 | 52 | 1.1R | MEA | [48] |
| Ti45Zr5Pd37Ni13 | 114 | 230 | 206 | 108 | 24 | 1.2R | MEA | [47] ST |
| Ti42Zr8Pd40Ni10 | - | - | - | - | - | 1.2R | MEA | [47] |
| Ti45Zr5Pd30Ni20 | - | - | - | - | - | 1.2R | MEA | [48] |
| Ti45Zr5Pd40Co10 | 303 | 361 | 339 | 298 | 22 | 1.1R | MEA | [48] |
| Ti45Zr5Pd40Ni8Co2 | 201 | 273 | 212 | 199 | 61 | 1.2R | MEA | [48] |
| Ti45Zr5Pd40Ni5Co5 | - | - | - | - | - | 1.2R | MEA | [48] |
| Ti45Zr5Pd40Ni2Co8 | 143 | 189 | 135 | 108 | 54 | 1.2R | MEA | [48] |
| Ti35Zr15Pd20Pt15Ni15 | - | - | - | - | - | 1.5R | High-entropy alloy HEA | [49] |
| Ti40Zr10Pd20Pt15Ni15 | - | - | - | - | - | 1.5R | HEA | [49] |
| Ti45Zr5Pd20Pt25Ni5 | 559 | 598 | 502 | 432 | 96 | 1.3R | MEA | [49] |
| Ti45Zr5Pd25Pt20Ni5 | 390 | 430 | 245 | 220 | 185 | 1.3R | MEA | [47] ST |
| Ti45Zr5Pd20Pt20Ni10 | 374 | 442 | 337 | 256 | 105 | 1.3R | MEA | [49] |
| Ti35Zr15Pd20Pt15Au15 | - | - | - | - | - | 1.5R | HEA | [50] |
| Ti35Zr15Pd20Pt15Co15 | - | - | - | - | - | 1.5R | HEA | [50] |
| Ti45Zr5Pd25Pt20Au5 | 520 | 590 | 515 | 453 | 75 | 1.3R | MEA | [50] |
| Ti45Zr5Pd25Pt20Co5 | 419 | 538 | 431 | 324 | 107 | 1.3R | MEA | [50] |
| Ti16.7Zr16.7Hf16.7Ni25Cu25 | 184 | 338 | 226 | 112 | 112 | 1.6R | HEA | [53] |
| Ti16.7Zr16.7Hf16.7Ni25Co10Cu15 | −23 | 71 | 36 | −80 | 35 | 1.8R | HEA | [54] |
| Ti30Hf20Pd15Ni35 | 537 | 686 | 525 | 479 | 161 | 1.3R | MEA | [55] |
| Ti25Hf25Pd25Ni25 | 680 | 720 | 620 | 580 | 100 | 1.4R | MEA | [55] |
| Ti16.7Zr16.7Hf16.7Pd25Ni25 | 740 | 780 | 660 | 620 | 120 | 1.6R | HEA | [55] |
| Alloys | Number of Alloys with Temperature Hysteresis over 100 °C | Number of Tested Alloys | Ratio of Alloys with Temperature Hysteresis over 100 °C (%) |
|---|---|---|---|
| Ternary alloy in Table 1 | 5 | 42 | 12 |
| LEA in Table 2 | 2 | 7 | 28 |
| MEA and HEA in Table 2 | 8 | 20 | 40 |
| Alloy | Test Temp., °C | The Difference from Martensite Transformation Temperature, °C | Applied Strain, % | Recoverable Strain, % | Strain Recovery Ratio, % | Ref. |
|---|---|---|---|---|---|---|
| Ti50Pd50 | 538 | - | 4.1 | 1.6 | 40 | [33] |
| Ti50Pd50 | 485 | Mf − 30 | 3.8 | 2.5 | 67 | [34] |
| Ti50Pd50 | 380 | - | 3.7 | 0.46 | 13 | [33] |
| Ti50Pd50 | 320 | - | 1.24 | 0.59 | 48 | |
| Ti48Pd50Hf2 | 380 | - | 2.8 | 0.4 | 14 | |
| Ti45Pd50Hf5 | 456 | As − 30 | 2.9 | 2.1 | 74 | |
| Ti45Pd50Hf5 | 440 | - | 2.7 | 2.3 | 80 | [34] |
| Ti45Pd50Hf5 | 380 | Mf − 50 | 1.9 | 1.5 | 80 | |
| Ti45Pd50Hf5 | 200 | - | 5.25 | 4.1 | 78 | |
| Ti40Pd50Hf10 | 380 | - | 3.0 | 2.4 | 80 | |
| Ti40Pd55Hf5 | 457 | Mf − 30 | 3.7 | 1.7 | 45 | [35] |
| Ti50Pd45Zr5 | 210 | - | 4.28 | 0.7 | 17 | |
| Ti50Pd45Zr5 | 295 | - | 3.1 | 0.55 | 18 | |
| Ti50Pd45Zr5 | 380 | - | 3.2 | 1.47 | 46 | |
| Ti47Pd48Zr5 | 301 | - | 4.2 | 3.6 | 85 | |
| Ti47Pd48Zr5 | 399 | - | 8.3 | 5.7 | 69 | |
| Ti47Pd48Zr5 | 380 | - | 2.2 | 1.1 | 50 | |
| Ti48Pd50Zr2 | 400 | - | 6.5 | 3.4 | 53 | |
| Ti48Pd50Zr2 | 380 | - | 3.1 | 1.3 | 43 | |
| Ti47Pd50Zr3 | 490 | As − 30 | 3.7 | 2.6 | 70 | |
| Ti47Pd50Zr3 | 415 | Mf − 30 | 3.3 | 2.9 | 88 | |
| Ti47Pd50Zr3 | 380 | - | 3.2 | 2.8 | 87 | |
| Ti45Pd50Zr5 | 415 | Mf − 30 | 4.4 | 3.5 | 81 | [34] |
| Ti45Pd50Zr5 | 426 | As − 30 | 3.1 | 2.58 | 84 | [33] |
| Ti45Pd50Zr5 | 460 | - | 8 | 5.2 | 65 | |
| Ti45Pd50Zr5 | 380 | - | 3.5 | 2,2 | 62 | |
| Ti45Pd50Zr5 | 380 | - | 4.1 | 3.9 | 94 | [33] |
| Ti43Pd52Zr5 | 380 | - | 2.2 | 1.75 | 81 | |
| Ti43Pd52Zr5 | 451 | As − 30 | 4.6 | 4.1 | 90 | |
| Ti40Pd55Zr5 | 490 | - | 7.2 | 2.0 | 27 | |
| Ti40Pd55Zr5 | 450 | - | 1.2 | 0.39 | 34 | |
| Ti40Pd55Zr5 | 380 | - | 1.9 | 0.4 | 22 | |
| Ti43Pd50Zr7 | 461 | As − 30 | 1.4 | 1.2 | 84 | |
| Ti43Pd50Zr7 | 413 | Mf − 30 | 3.6 | 3.6 | 100 | |
| Ti43Pd50Zr7 | 380 | - | 3.2 | 2.9 | 90 | |
| Ti40Pd50Zr10 | 225 | - | 7.7 | 4.8 | 63 | [33] |
| Ti40Pd50Zr10 | 380 | - | 1.4 | 1.4 | 100 | |
| Ti40Pd55Zr5 | 408 | Mf − 30 | 4 | 1.2 | 30 | [35] |
| Ti45Pd50V5 | 413 | Mf − 30 | 2.4 | 1.2 | 60 | [34] |
| Ti40Pd55V5 | 368 | Mf − 30 | 3 | 0.3 | 10 | [35] |
| Ti45Pd50Nb5 | 378 | Mf − 30 | 3.1 | 1.1 | 37 | [34] |
| Ti40Pd55Nb5 | 330 | Mf − 30 | 3 | 0.3 | 10 | [35] |
| Ti45Pd50Ta5 * | 483 | Mf − 30 | 3.0 | 1.1 | 35 | [34] |
| Ti45Pd50Cr5 | 228 | Mf − 30 | 3.3 | 0.4 | 12 | [34] |
| Ti45Pd50Mo5 | 256 | Mf − 30 | 2.8 | 0.4 | 14 | [34] |
| Ti45Pd50W5 * | 474 | Mf − 30 | 2.3 | 0.5 | 22 | [34] |
| Ti50Pd46Ru2 | 320 | - | - | - | 40 | [37] |
| Ti50Pd46Ru4 | 320 | - | 3.7 | 2.0 | 52 | |
| Ti50Pd42Ru8 | 320 | - | 3.4 | 2.5 | 72 | |
| Ti50Pd48Ir2 | 320 | - | 2.6 | 1.22 | 47 | [37] |
| Ti50Pd46Ir4 | 320 | - | 2.9 | 1.49 | 51 | [37] |
| Ti50Pd46Ir4 | 454 | Mf − 30 | 1.0 | 1.0 | 100 | |
| Ti50Pd46Ir4 | 427 | Mf − 60 | 2.9 | 0.55 | 19 | |
| Ti50Pd42Ir8 | 320 | - | 2.17 | 1.25 | 57 | [37] |
| Ti50Pd42Ir8 | 408 | Mf − 30 | 1.5 | 1.3 | 87 | |
| Ti50Pd42Ir8 | 427 | Mf − 60 | 6.36 | 2.05 | 32 | |
| Ti50Pd38Ir12 | 401 | Mf − 30 | 1.4 | 1.01 | 72 | |
| Ti50Pd48Co2 | 320 | - | 2.35 | 1.36 | 58 | [37] |
| Ti50Pd46Co4 | 320 | - | 3.2 | 1.4 | 47 | [37] |
| Ti50Pd42Co8 | 320 | - | 3.3 | 1.0 | 31 | [37] |
| Ti50Pt50 | 910 | Mf − 50 | 6 | 0 | 0 | [38] |
| Ti50Pt50 | 850 | - | - | - | 11 | [39] |
| Ti50Pt45Ir5 | 850 | - | - | - | 10 | [39,40] |
| Ti50Pt37.5Ir12.5 | 928 | Mf − 50 | 17 | 2 | 12 | [38] |
| Ti50Pt37.5Ir12.5 | 850 | - | - | - | 51 | [39] |
| Ti50Pt25Ir25 | 1018 | Mf − 50 | 17 | 4 | 23 | [38] |
| Ti50Pt25Ir25 | 850 | - | - | - | 57 | [39] |
| Ti50Pt20Ir30 | 850 | - | - | - | 36 | [39] |
| Ti50Pt12.5Ir37.5 | 1119 | Mf − 50 | 15 | 0 | 0 | [38] |
| Ti50Pt12.5Ir37.5 | 850 | - | - | - | 21 | [39] |
| Ti55Pt35Ir10 | 850 | - | 4.8 | 1 | 20 | [41] |
| Ti56Pt22Ir22 | 850 | - | 2.5 | 1.4 | 56 | [41] |
| Ti58Pt10Ir32 | 850 | - | 1.9 | 1.4 | 73 | [41] |
| Ti50Pt45Ru5 | 802 | Mf − 50 | 2.9 | 1.3 | 45 | [40,42] |
| Ti45Pt50Zr5 | 790 | Mf − 50 | 2.1 | 1.2 | 58 | [40,42] |
| Ti50Pt45Co5 | 801 | Mf − 50 | 2.5 | 0.9 | 36 | [40,43] |
| Ti45Pt50Hf5 | 806 | Mf − 50 | 2.4 | 1.4 | 58 | [40,44] |
| Alloy | Test Temp., °C | The Difference from Martensite Transformation Temperature, °C | Applied Strain, % | Recoverable Strain, % | Strain Recovery Ratio, % |
|---|---|---|---|---|---|
| Ti45Pd45Ir5Zr5 | 368 | Mf − 30 | 2.8 | 2.7 | 96 |
| Ti45Pd35Ir15Zr5 | 226 | Mf − 30 | 2.6 | 2.2 | 85 |
| Alloy | Test Temp., °C | The Difference from Martensite Transformation Temperature, °C | Detwining Stress, MPa | 0.2% Flow Stress, MPa | Ref. |
|---|---|---|---|---|---|
| Ti50Pd50 | 617 | Af + 30 | - | 82 | [33] |
| Ti50Pd50 | 538 | As − 30 | 231 | 293 | [33] |
| Ti50Pd50 | 485 | Mf − 30 | 249 | 617 | |
| Ti45Pd50Hf5 | 534 | Af + 30 | - | 267 | [34] |
| Ti45Pd50Hf5 | 456 | As − 30 | 152 | 794 | [34] |
| Ti40Pd55Hf5 | 581 | Af + 30 | - | 341 | [35] |
| Ti40Pd55Hf5 | 441 | Mf − 30 | 725 | 1300 | [35] |
| Ti47Pd48Zr5 | 301 | Mf − 30 | 301 | 1163 | |
| Ti47Pd48Zr5 | 550 | Af + 30 | - | 628 | |
| Ti47Pd48Zr5 | 399 | As − 30 | 302 | 1449 | |
| Ti48Pd50Zr2 | 400 | As − 30 | 631 | 1504 | |
| Ti48Pd50Zr2 | 563 | Af + 30 | - | 467 | |
| Ti47Pd50Zr3 | 563 | Af + 30 | - | 183 | |
| Ti47Pd50Zr3 | 490 | As − 30 | 219 | 666 | |
| Ti47Pd50Zr3 | 439 | Mf − 30 | 234 | 863 | |
| Ti45Pd50Zr5 | 538 | Af + 30 | - | 274 | [33] |
| Ti45Pd50Zr5 | 426 | As − 30 | 161 | 722 | [33] |
| Ti45Pd50Zr5 | 415 | Mf − 30 | 298 | 937 | |
| Ti43Pd50Zr7 | 536 | Af + 30 | - | 245 | |
| Ti43Pd50Zr7 | 461 | As − 30 | 255 | 733 | |
| Ti43Pd50Zr7 | 413 | Mf − 30 | 227 | 954 | |
| Ti40Pd50Zr10 | 337 | Af + 30 | - | 386 | [33] |
| Ti40Pd50Zr10 | 225 | As − 30 | 331 | 894 | [33] |
| Ti43Pd52Zr5 | 451 | As − 30 | 371 | 1080 | |
| Ti43Pd52Zr5 | 337 | Mf − 30 | 568 | 1378 | |
| Ti40Pd55Zr5 | 623 | Af + 30 | - | 386 | [35] |
| Ti40Pd55Zr5 | 408 | Mf − 30 | 1030 | 1492 | [35] |
| Ti45Pd50V5 | 591 | Af + 30 | - | 644 | [34] |
| Ti45Pd50V5 | 413 | Mf − 30 | 521 | 1252 | [34] |
| Ti40Pd55V5 | 589 | Af + 30 | 643 | 386 | [35] |
| Ti40Pd55V5 | 368 | Mf − 30 | 744 | 386 | [35] |
| Ti45Pd50Nb5 | 625 | Af + 30 | - | 389 | [34] |
| Ti45Pd50Nb5 | 378 | Mf − 30 | 329 | 836 | [34] |
| Ti40Pd55Nb5 | 565 | Af + 30 | 601 | 386 | [35] |
| Ti40Pd55Nb5 | 330 | Mf − 30 | 651 | 386 | [35] |
| Ti45Pd50Ta5 * | 625 | Af + 30 | - | 287 | [34] |
| Ti45Pd50Ta5 * | 483 | Mf − 30 | 462 | 941 | [34] |
| Ti45Pd50Cr5 | 333 | Af + 30 | - | 273 | [34] |
| Ti45Pd50Cr5 | 228 | Mf − 30 | 317 | 1000 | [34] |
| Ti45Pd50Mo5 | 503 | Af + 30 | - | 364 | [34] |
| Ti45Pd50Mo5 | 256 | Mf − 30 | 512 | 1239 | [34] |
| Ti45Pd50W5 * | 615 | Af + 30 | - | 232 | [34] |
| Ti45Pd50W5 * | 474 | Mf − 30 | 591 | 1008 | [34] |
| Ti50Pd48Ru2 | 320 | - | - | 301 | |
| Ti50Pd46Ru4 | 520 | Af + 30 | - | 150 | |
| Ti50Pd46Ru4 | 320 | - | 196 | 598 | |
| Ti50Pd46Ir4 | 580 | Af + 30 | - | 100 | |
| Ti50Pd46Ir4 | 454 | Mf − 30 | 260 | 740 | |
| Ti50Pd46Ir4 | 320 | - | 280 | 800 | |
| Ti50Pd42Ir8 | 551 | Af + 30 | 65 | 150 | |
| Ti50Pd42Ir8 | 408 | Mf − 30 | 320 | 720 | |
| Ti50Pd38Ir12 | 521 | - | 215 | 744 | |
| Ti50Pd38Ir12 | 401 | Mf − 30 | 360 | 850 | |
| Ti50Pd46Co4 | 520 | Af + 30 | - | 65 | |
| Ti50Pd46Co4 | 320 | - | 200 | 710 | |
| Ti50Pt50 | 1100 | Af + 50 | - | 27 | [38] |
| Ti50Pt50 | 910 | Mf − 50 | - | 320 | [38] |
| Ti50Pt37.5Ir12.5 | 1153 | Af + 50 | - | 42 | [38] |
| Ti50Pt37.5Ir12.5 | 928 | Mf − 50 | 300 | 640 | [38] |
| Ti50Pt25Ir25 | 1240 | Af + 50 | - | 35 | [38] |
| Ti50Pt25Ir25 | 1018 | Mf − 50 | 230 | 450 | [38] |
| Ti50Pt12.5Ir37.5 | 1268 | Af + 50 | - | 170 | [38] |
| Ti50Pt12.5Ir37.5 | 1119 | Mf − 50 | 285 | 435 | [38] |
| Ti50Pt45Ru5 | - | Af + 50 | - | 100 | [40,42] |
| Ti50Pt45Ru5 | 800 | Mf − 50 | 355 | 712 | [42] |
| Ti45Pt50Zr5 | - | Af + 50 | - | 111 | [40,42] |
| Ti45Pt50Zr5 | 790 | Mf − 50 | 600 | 1468 | [40,42] |
| Ti50Pt45Co5 | - | Af + 50 | - | 72 | [40,43] |
| Ti50Pt45Co5 | - | Mf − 50 | 407 | 523 | [40,43] |
| Ti45Pt50Hf5 | - | Af + 50 | - | 149 | [40,44] |
| Ti45Pt50Hf5 | - | Mf − 50 | 565 | 1113 | [40,44] |
| Alloy | Test Temp., °C | The Difference from Martensite Transformation Temperature, °C | Detwining Stress, MPa | 0.2% Flow Stress, MPa | Ref. |
|---|---|---|---|---|---|
| Ti45Pd45Pt5Zr5 | - | Af + 30 | - | 281 | [45] |
| Ti45Pd45Pt5Zr5 | 425 | Mf − 30 | 246 | 877 | [45] |
| Ti45Pd35Pt15Zr5 | - | Af + 30 | - | 315 | [45] |
| Ti45Pd35Pt15Zr5 | 442 | Mf − 30 | 387 | 1080 | [45] |
| Ti45Pd25Pt25Zr5 | - | Af + 30 | - | 231 | [45] |
| Ti45Pd25Pt25Zr5 | 509 | Mf − 30 | 436 | 1205 | [45] |
| Ti45Zr5Pd20Ni5Pt25 | 628 | Af + 30 | - | 322 | [49] |
| Ti45Zr5Pd20Ni5Pt25 | 402 | Mf − 30 | - | 1266 | [49] |
| Ti45Zr5Pd20Ni10Pt20 | 472 | Af + 30 | - | 610 | [49] |
| Ti45Zr5Pd20Ni10Pt20 | 226 | Mf − 30 | - | 1615 | [49] |
| Ti45Zr5Pd25Pt20Au5 | 620 | Af + 30 | - | 267 | [50] |
| Ti45Zr5Pd25Pt20Au5 | 423 | Mf − 30 | - | 1169 | [50] |
| Ti45Zr5Pd25Pt20Co5 | 568 | Af + 30 | - | 269 | [50] |
| Ti45Zr5Pd25Pt20Co5 | 294 | Mf − 30 | - | 741 | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamabe-Mitarai, Y. TiPd- and TiPt-Based High-Temperature Shape Memory Alloys: A Review on Recent Advances. Metals 2020, 10, 1531. https://doi.org/10.3390/met10111531
Yamabe-Mitarai Y. TiPd- and TiPt-Based High-Temperature Shape Memory Alloys: A Review on Recent Advances. Metals. 2020; 10(11):1531. https://doi.org/10.3390/met10111531
Chicago/Turabian StyleYamabe-Mitarai, Yoko. 2020. "TiPd- and TiPt-Based High-Temperature Shape Memory Alloys: A Review on Recent Advances" Metals 10, no. 11: 1531. https://doi.org/10.3390/met10111531
APA StyleYamabe-Mitarai, Y. (2020). TiPd- and TiPt-Based High-Temperature Shape Memory Alloys: A Review on Recent Advances. Metals, 10(11), 1531. https://doi.org/10.3390/met10111531





